GATE 2024 Life Sciences Question Paper PDF is available here. IISc Banglore conducted GATE 2024 Life Sciences exam on February 10 in the Afternoon Session from 2:30 PM to 5:30 PM. Students have to answer 65 questions in GATE 2024 Life Sciences Question Paper carrying a total weightage of 100 marks. 10 questions are from the General Aptitude section and 55 questions are from Core Discipline.
GATE 2024 Life Sciences Question Paper with Solutions PDF
| GATE 2024 Life Sciences Question Paper with Solutions PDF | Download PDF | Check Solutions |

If ‘\( \to \)’ denotes increasing order of intensity, then the meaning of the words \([ walk \to jog \to sprint ]\) is analogous to \([ bothered \to \_\_\_\_\_\_ \to daunted ]\). Which one of the given options is appropriate to fill the blank?
Two wizards try to create a spell using all the four elements, water, air, fire, and earth. For this, they decide to mix all these elements in all possible orders. They also decide to work independently. After trying all possible combinations of elements, they conclude that the spell does not work. How many attempts does each wizard make before coming to this conclusion, independently?
In an engineering college of 10,000 students, 1,500 like neither their core branches nor other branches. The number of students who like their core branches is \( \frac{1}{4} \) of the number of students who like other branches. The number of students who like both their core and other branches is 500. The number of students who like their core branches is:
For positive non-zero real variables \( x \) and \( y \), if \[ \ln \left( \frac{x + y}{2} \right) = \frac{1}{2} \left[ \ln(x) + \ln(y) \right], \]
then, the value of \( \frac{x}{y} + \frac{y}{x} \) is:
In the sequence \(6, 9, 14, x, 30, 41\), a possible value of \(x\) is:
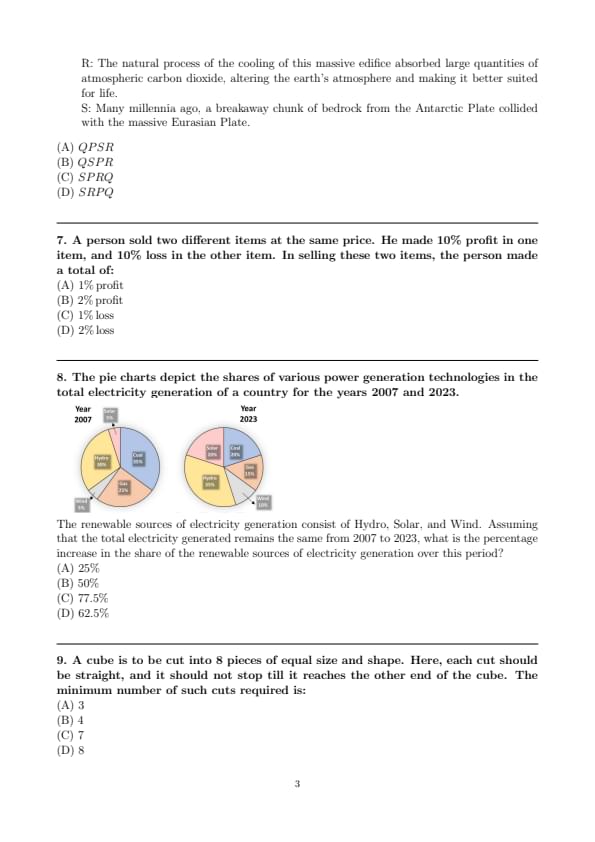
Sequence the following sentences in a coherent passage.
P: This fortuitous geological event generated a colossal amount of energy and heat that resulted in the rocks rising to an average height of 4 km across the contact zone.
Q: Thus, the geophysicists tend to think of the Himalayas as an active geological event rather than as a static geological feature.
R: The natural process of the cooling of this massive edifice absorbed large quantities of atmospheric carbon dioxide, altering the earth’s atmosphere and making it better suited for life.
S: Many millennia ago, a breakaway chunk of bedrock from the Antarctic Plate collided with the massive Eurasian Plate.
A person sold two different items at the same price. He made 10% profit in one item, and 10% loss in the other item. In selling these two items, the person made a total of:
The pie charts depict the shares of various power generation technologies in the total electricity generation of a country for the years 2007 and 2023.

The renewable sources of electricity generation consist of Hydro, Solar, and Wind. Assuming that the total electricity generated remains the same from 2007 to 2023, what is the percentage increase in the share of the renewable sources of electricity generation over this period?
A cube is to be cut into 8 pieces of equal size and shape. Here, each cut should be straight, and it should not stop till it reaches the other end of the cube. The minimum number of such cuts required is:
In the \(4 \times 4\) array shown below, each cell of the first three rows has either a cross (X) or a number. The number in a cell represents the count of the immediate neighboring cells (left, right, top, bottom, diagonals) NOT having a cross (X). Given that the last row has no crosses (X), the sum of the four numbers to be filled in the last row is:

The CORRECT order of electronegativity is:
Which one of the following is the CORRECT representation of the variation of the Gibbs free energy (\( G \)) of a substance with temperature (\( T \)) at constant pressure?

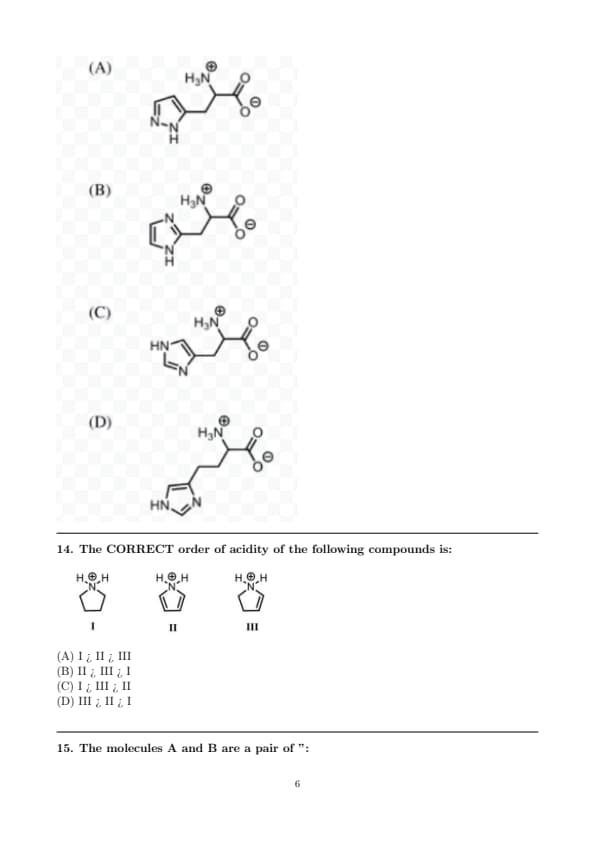
Among the following, the structure representing histidine is:

The CORRECT order of acidity of the following compounds is:

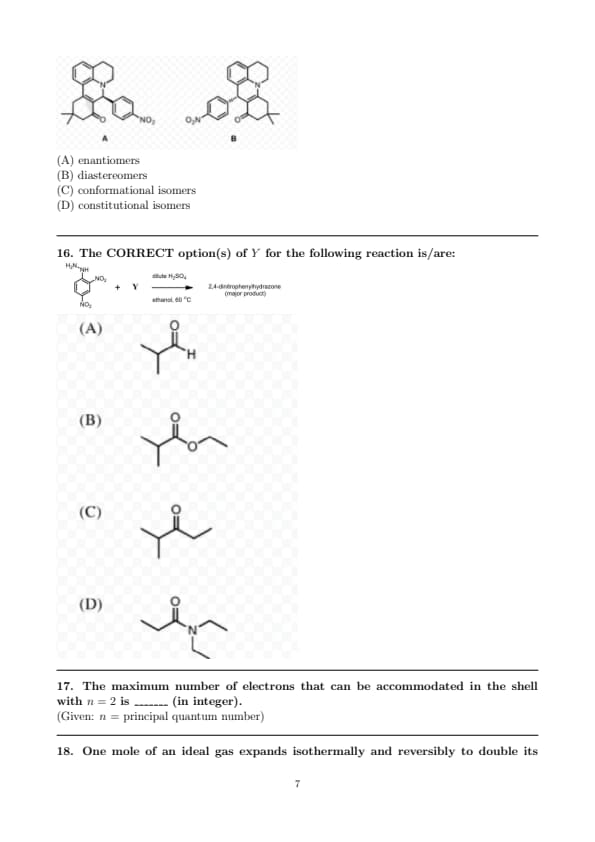
The molecules A and B are a pair of":

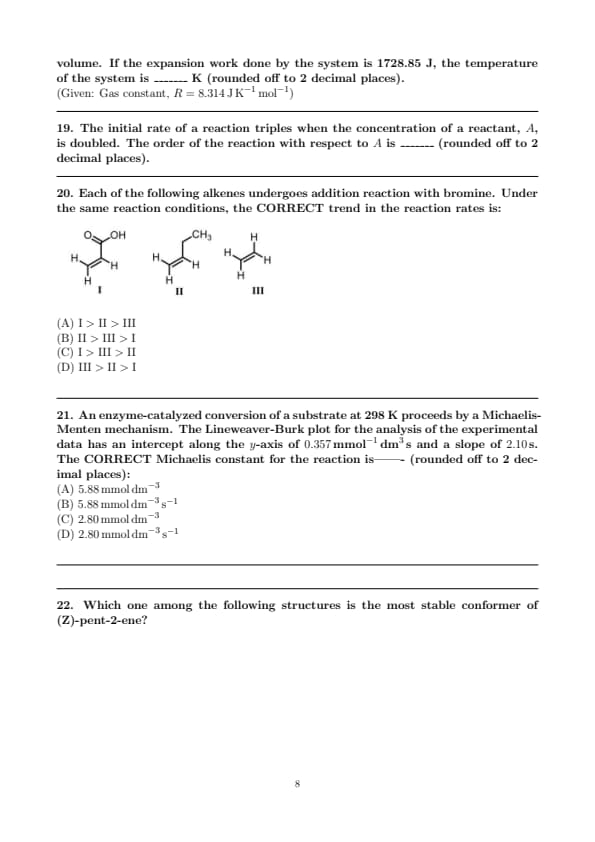
The CORRECT option(s) of \( Y \) for the following reaction is/are:


The maximum number of electrons that can be accommodated in the shell with \( n = 2 \) is _______ (in integer).
(Given: \( n \) = principal quantum number)
One mole of an ideal gas expands isothermally and reversibly to double its volume. If the expansion work done by the system is 1728.85 J, the temperature of the system is _______ K (rounded off to 2 decimal places).
(Given: Gas constant, \( R = 8.314 \, \mathrm{J \, K^{-1} \, mol^{-1}} \))
The initial rate of a reaction triples when the concentration of a reactant, \( A \), is doubled. The order of the reaction with respect to \( A \) is _______ (rounded off to 2 decimal places).
Each of the following alkenes undergoes addition reaction with bromine. Under the same reaction conditions, the CORRECT trend in the reaction rates is:

An enzyme-catalyzed conversion of a substrate at 298 K proceeds by a Michaelis-Menten mechanism. The Lineweaver-Burk plot for the analysis of the experimental data has an intercept along the \( y \)-axis of \( 0.357 \, mmol^{-1} \, dm^{3} \, s \) and a slope of \( 2.10 \, s \). The CORRECT Michaelis constant for the reaction is------- (rounded off to 2 decimal places):
Which one among the following structures is the most stable conformer of (Z)-pent-2-ene?

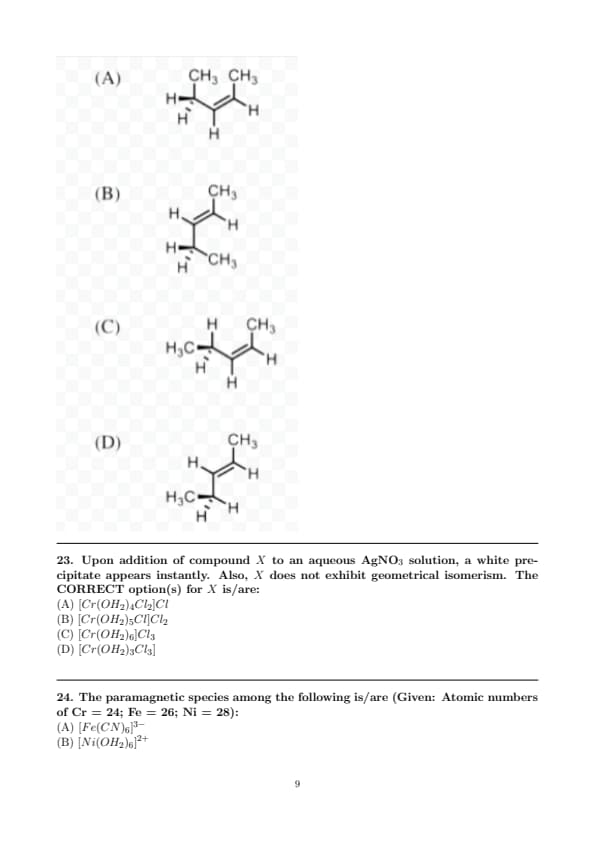
Upon addition of compound \( X \) to an aqueous AgNO\(_3\) solution, a white precipitate appears instantly. Also, \( X \) does not exhibit geometrical isomerism. The CORRECT option(s) for \( X \) is/are:
The paramagnetic species among the following is/are (Given: Atomic numbers of Cr = 24; Fe = 26; Ni = 28):
The molecule(s) with non-zero dipole moment is/are:
The ionic product of water at \(40 \,^{\circ}\mathrm{C}\) is \(2.92 \times 10^{-14} \, \mathrm{M}^2\). The pH of water at \(40 \,^{\circ}\mathrm{C}\) is _______ (rounded off to 2 decimal places).
Given the standard reduction potentials (\(E^\Theta\)) for the half-cell reactions below, the standard Gibbs free energy of the dissolution of silver chloride in water, at 298 K, is ---- J mol\(^{-1}\) (rounded off to nearest integer).
\[ (Given: Faraday constant, F = 96500 \, C mol^{-1}); \, J = C \times V) \] \[ AgCl(s) + e^{-} \rightarrow Ag(s) + Cl^{-}(aq) ; \, E^\Theta = 0.22 \, V at 298 K \] \[ Ag^{+}(aq) + e^{-} \rightarrow Ag(s) \, ; \, E^\Theta = 0.80 \, V at 298 K \]
Which one of the following pairs of amino acids is NOT incorporated in a polypeptide chain?
Mammalian cells cultured at low temperature (25 to 30 °C) lead to an increased sterol content in the membrane. Elevated sterols in the membrane result in
Which one of the following metabolic intermediates is common to glycolysis, nucleotide synthesis, and glycogen synthesis?
In mammals, hematopoietic stem cells that give rise to different types of blood cells are known as:
Which one or more of the following statements correctly describe(s) the addition of N-nucleotides during the rearrangement of the immunoglobulin heavy chain-encoding gene?
A newly identified viral protein contains one long α-helix spanning 60 amino acid residues. The number of main chain H-bonds formed in this helix is _______. (Answer in integer)
In a lactic acid solution at pH 4.8, the concentrations of lactic acid and lactate are 0.01 M and 0.087 M, respectively. The calculated pKa of lactic acid is _______. (Round off to one decimal place)
If a 10 mM solution of a biomolecule in a cuvette of path length 10 mm absorbs 90% of the incident light at 280 nm, the molar extinction coefficient of the biomolecule at this wavelength is _______ M\(^{-1}\)cm\(^{-1}\). (Round off to two decimal places)
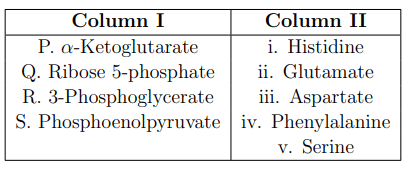
Metabolic intermediates provide the backbone for the synthesis of amino acids. Match the metabolic intermediates listed in Column I with their corresponding amino acids given in Column II.
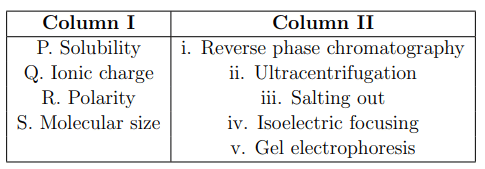
Which one of the following is the correct match between the molecular properties listed in Column I and the corresponding biochemical separation methods in Column II?
Which one or more of the following statements is/are correct regarding the electromotive force generated by the electron transfer chain?
Which one or more of the following statements is/are correct regarding the transport and retention of proteins in different cell organelles?
Which one or more of the following statements correctly describe(s) fluorescence spectroscopy?
Which one or more of the following statements is/are correct in the processing of pre-mRNA in eukaryotes?
Which one or more of the following statements correctly describe(s) the changes upon the addition of puromycin during eukaryotic translation?
Factor H, a complement regulatory protein in plasma, binds C3b and:
In Michaelis-Menten's equation, if \([S] = 15 K_m\), then the ratio \(\frac{V_0}{V_{max}}\) is _______. (Round off to three decimal places)
A 5250 base-pair long plasmid with 10 negative supercoils would have a linking number of _______, considering 10.5 base pairs per turn for B DNA. (Answer in integer)
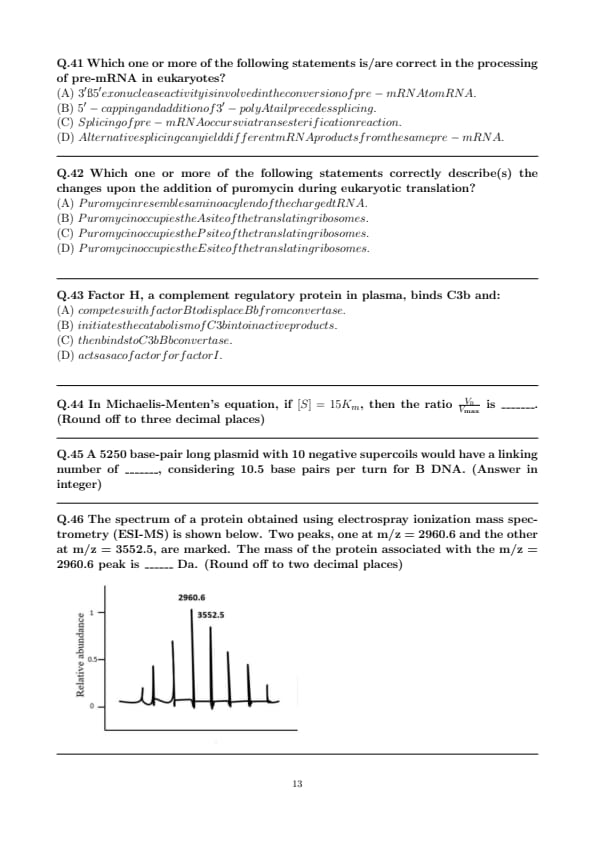
The spectrum of a protein obtained using electrospray ionization mass spectrometry (ESI-MS) is shown below. Two peaks, one at m/z = 2960.6 and the other at m/z = 3552.5, are marked. The mass of the protein associated with the m/z = 2960.6 peak is ______ Da. (Round off to two decimal places)

Which one of the following plant families does apple (Malus domestica) belong to?
The collateral and open type of vascular bundle with endarch xylem strand is usually found in
Which of the following tissue types is/are established during embryogenesis in wild-type Arabidopsis thaliana?
Which of the following plant natural products is/are cyanogenic glycosides?
Which of the following plant diseases is/are caused by nematode?
Which of the following selectable marker genes is/are used for herbicide tolerance during genetic transformation of plants?
Which of the following statements is/are CORRECT with reference to rubber production from plants?
In the Calvin-Benson cycle, to produce 1 molecule of glyceraldehyde 3-phosphate by fixing 3 molecules of carbon dioxide, 9 molecules of ATP and ____ molecules (in integer) of NADPH are typically utilized.
In wild-type Arabidopsis thaliana, the four types of floral organs (sepal, petal, stamen, carpel) are arranged in concentric whorls from outside to inside. With reference to the ABC model of floral organ patterning, match the homeotic mutants in Group 1 with their respective arrangements of organs in the four whorls given in Group 2.

- (A) \( P–iv, Q–ii, R–i \)
- (B) \( P–iii, Q–i, R–ii \)
- (C) \( P–ii, Q–i, R–iii \)
- (D) \( P–iii, Q–i, R–iv \)
Match the inhibitors in Group 1 with their respective targets in Group 2.

With reference to Agrobacterium tumefaciens mediated plant transformation, match the virulence factors in Group 1 with their protein types in Group 2.

Match the plant products in Group 1 with the plant species in Group 2 that produce them and the respective plant parts in Group 3 where they accumulate the most.

Match the types of ecological interactions in Group 1 with their respective definitions in Group 2.

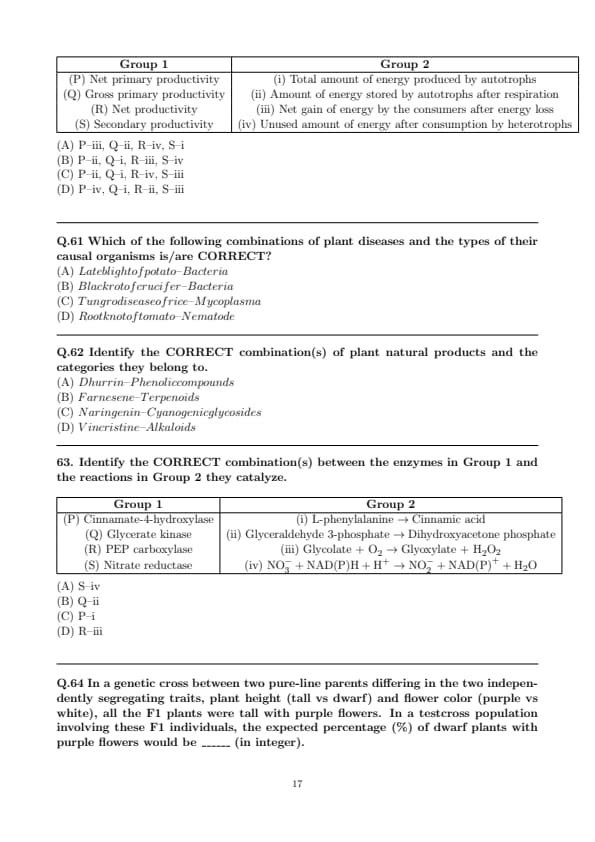
Match the types of ecological energy productivity in Group 1 with their respective definitions in Group 2.

Which of the following combinations of plant diseases and the types of their causal organisms is/are CORRECT?
Identify the CORRECT combination(s) of plant natural products and the categories they belong to.
Identify the CORRECT combination(s) between the enzymes in Group 1 and the reactions in Group 2 they catalyze.

In a genetic cross between two pure-line parents differing in the two independently segregating traits, plant height (tall vs dwarf) and flower color (purple vs white), all the F1 plants were tall with purple flowers. In a testcross population involving these F1 individuals, the expected percentage (%) of dwarf plants with purple flowers would be ______ (in integer).
The mRNA of a hypothetical plant gene \( HSDU \) is 800-nucleotide long and encodes a protein of 160 amino acid residues. The calculated length of \( HSDU \) CDS would be ____ nucleotides (in integer).
Which one of the following bacterial species can cause atypical pneumonia?
Which one of the following organisms has axial filaments?
Who among the following scientists was the pioneer in development of chemotherapy?
In which of the following processes, glutaraldehyde is used as a sterilizing agent?
The most abundant class of immunoglobulins in serum is ________.
Which one of the following double-stranded sequences will NOT be recognized by a Type IIP restriction endonuclease?
Which one of the following use inorganic compounds as an energy source?
Which one of the following represents the abundance of the organisms found in soil?
Match the antibiotics in Group I with the microorganisms that produce them in Group II.

Which one of the following redox couples has the highest tendency to donate electrons?
Which of the following is/are active transport mechanism(s) in prokaryotes where the substance is chemically altered during transport across the membrane?
Which of the following cocci is/are examples of division in one plane?
Which of the following event(s) occur(s) during translation in prokaryotes?
Which of the following is/are consequence(s) of nitrous acid (HNO\(_2\)) mediated deamination?
At root nodules, which of the following C4 organic acid(s) is/are transported across the symbiosome membrane and into bacteroids?
Which of the following is/are TRUE about the Escherichia coli chromosome?
At \( t = 0 \), the bacterial cell number is 10,000 cells/mL. At \( t = 480 \) minutes, the cell number increased to 320,000 cells/mL. The mean generation time during this exponential growth period, rounded off to the nearest integer is ____ minutes.
A landfill sample was analyzed by dilution and plating techniques for viable bacterial count. When one gram of the landfill sample was diluted \(1 \times 10^4\) (w/v) it yielded 400 CFU. The viable bacterial count (in million, rounded off to the nearest integer) in one gram landfill sample is ____.
A fluorescence microscope with an objective lens of numerical aperture (NA) 1.5 is used with light of wavelength (\(\lambda\)) 600 nanometers. The lateral resolution limit of this microscope rounded off to the nearest integer, is ____ nanometers.
Which one of the following statements about gene expression is INCORRECT?
Which one of the following tissues/organs is least likely to experience graft rejection when transplanted from a person to an unrelated person?
Codon bias is correlated with the relative frequencies of which one of the following types of RNA?
CREB1 is a eukaryotic transcription factor. In which one of the following compartments of the cell is CREB1 predominantly localized?
In certain species of salamanders, male-female pairs have multiple mating partners in a breeding season. Which one of the following terminologies accurately describes this mating system?
Which one of the following statements describes the key function of human sweat glands?
Urease enzyme catalyzes the conversion of urea into ammonia and carbon dioxide. Which one of the following organisms expresses urease enzyme?
The human genetic code is triplet in nature with 64 codons made using four nucleotides. If the human genetic code was doublet in nature, the number of codons theoretically possible from four nucleotides is ____. (Answer in integer)
Which one of the following statements is NOT TRUE of glycosaminoglycans?
Which one of the options correctly matches the human tissues/organs with their embryonic germ layers of origin?

Consider a large population of a finch species, where both small and big beak sizes are advantageous, and an intermediate beak size is maladaptive. Over a period of 10 years, which one of the following evolutionary processes is most likely to operate on the beak size of this finch population?
When the blood glucose level of a healthy person is 100 mg/dL, which one of the following options is most likely to represent the level of glucose in the urine of that person?
Which one of the following rooted tree topologies best describes the primate phylogeny?

Consider a species of brightly colored beetle. Which one or more of the following observations suggest(s) that this species is aposematic?
The embryos of which one or more of the following animals show meroblastic cleavage?
Which one or more of the following parasites is/are typically transmitted by mosquitoes as vector?
Consider the following nucleotide sequence:
5'-GCGCCCAUGGCGUCGCUACGUCGCGCUCACGCGGACGAUCGUACGUAAUGAUGAA-3'
Assume canonical initiation, canonical termination, no post-translational modification, and the average molecular mass of an amino acid to be 110 daltons.
The theoretical molecular mass of the polypeptide translated from the above nucleotide sequence is ____ daltons. (Answer in integer)
The pKa of a buffer solution with pH of 5, consisting of 0.4 M sodium acetate and 0.04 M acetic acid, is ____. (Answer in integer)
Consider a healthy person with the following lung volumes:
Residual volume = 900 mL
Expiratory reserve volume = 800 mL
Tidal volume = 200 mL
If the Total lung capacity is 5500 mL, then the Inspiratory reserve volume of the person is ____ mL. (Answer in integer)
Which one of the following fungi produces aflatoxins?
Under standard conditions in animal feeding studies, the weight gained (in grams) per gram of protein consumed by an animal is termed as
Xerophthalmia is caused due to the deficiency of:
Which one of the following steps is used to remove phosphatides from crude oil in the refining process?
The unique flavor of chocolate and cocoa is due to the formation of:
Which one of the following statements regarding Hazard Analysis Critical Control Point (HACCP) plan is NOT correct?
The product of cabbage fermentation by Leuconostoc mesenteroides is:
Which one of the following absorbents is NOT used as an ethylene absorber in active packaging of fruits and vegetables?
Which one of the following statements regarding moisture sorption isotherms of a dried food is NOT correct?
Processing of fluid milk at \( 72^\circ C \) for 15 seconds is termed as:
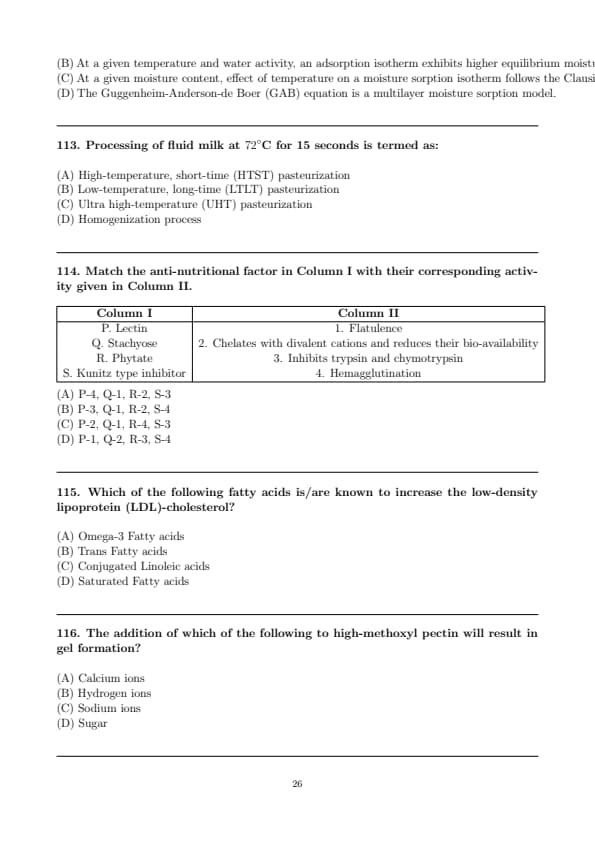
Match the anti-nutritional factor in Column I with their corresponding activity given in Column II.

Which of the following fatty acids is/are known to increase the low-density lipoprotein (LDL)-cholesterol?
The addition of which of the following to high-methoxyl pectin will result in gel formation?
Which of the following steps in food processing is/are used to reduce the acrylamide formation in food products?
Which of the following enzymes is/are used for the production of high fructose syrup (HFS) from corn starch?
Which of the following is/are typical characteristic(s) of a fungal cell?
Which of the following statements is/are correct regarding food and waterborne diseases and the class of causative microorganisms?
Which of the following statements is/are true?
A 10 kg tomato pulp is concentrated from an initial moisture content of \( 90% \) (wet weight basis) to \( 35% \) (wet weight basis). The weight of the concentrate in kg is _____ (round off to 2 decimal places).

























Comments