GATE 2023 Life Sciences (XL) Question Paper PDF is available here for download. IIT Kanpur conducted GATE 2023 Life Sciences exam on February 11, 2023 in the Forenoon Session from 09:30 AM to 12:30 PM. Students have to answer 65 questions in GATE 2023 Life Sciences Question Paper carrying a total weightage of 100 marks. 10 questions are from the General Aptitude section and 55 questions are from Core Discipline.
GATE 2023 Life Sciences (XL) Question Paper with Solutions PDF
| GATE 2023 Life Sciences (XL) Question Paper with Solutions | Check Solutions |

The village was nestled in a green spot, ______ the ocean and the hills.
Disagree : Protest : : Agree : ______ (By word meaning)
A ‘frabjous’ number is defined as a 3 digit number with all digits odd, and no two adjacent digits being the same. For example, 137 is a frabjous number, while 133 is not. How many such frabjous numbers exist?
Which one among the following statements must be TRUE about the mean and the median of the scores of all candidates appearing for GATE 2023?
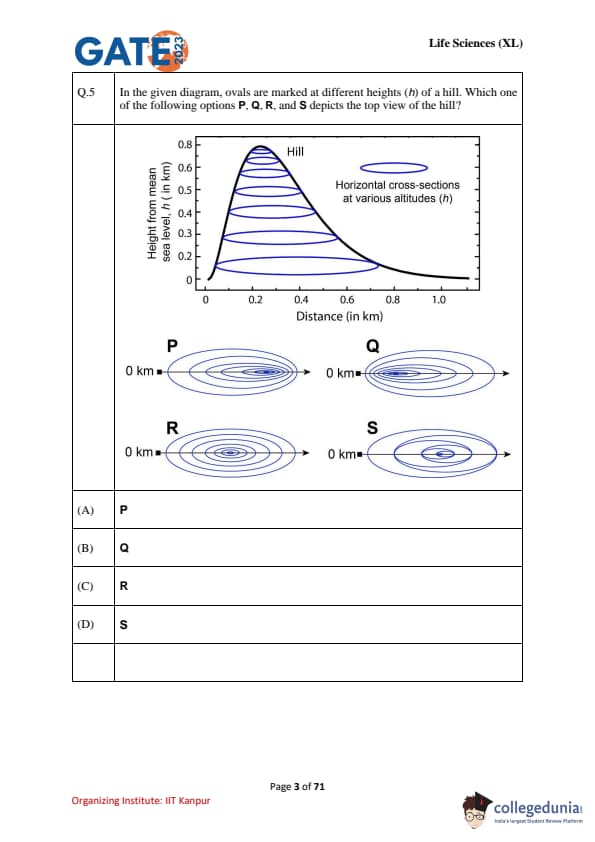
In the given diagram, ovals are marked at different heights (h) of a hill. Which one of the following options P, Q, R, and S depicts the top view of the hill?

Residency is a famous housing complex with many well-established individuals among its residents. A recent survey conducted among the residents of the complex revealed that all of those residents who are well established in their respective fields happen to be academicians. The survey also revealed that most of these academicians are authors of some best-selling books. Based only on the information provided above, which one of the following statements can be logically inferred with certainty?
Ankita has to climb 5 stairs starting at the ground, while respecting the following rules:
1. At any stage, Ankita can move either one or two stairs up.
2. At any stage, Ankita cannot move to a lower step.
Let \(F(N)\) denote the number of possible ways in which Ankita can reach the \(N^{th}\) stair. For example, \(F(1) = 1\), \(F(2) = 2\), \(F(3) = 3\). The value of \(F(5)\) is _______.
The information contained in DNA is used to synthesize proteins that are necessary for the functioning of life. DNA is composed of four nucleotides: Adenine (A), Thymine (T), Cytosine (C), and Guanine (G). The information contained in DNA can then be thought of as a sequence of these four nucleotides: A, T, C, and G. DNA has coding and non-coding regions. Coding regions—where the sequence of these nucleotides are read in groups of three to produce individual amino acids—constitute only about 2% of human DNA. For example, the triplet of nucleotides CCG codes for the amino acid glycine, while the triplet GGA codes for the amino acid proline. Multiple amino acids are then assembled to form a protein.
Based only on the information provided above, which of the following statements can be logically inferred with certainty?
(i) The majority of human DNA has no role in the synthesis of proteins.
(ii) The function of about 98% of human DNA is not understood.
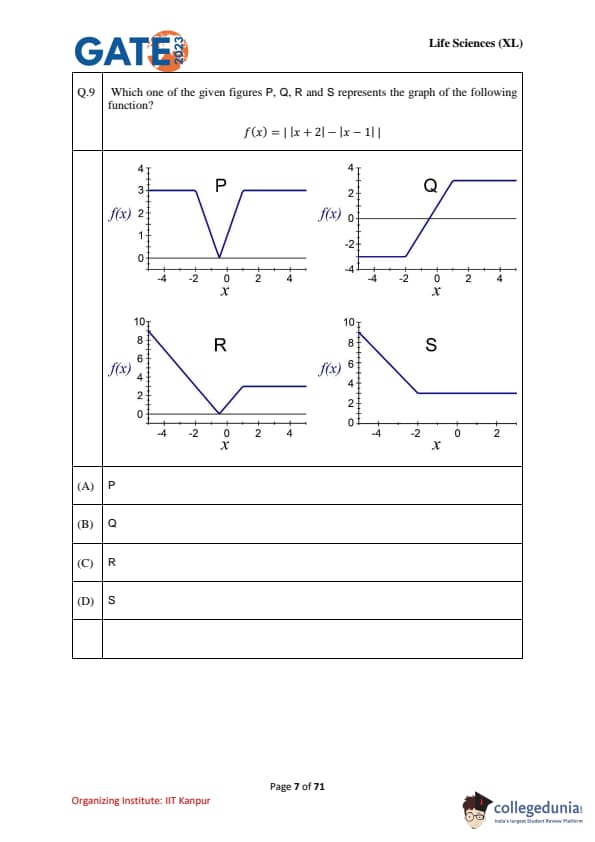
Which one of the given figures P, Q, R, and S represents the graph of the following function? \[ f(x) = |x + 2| - |x - 1| \]

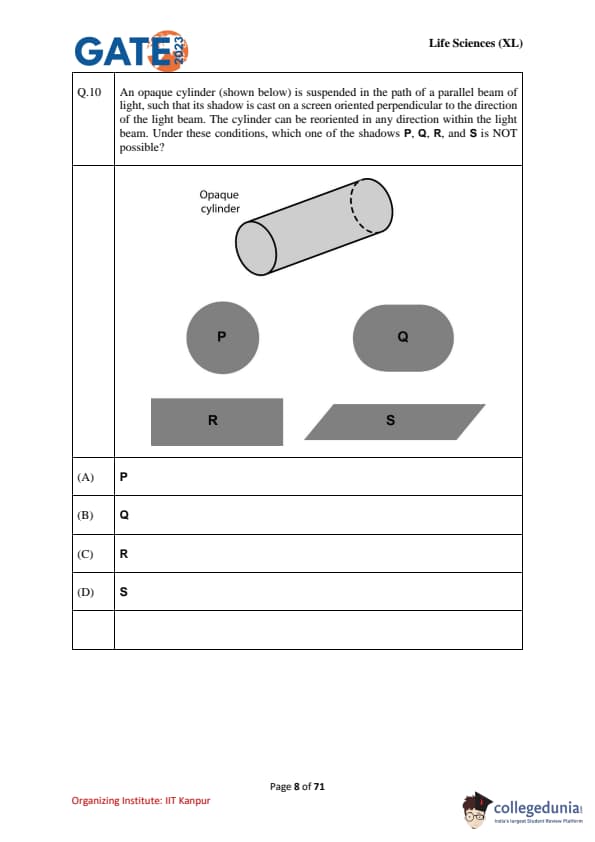
An opaque cylinder (shown below) is suspended in the path of a parallel beam of light, such that its shadow is cast on a screen oriented perpendicular to the direction of the light beam. The cylinder can be reoriented in any direction within the light beam. Under these conditions, which one of the shadows P, Q, R, and S is NOT possible?

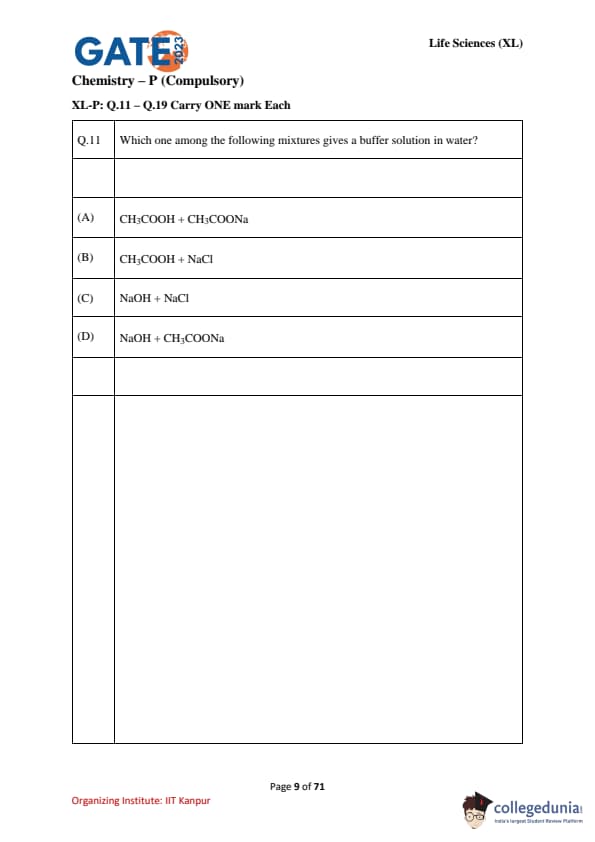
Which one among the following mixtures gives a buffer solution in water?
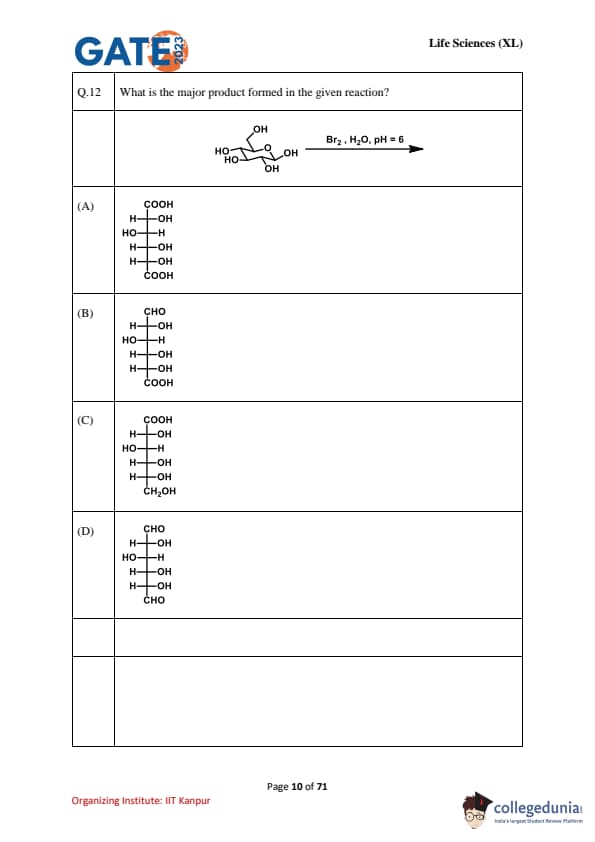
What is the major product formed in the given reaction?
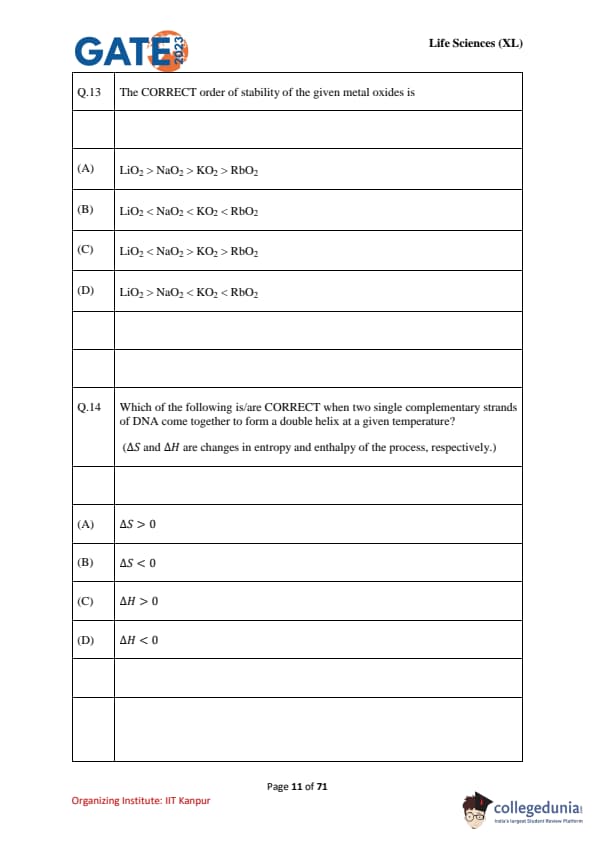
Which of the following is/are CORRECT when two single complementary strands of DNA come together to form a double helix at a given temperature? \((\Delta S and \Delta H are changes in entropy and enthalpy of the process, respectively.)\)
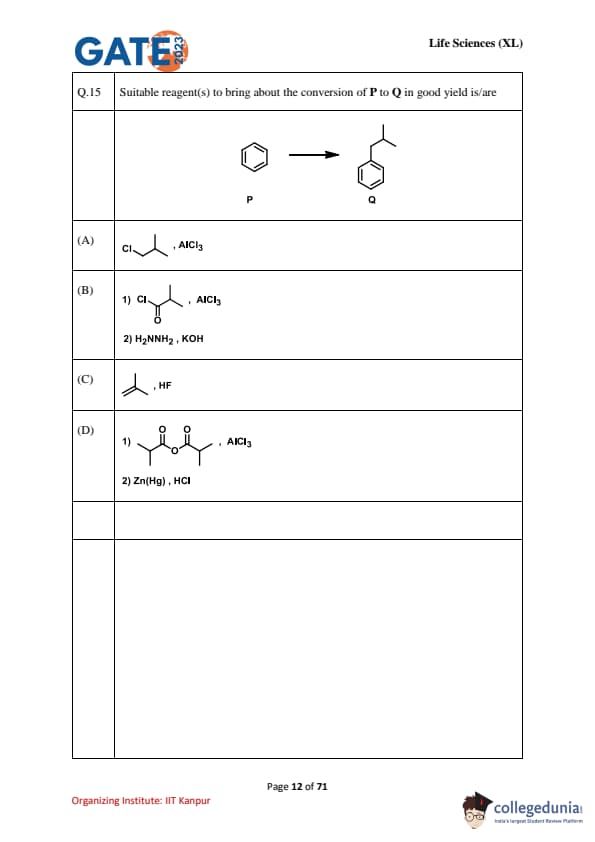
Suitable reagent(s) to bring about the conversion of P to Q in good yield is/are

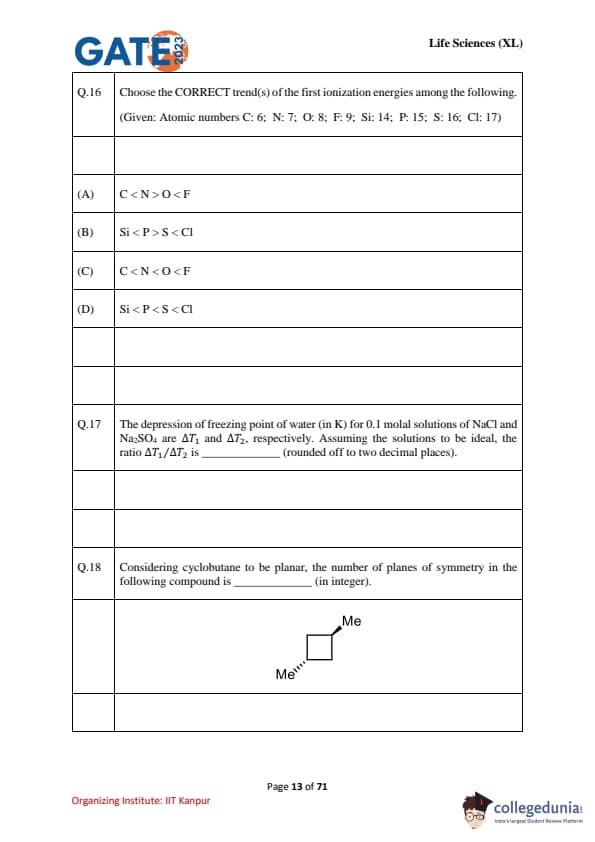
Choose the CORRECT trend(s) of the first ionization energies among the following. (Given: Atomic numbers C: 6; N: 7; O: 8; F: 9; Si: 14; P: 15; S: 16; Cl: 17)
The depression of freezing point of water (in K) for 0.1 molal solutions of NaCl and Na\(_2\)SO\(_4\) are \(\Delta T_1\) and \(\Delta T_2\), respectively. Assuming ideal solutions, find the ratio \(\Delta T_1/\Delta T_2\) (rounded off to two decimal places).
Considering cyclobutane to be planar, the number of planes of symmetry in the following compound is ____ (in integer).
The dipole moment (\(\mu\)) of BrF is 1.42 D and the bond length is 176 pm. The atomic charge distribution (\(q\)) in the molecule is ____ (rounded off to two decimal places). (Given: 1 D = \(3.34 \times 10^{-30}\) C m; electronic charge \(e = 1.60 \times 10^{-19}\) C).
Consider two different paths in which the volume of an ideal gas doubles isothermally:
(i) Reversible expansion (work done = \( w_{rev} \))
(ii) Irreversible expansion, with the external pressure equal to the final pressure of the gas (work done = \( w_{irrev} \))
Here, \[ \dfrac{w_{rev}}{w_{irrev}} = ? \]
A mixture of four peptides, PKKRK, RGERV, RYRGV and LVVYP, is loaded onto an ion-exchange column at pH = 7.2. If carboxymethyl (CM) cellulose is used as the stationary phase of this column, then which peptide elutes first?

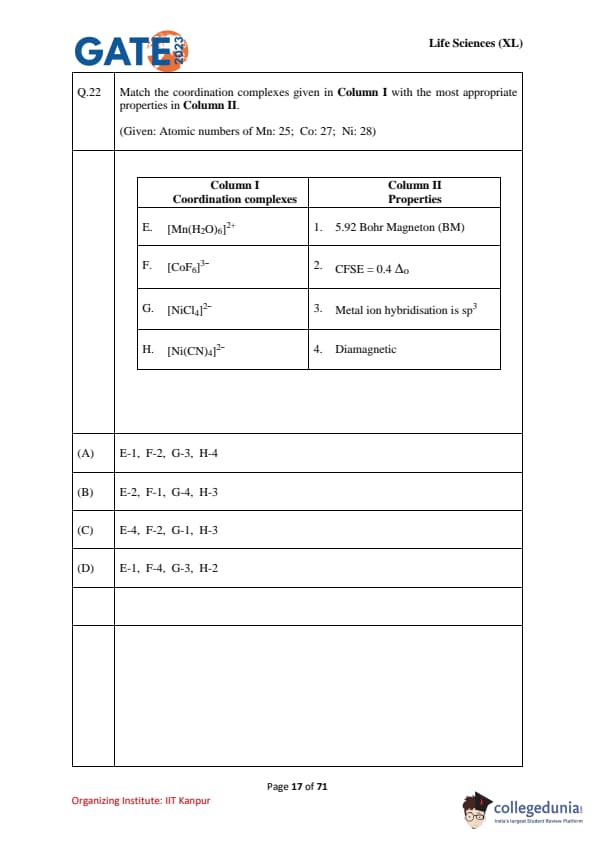
Match the coordination complexes given in Column I with the most appropriate properties in Column II.

(Given: Atomic numbers of Mn: 25; Co: 27; Ni: 28)
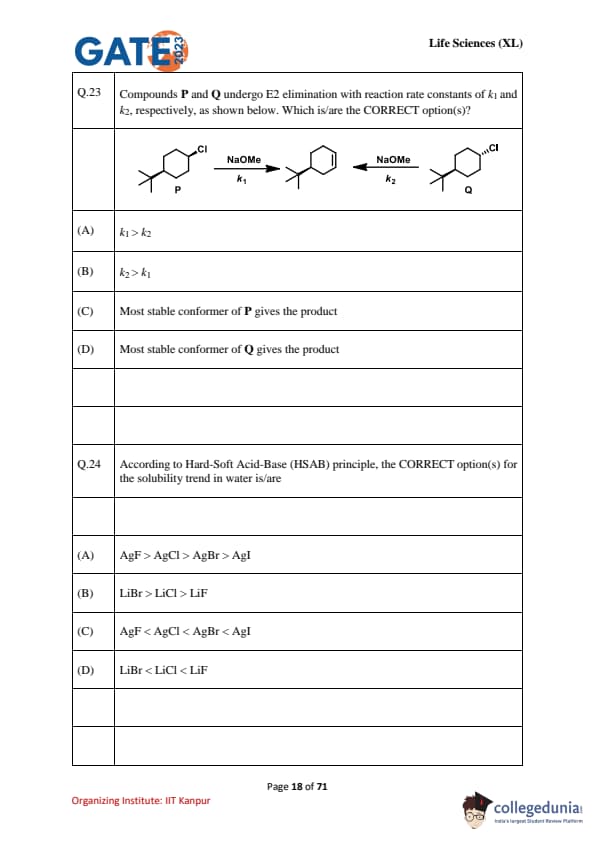
Compounds P and Q undergo E2 elimination with reaction rate constants of \(k_1\) and \(k_2\), respectively, as shown below. Which is/are the CORRECT option(s)?

According to Hard–Soft Acid–Base (HSAB) principle, the CORRECT option(s) for the solubility trend in water is/are
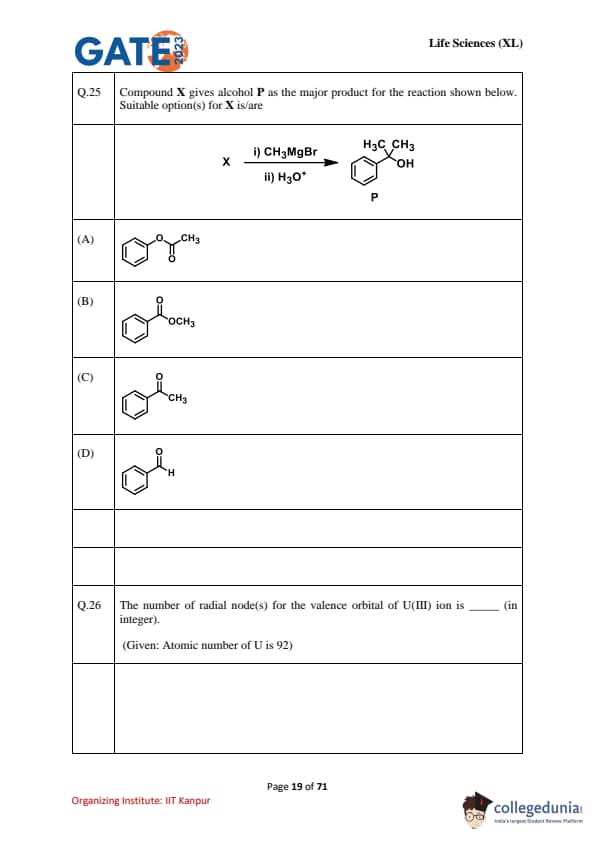
Compound \(X\) gives alcohol \(P\) as the major product for the reaction: \;i) CH\(_3\)MgBr \quad ii) H\(_3\)O\(^+\). Suitable option(s) for \(X\) is/are. \;(\(P\) is the benzylic \emph{tertiary alcohol bearing two CH\(_3\) groups)

The number of radial node(s) for the valence orbital of U(III) ion is ____ (in integer). (Given: Atomic number of U is 92)
\(E^\circ=1.10\ V\) for the cell: \( \mathrm{Zn(s)+Cu^{2+}(aq) \rightleftharpoons Zn^{2+}(aq)+Cu(s)}\). At \(298\ K\), the equilibrium constant is \(y\times 10^{37}\). Find \(y\) (to two decimals). (Given: \(F=96485\ C mol^{-1}\), \(R=8.314\ J K^{-1}mol^{-1}\)).
Determine the correctness or otherwise of the following Assertion [a] and the Reason [r].
Assertion [a]: On a per carbon basis, palmitic acid yields more ATP than glucose.
Reason [r]: Carbons in palmitic acid are more reduced than those in glucose.
When cell components are fractionated by sedimentation, the correct order (from lower to higher gravitational force, g) in which the components get separated is _________.
In a population, the probability of a susceptible individual getting infected with SARS-CoV-2 is low when a majority of individuals in the population becomes immune to this virus. This phenomenon is known as _____.
Given below are four reactions of the glycolytic pathway catalyzed by the enzymes E1, E2, E3, and E4, as indicated. Which of these enzymes is/are NOT part of the gluconeogenesis pathway?
{(i) Fructose-6-phosphate \(\xrightarrow{E1}\) Fructose-1,6-bisphosphate
(ii) Fructose-1,6-bisphosphate \(\xrightarrow{E2}\) DHAP + GAP
(iii) 3-Phosphoglycerate \(\xrightarrow{E3}\) 2-Phosphoglycerate
(iv) Phosphoenolpyruvate \(\xrightarrow{E4}\) Pyruvate)
Which of the following molecules is/are second messenger(s) produced by the phosphoinositide signaling cascade?
A protein has seven cysteine residues. The maximum number of disulfide bonds of different combinations that can possibly be formed by these seven cysteine residues is ______ (in integer).
A lyophilized sample of 20 nanomoles of an oligonucleotide is dissolved in water and the volume of the solution is made up to 200 \(\mu\)L. The concentration (in \(\mu\)M) of the oligonucleotide in this solution is ____ (in integer).
DNA in a 1 cm long chromatin contains \(5 \times 10^{9}\) base pairs. The fold compaction of this DNA within the chromatin is ____ (in integer).
Intracellular concentrations of ATP, ADP, and inorganic phosphate in four cell types are given below. Which one of these cell types has the most negative \(\Delta G\) for ATP hydrolysis?
\begin{tabular{|c|c|c|c|
\hline
Cell type & ATP (mM) & ADP (mM) & Inorganic phosphate (mM)
\hline
L & 3.0 & 1.8 & 5.0
\hline
K & 3.9 & 1.3 & 3.0
\hline
B & 2.7 & 0.7 & 2.7
\hline
M & 7.2 & 0.9 & 8.0
\hline
\end{tabular
Which one of the following amino acids has more than two acid-base groups?
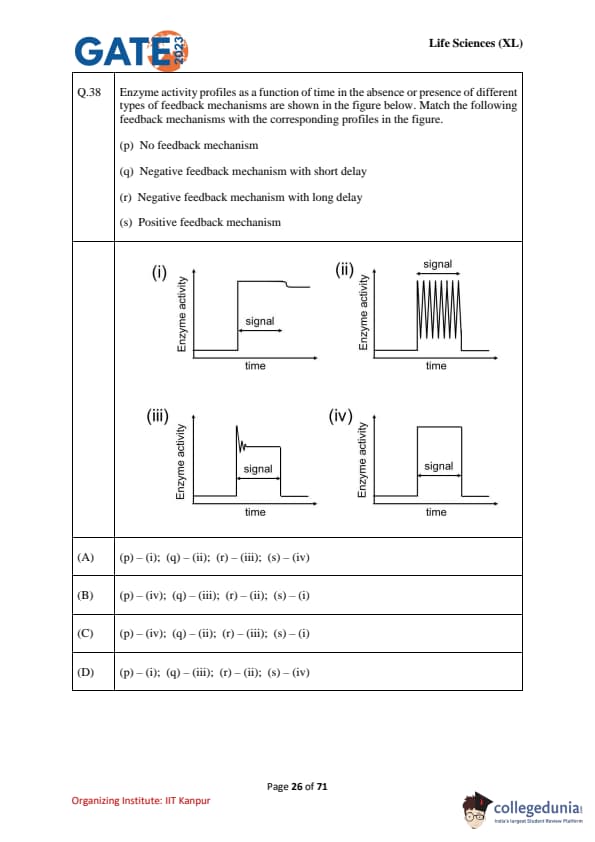
Enzyme activity profiles as a function of time in the absence or presence of different types of feedback mechanisms are shown in the figure below. Match the following feedback mechanisms with the corresponding profiles in the figure.
(p) No feedback mechanism
(q) Negative feedback mechanism with short delay
(r) Negative feedback mechanism with long delay
(s) Positive feedback mechanism
A linear DNA fragment of 5 kb gives 2.5 kb, 1.5 kb, and 1.0 kb fragments on complete EcoRI digestion; the same 5 kb fragment gives 3.5 kb and 1.5 kb fragments on complete XbaI digestion. What set of fragments will appear when the 5 kb piece is fully digested with EcoRI and XbaI simultaneously?
Match the cell types in Group I with the processes in Group II.
Group I
(p) NK cells
(q) B cells
(r) Mast cells
(s) Neutrophils
Group II
(i) Antibody production
(ii) First cells to be recruited at the site of infection
(iii) Antibody-dependent cell-mediated cytotoxicity
(iv) Histamine production
Four statements about lipids are given below as options. Choose the statement(s) which is/are CORRECT.
Which of the following technique(s) can be used to separate proteins according to their molecular weights from a mixture of proteins?
B cells produce two forms of an immunoglobulin: (i) membrane-bound form, known as B cell receptor (BCR) and (ii) soluble form, known as antibody. Which of the following statements is/are CORRECT about BCR and antibody produced by the same B cell?
A 100 ml solution of pH 10 was well-mixed with a 100 ml solution of pH 4. The pH of the resultant 200 ml solution is _____ (rounded off to two decimal places).
An organism uses only the glycerophosphate shunt pathway to transport cytosolic NADH to mitochondria. For every two electrons transported, complex I, complex III, and complex IV of the electron transport chain in this organism transport 2.5, 1.5, and 2.0 protons (H\(^+\)), respectively. The H\(^+\) to ATP ratio of F\(_0\)F\(_1\)-ATPase of this organism is 4.0. Terminal electron acceptor is oxygen.
The number of ATP molecules synthesized by oxidizing NADH from glycolysis is \underline{\hspace{2cm (rounded off to two decimal places).
If the extracellular concentration of Na\(^+\) is ten times its intracellular concentration, then the sodium equilibrium potential at \(20^\circ\)C in mV is \hspace{2cm (rounded off to two decimal places). Assume the membrane is permeable only to Na\(^+\) ions. [Use \(R=1.987\ \mathrm{cal\,deg^{-1\,mol^{-1}}\), \(F=23062\ \mathrm{cal\,mol^{-1}\,V^{-1}}\)]
Which one of the following statements on Casparian strips is correct?
Rotenone is a chemical often used to kill insect pests on crop plants and fishes in lakes. Rotenone acts by inhibiting electron transport from the NADH dehydrogenase enzyme in Complex I to ubiquinone in the mitochondrial electron transport chain. Which one of the following explains why plants can tolerate rotenone application?
Although {Pseudomonas syringae} infection in plants is actively inhibited by the endogenous salicylic acid (SA) of host origin, a successful infection is still established because the bacterium secretes coronatine, an effector molecule. Which one of the following best describes the mode of action of coronatine?
The schematic depicts an unexpanded plant cell within a hypocotyl with the arrangement of cellulose microfibrils marked on its cell wall.

Which one or more of the following statements is/are NOT CORRECT with respect to pollen development in angiosperm?
Regulation of phosphoenolpyruvate carboxylase (PEPCase) governs CO\textsubscript{2} fixation in both C4 and CAM (crassulacean acid metabolism) plants. Which one or more of the following statements with respect to PEPCase activity is/are CORRECT?
Which one or more processes listed below DOES NOT produce carbon dioxide during fermentation?
The ovule of a diploid species with \(2n = 8\) undergoes double fertilization. If the pollen is contributed by an individual with meiotic nondisjunction, the chromosome number of the zygote will be _____.
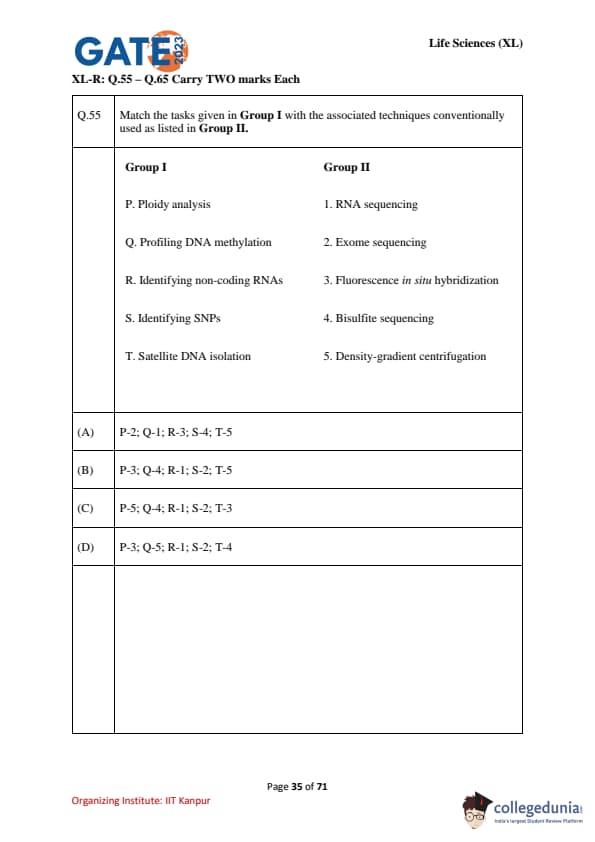
Match the tasks given in Group I with the associated techniques conventionally used as listed in Group II.

Periderm is a protective tissue found in stems and roots of gymnosperm and woody dicotyledons. It contributes to the increased thickness by secondary growth. Match the peridermal components given in Group I with the cell/tissue types given in Group II.

Match the following rice diseases in Group I with their causal agents in Group II.

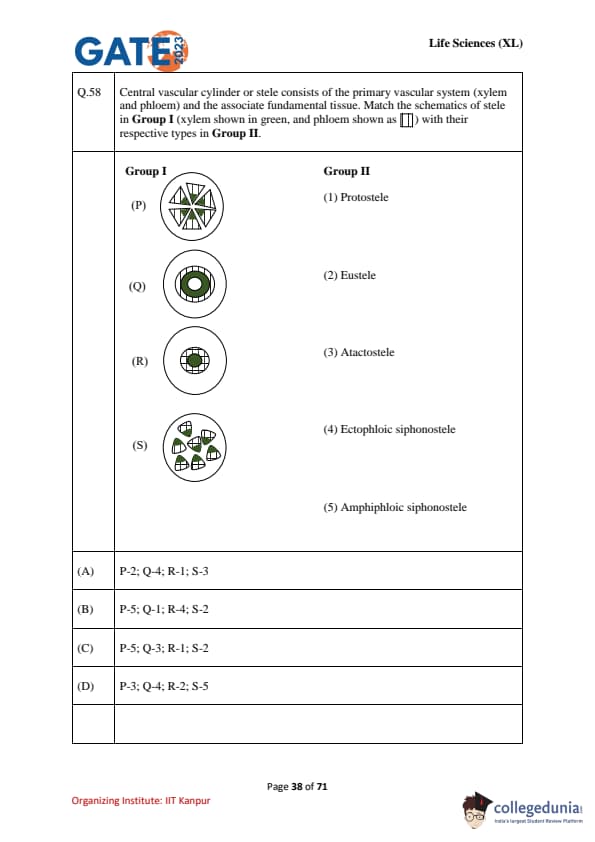
Central vascular cylinder or stele consists of the primary vascular system (xylem and phloem) and the associated fundamental tissue. Match the schematics of stele in Group I (xylem shown in green, and phloem shown as with their respective types in Group II.
Consider four observations about the FT gene and flowering transition in the shoot apical meristem (SAM) of {Arabidopsis thaliana}:
(i) The {FT promoter is active in leaves alone.
(ii) The {ft null mutation causes delayed flowering of the SAM.
(iii) Expressing a recombinant FT protein fused to a nuclear localization signal (NLS) under the endogenous promoter does not rescue the delayed-flowering phenotype of the {ft null mutant.
(iv) Downregulation of {FT transcript in the SAM by RNAi in a wild-type background does not alter flowering.
Which conclusion best explains these observations?
Which one of the options given correctly matches the alkaloids in Group I with their source plants in Group II?

A drought tolerant rice genotype was found to be associated with a missense mutation in the gene A. Which one or more of the following experiments is/are appropriate to validate whether the mutation in A is the causal factor for drought tolerance?
Blue light can directly induce opening of stomata. Blue light also triggers photosynthesis in the guard cells, which indirectly induces stomatal opening. Which one or more of the following experimental approaches would test the direct effect of blue light on stomatal opening?
In a diploid angiosperm species, flower colour is regulated by the R gene. RR and Rr genotypes produce red flowers, whereas the rr genotype produces white flowers. If two individual plants are randomly selected from a large segregating population of a genetic cross between RR and rr parents, the probability of both the plants producing red flowers is _____. (Rounded off to two decimal places)
A cytoplasmic male-sterile female plant with the restorer (nuclear) genotype rr is crossed to a male-fertile male plant with the genotype RR. Both RR and Rr can restore the fertility, whereas rr cannot. When an F1 female plant with Rr genotype was test-crossed to a male-fertile male plant with the rr genotype, the percentage of the population that is male fertile would be _____. (Answer in integer)
The frequencies for autosomal alleles A and a are \(p = 0.5\) and \(q = 0.5\), respectively, where A is dominant over a. Under the assumption of random mating, the mating frequency among dominant parents is _________ (Rounded off to two decimal places).
Monkeypox is caused by a
Which one of the following converts sulfate to hydrogen sulfide?
Which one of the statements about bacterial flagella is correct?
Microbial plastics are made from:
The correct sequence of metabolic intermediates in the Krebs cycle is:
Catabolite repression in bacteria is regulated by the concentration of
Phagocytosis was first described by
Which one of the following statements about batch culture of microbes is NOT correct?
Match the test in Group I with its application in Group II

Which one of the following is NOT correct about antibiotic resistance mechanism in microbes?
A suspension of photosynthetic green algae was illuminated in the presence of \(^{14}\)CO\(_2\) for few seconds. The first metabolite in the Calvin cycle to be radiolabeled will be
Determine the correctness or otherwise of the following Assertion [a] and the Reason [r].
Assertion [a]: Endospore can survive heat that would rapidly kill vegetative cells of the same species.
Reason [r]: In endospore, the protoplasm is reduced to minimum volume as a result of the accumulation of calcium-dipicolinic acid complexes and small acid-soluble spore proteins, which forms a cytoplasmic gel and a thick cortex.
Which one of the following conjugations will result in formation of merodiploids?
Which of the following genus is/are a spirochete(s)?
Which of the following is/are non-membrane bound inclusion bodies?
Which of the following antibiotics is/are isolated from \emph{Streptomyces} spp.?
Which of the following statements about the primary and secondary adaptive immune responses to an antigen is/are correct?
The spontaneous, and induced mutations in bacteria can be distinguished by
During the exponential growth, it took 6 hours for the population of bacterial cells to increase from \(2.5 \times 10^{6}\) to \(5 \times 10^{8}\). The generation time of the bacterium, rounded off to the nearest integer, is _____ minutes.
Which one of the following animals has “Book Lungs” as a respiratory organ?
Which one of the following describes the “innate behavior” of an animal?
Which one of the following represents a true “Ecological population”?
Which of the following animals show “Bottle cells” during the gastrulation stage of development?
The organisms that obtain energy from inorganic compounds are known as:
Which of the following is/are the causative agent(s) of Filariasis?
In a population of 1000 wild dogs in a grassland, 360 and 480 dogs had black body colour with genotypes BB and Bb, respectively. In the same population, remaining dogs were white in colour with a genotype of bb. Based on this data, the frequency of allele “b” in the population is _____ (round off to one decimal place).
A mature rat sperm cell has 2.5 µg of genomic DNA that is equivalent of a haploid genome. Compared to this sperm cell, the amount of genomic DNA (in µg) in a somatic cell, which is in the G2 phase of cell cycle, will be _____ (in integer).
In an experiment, excess amount of {bicoid} mRNA (more than wild-type expression level) was injected into the posterior pole of a wild-type Drosophila embryo at pre-blastodermal stage. Out of the following options, which one represents the best expected phenotype in the resulted developing embryo?
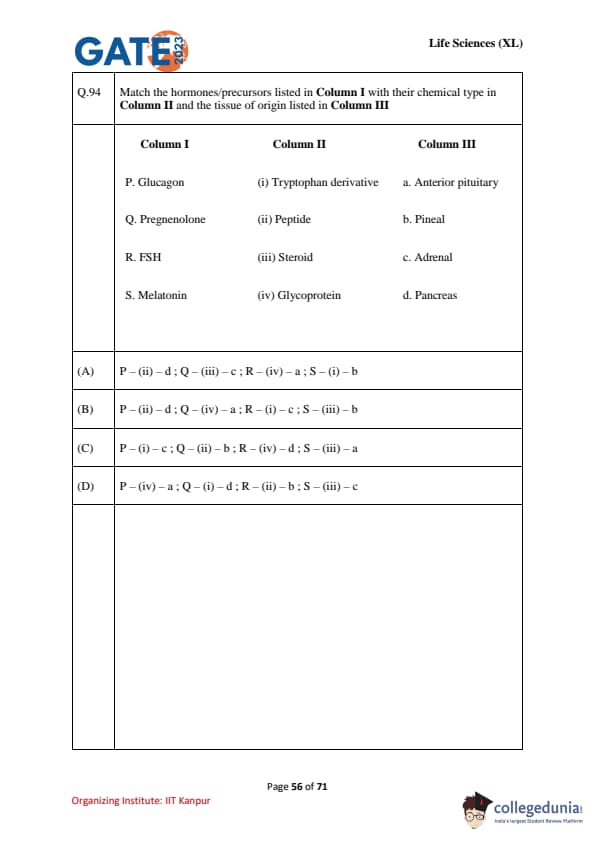
Match the hormones/precursors listed in Column I with their chemical type in Column II and the tissue of origin listed in Column III.

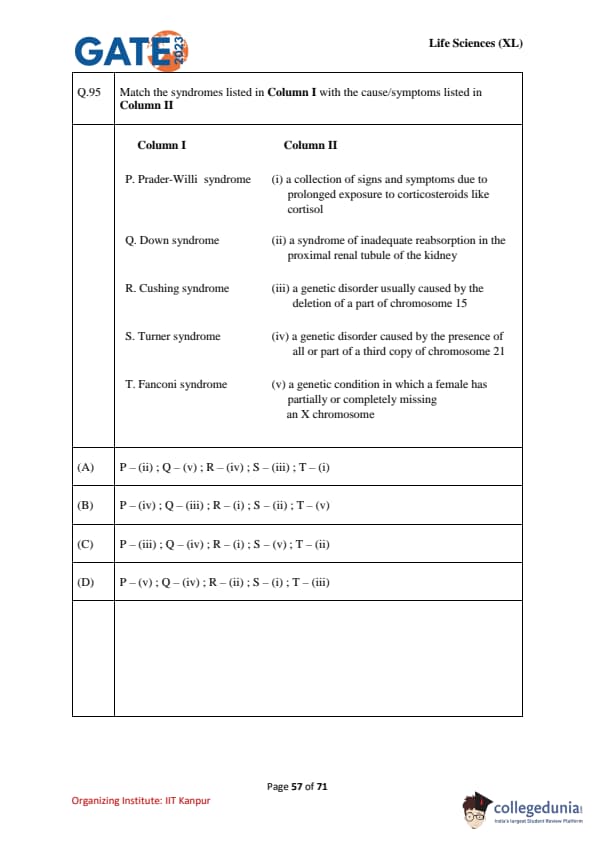
Match the syndromes listed in Column I with the cause/symptoms listed in Column II.

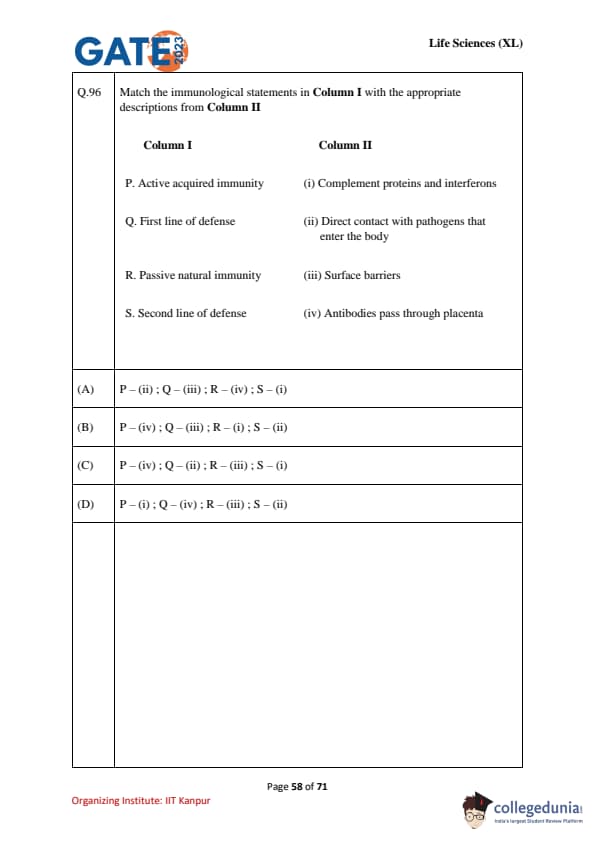
Match the immunological statements in Column I with the appropriate descriptions from Column II.

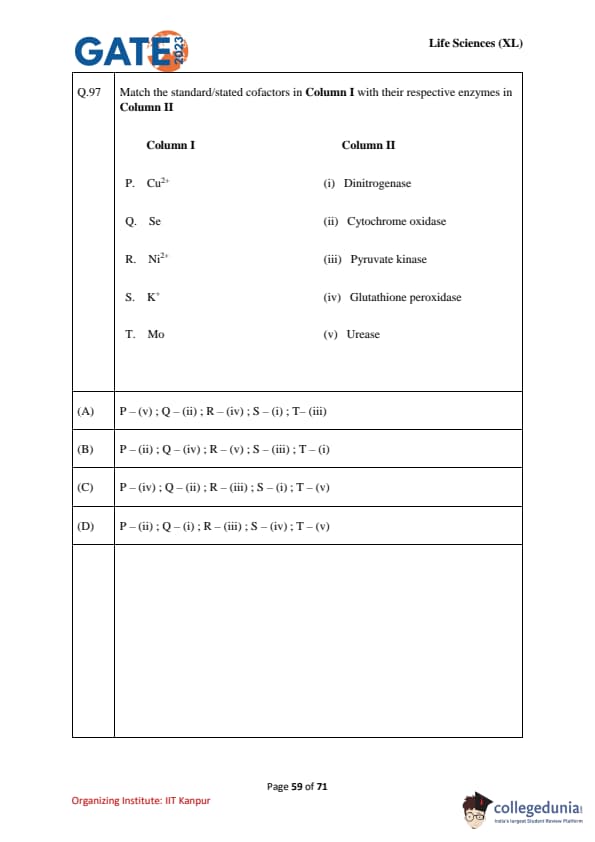
Match the standard/stated cofactors in Column I with their respective enzymes in Column II

The presence of excess glucose has been known to prevent the induction of {lac} operon as well as other operons controlling enzymes involved in carbohydrate metabolism in {E. coli}. Which of the following processes define(s) the phenomenon?
Which of the following techniques is/are used for determining the three-dimensional structure of proteins?
Among the following statements, which is/are TRUE regarding the replication of DNA?
Which of the following statements is/are TRUE for Colchicine?
Wild-type \emph{Drosophila} females having three linked genes (AABBCC) were crossed with triple recessive mutant (aabbcc) males. The F\textsubscript{1} females (AaBbCc) were backcrossed with aabbcc males. The following F\textsubscript{2} progeny numbers were obtained (total = 1000). The gene order is ABC. Find the recombination map distance (in cM) between A and C (round to one decimal place).
\begin{tabular{l r
AaBbCc & 241
Aabbcc & 112
aaBbCc & 103
aabbcc & 252
aaBbcc & 17
aabbCc & 134
AabbCc & 14
AaBbcc & 127
\end{tabular
The length of a double helical DNA molecule is 13.6 km. If the DNA double helix weighs \(1 \times 10^{-18}\) g per 1000 nucleotide pairs and rise per base pair is 3.4 Å, then weight of the double helical DNA molecule (in nanogram) will be _____ (in integer).
Choose the correct group of fat soluble vitamins.
The synthesis of thyroxine T4 in the human body requires
Which among the following is NOT an essential amino acid?
The time required for stipulated destruction of a microbial population at a given temperature is
Which among the following statements is NOT correct?
Calculate the efficiency in percent (rounded off to 1 decimal place) of an oil expeller which yields 37 kg oil containing 5% solid impurities from 100 kg mustard seeds. The oil content of the mustard seed is 38%.
Orange juice stored under ambient conditions shows vitamin C degradation following first-order kinetics with degradation constant \(k = 5.2 \times 10^{-3} \ day^{-1}\). What is the half-life of vitamin C in days (integer)?
The weight of 10 kg dried cauliflower containing 5% moisture (wet basis) after rehydration is 60 kg. If the fresh cauliflower contained 87% moisture (wet basis), calculate the coefficient of rehydration (rounded off to 2 decimal places).
Some of the industrial products are produced by fermentation processes. Identify the correct pair of product and fermentative microorganism.
Choose the correct statement(s) about the enzyme and its application in food processing reaction.
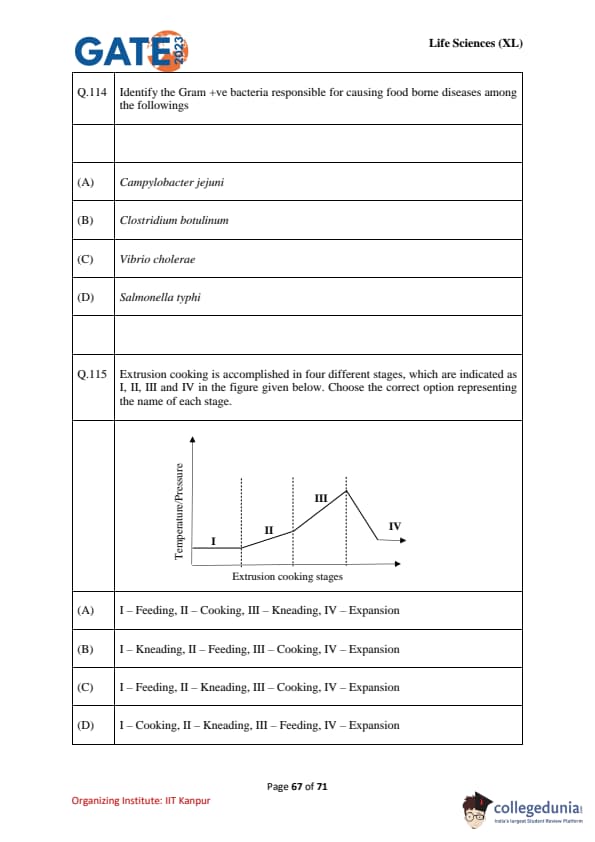
Identify the Gram +ve bacteria responsible for causing food borne diseases among the followings.
Extrusion cooking is accomplished in four different stages, which are indicated as I, II, III and IV in the figure given below. Choose the correct option representing the name of each stage.

Match the method/value used for measuring lipid characteristics in Column I with the corresponding properties indicated by them, in Column II.

Match the peeling technique in Column I with the vegetable, for which it is used in industry, given in Column II.

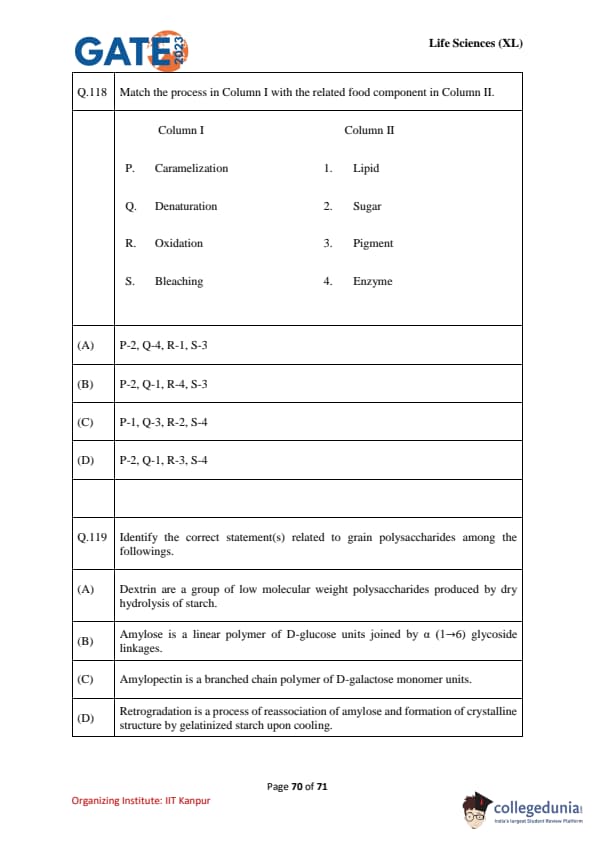
Match the process in Column I with the related food component in Column II.

Identify the correct statement(s) related to grain polysaccharides among the following.
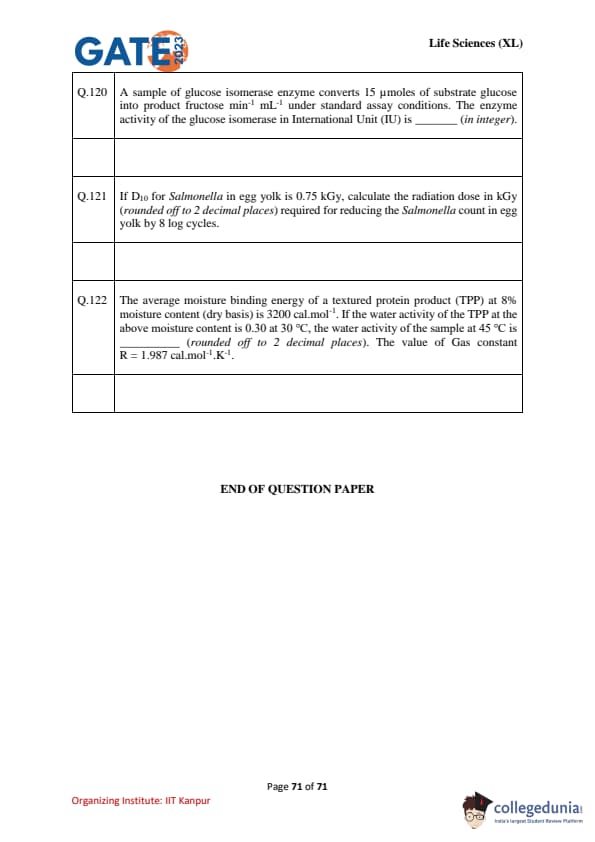
A sample of glucose isomerase enzyme converts 15 \(\mu\)moles of substrate glucose into fructose per min per mL under standard assay conditions. The enzyme activity in International Unit (IU) is _________ (in integer).
If \(D_{10}\) for {Salmonella in egg yolk is 0.75--1.80 kGy (data vary by matrix and strain), calculate the radiation dose in kGy (rounded off to 2 decimal places) required for reducing the {Salmonella count in egg yolk by 8 log cycles.
The average moisture binding energy of a textured protein product (TPP) at 8% moisture content (dry basis) is 3200 cal\,mol\(^{-1}\). If the water activity of the TPP at the above moisture content is 0.30 at \(30^{\circ}\)C, the water activity of the sample at \(45^{\circ}\)C is ______ (rounded off to 2 decimal places). Gas constant \(R=1.987\) cal\,mol\(^{-1}\)\,K\(^{-1}\).

































Also Check:
| Previous Year GATE Life Sciences Question Papers | GATE 2023 Life Sciences Paper Analysis |
| GATE Life Sciences Exam Pattern | GATE Life Sciences Syllabus |





Comments