Arpita Srivastava Content Writer
Content Writer
Amines are organic compounds that contain nitrogen atoms with a lone pair. These are derived, either in principle or in practice, from ammonia (NH3). These compounds are derived by replacing one or more hydrogen atoms of ammonia molecules with alkyl groups.
- Amines resemble the structural shape of ammonia.
- Like ammonia, it also has three hydrogen atoms bonded to nitrogen.
- They are found in proteins, vitamins, alkaloids and hormones.
- It is also found in cheese, chocolates, beer, yeast extracts and fish products.
- Amines are classified on the basis of nature and number of nitrogen atoms.
- The structure of the compound resembles that of a flattened triangular pyramid.
- Nitrogen atoms attached in amines are trivalent and have an unshared pair of electrons.
Key Terms: Amines, Ammonia, Alkyl Group, Nomenclature, Lone Pair, Alkaloids, Carbon, Hydrogen, Proteins, Nitrogen, Amino Acid, Functional Groups
What are Amines?
[Click Here for Sample Questions]
Amine is a type of compound that is derived from ammonia (NH3). In organic chemistry, they are basically classified as the functional groups of the organic nitrogen compounds that contain nitrogen atoms with a lone pair.
- A few of the naturally occurring amines include alkaloids.
- These are found in plants, such as histamine and catecholamine neurotransmitters.
- Neurotransmitters include dopamine, epinephrine, and norepinephrine.
- Hydrogen is replaced by the alkyl or aryl group.
- Hence, amines are also known as alkylamines and arylamines, respectively.
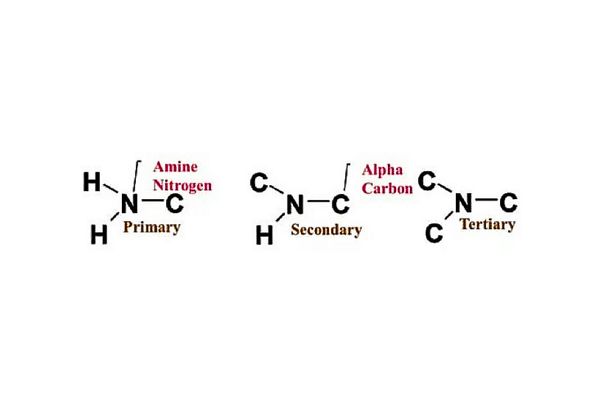
- They are classified as primary, secondary or tertiary category.
- It depends upon the number of carbons bonded directly to the nitrogen atom.
- Primary amines have one carbon binding to the nitrogen.
- The secondary amines have to have carbons bonded to the nitrogen.
- The tertiary amines are three carbons bonded to the nitrogen.

Amines
The video below explains this:
Amines Detailed Video Explanation:
Read More:
Types of Amines
[Click Here for Previous Year Questions]
On the basis of the number of hydrogen atoms replaced in NH3 molecules, amines are classified as Primary (1o Amine), Secondary (2o Amine), and Tertiary (3o Amine). The different classification of amines are as follows:
Primary Amines
When one hydrogen atom in ammonia is substituted by an alkyl or aromatic group then primary amines are formed. Amino acids and methyl amine are some examples of primary alkyl amines why aromatic amines include aniline.
Secondary Amines
Amines that have two organic substitutes either alkyl or aryl ones or both and are bound to the nitrogen together with one hydrogen are termed as secondary amines. Dimethylamine is a secondary amine.
Tertiary Amines
Amines where the nitrogen consists of three organic substitutes are tertiary amines. Trimethylamine and EDTA are the examples of tertiary amines.
Types of Amines
Nomenclature of Amines
[Click Here for Sample Questions]
There is a definite set of rules to name a compound, which is called nomenclature. The three things required for the Nomenclature of Amines include finding a parent chain of compounds, finding a functional group (suffix), and finding substituents as prefixes. There are two ways of naming an amine, which are as follows:
Common Name
In the common name system, an aliphatic amine is named by prefixing the alkyl group to amine, i.e., alkylamine, as one word (e.g., methylamine). The criteria are different in the case of secondary and tertiary amines.
- In secondary and tertiary amines, the alkyl groups are added in alphabetical order, which is followed by the word amine.
- The prefix tri or di is appended before the name of the alkyl group in secondary and tertiary amines.
- In the case of arylamines, the NH2 is attached to the aromatic compound.
IUPAC Name
In the IUPAC system, amines are named as alkanamines that are derived by the replacement of 'e' of alkane by the word amine.
- If more than one amino groups are present at different positions in the parent chain, their positions are specified by carbon atoms bearing NH2 groups.
- Suitable prefix such as di, tri, etc is attached to the amine.
- The letter 'e' of the suffix of the hydrocarbon part is retained.

Nomenclature of amines
Structure of Amines
[Click Here for Previous Year Questions]
The different structures of amines are as follows:
Alkyl Amines
Alkylamines consist of tetrahedral nitrogen centres where the C-N-C and C-N-H bond angle is 109 degree. The distance between C-N bonds is smaller as compared to the C-C range.
- The amines can also have a chiral property where the centre of nitrogen holds for replacements that create the lone pairs.

Alkyl Amines
Aromatic Amine
Nitrogen almost has a planar structure in aromatic amines due to the mixture of the lone pair with the aryl substituent. The C-N range is shorter. In Aniline, the distance between C-N bonds is the same as the distances between C-C bonds.

Aromatic Amine
Preparation of Amine
[Click Here for Sample Questions]
There are various methods used to prepare amines which are as follows:
Preparation from Halogenoalkanes
The process of making of amines from halogenoalkanes is carried out in a sealed tube. In this, the halogenoalkanes compound is heated with the concentrated solution of ammonia.
- The mixture is heated in the presence of ethanol.
- It cannot be heated in reflux; otherwise, ammonia will get released in the form of gas.
- The reaction is carried out in two stages.
- In the first stage, ethyl ammonium bromide salt is formed.
- In the second stage, a reverse reaction between salt and ammonia is formed.
CH3CH2Br + NH3 -----------→ CH3CH2NH3+Br-
CH3CH2NH3+Br- + NH3 -----------→ CH3CH2NH2 + NH4+Br-
Reduction of Nitriles
In this method, nitriles are treated with lithium aluminium hydride solution. This method is used when carbon atom are more than the starting amine.
Reduction of Nitriles
Gabriel Phthalimide Synthesis
Gabriel phthalimide synthesis is the third method used for the preparation of amines. In this method phthalimide reacts with the solution of ethanol and potassium hydroxide.
- This result in the removal of N-H proton with imide ion.
- Later it is heated with alkyl halide to give the N-alkyl phthalimide.
- It is also known as alkylation of phthalimide.
Gabriel phthalimide synthesis
Physical Properties of Amines
[Click Here for Sample Questions]
The physical properties of amines are as follows:
- Amine compounds can easily formed with the help of hydrogen bonds.
- They are soluble in water, whose solubility decreases with an increase in carbon atoms.
- Aromatic compounds are less soluble in water.
- Aromatic amines will donate lone electrons to the benzene ring.
- The lower class of amines are gaseous in nature with a fish like odour.
Basicity of Amines
[Click Here for Sample Questions]
Amines are very strong bases. The basicity of the amines is varied by the molecule and the presence of the lone pair of nitrogen electrons. The primary and secondary amines are the weak acids as the pKa value of ammonia is 34.
- Thus, amine acidity is measured by the pKarather than its conjugate acid.
- The acid-base relationships lies in the need for differing intensity organic bases as reagents suited to the particular reaction requirements.
- Simple amine water solubility is larger due to hydrogen bonding capacity.
- The bonding is formed between the protons on the water molecules and the lone electron pairs.
Factors that affects the basicity of amines
Factors that affects the basicity of amines compounds are as follows:
- The basicity of the amines is increased by the presence of electron donating group.
- It is decreased by the electron withdrawing group.
- For example- Nitro group.
Uses of Amines
[Click Here for Previous Year Questions]
Various uses of amines include
- Amines are widely used in the manufacturing of azo dyes.
- They are used in drugs.
- It is used in gas treatment during the removal of carbon dioxide from natural gas, etc.
- Amines are used as corrosion inhibitors.
- They are mainly used in the synthesis of many products.
Things to Remember
- Amines are an organic compound that is obtained from ammonia.
- It consists of a nitrogen atom attached with a lone pair of electrons.
- Amines are used in the rubber, dye, and pharmaceutical industries.
- It is also known as alkylamines and arylamines.
- Nitroalkanes are compounds that are used as a propellant in rockets.
Read More:
| Class 12 Chemistry Related Concepts | ||
|---|---|---|
| Etard reaction | Williamson Ether Synthesis | Electrophilic Aromatic Substitution |
| Ester Hydrolysis | Reimer Tiemann Reaction | Esterification |
Previous Year Questions
Sample Questions
Ques: Give a chemical test to differentiate between ethylamine and aniline? (3 marks)
Ans: Azo dye test involves the reaction of any aromatic primary amine with HNO2(NaNO2 + dil. HCl) at 273 – 278 K which is followed by a treatment with an alkaline solution of 2-naphthol when a brilliant yellow, orange and red coloured dye is obtained.

Ques: Arrange the following in increasing order based on their basic strength in aqueous solution: CH3. NH2, (CH3)3N, (CH3)2NH? (1 mark)
Ans: The order in which compounds are arranged in increasing order of basic strength are as follows:
![]()
Ques: Complete the following reaction equation: (2 marks)
Ans: The complete reaction is as follows:

Ques: Write the IUPAC name of the following compunds? (2 marks)
(A)


Ans: (A) The IUPAC name of the given compound is tribromoaniline.
(B) The IUPAC name of the given compound is N, N-Dimethylbutanamine.
Ques: Describe the following with relevant chemical equation in each case:
(A) Carbylamine reaction
(B) Hofmann’s Bromamide reaction? (3 marks)
Ans: (A) Carbylamine reaction: Aliphatic and aromatic primary amines on heating with chloroform and ethanolic KOH from isocyanides or carbylamines that are foul smelling sybstances. This reaction is called carbylamines reaction.
(B) Hofmann’s bromamide reaction: By treating an amide with Br2 in an aqueous or alcoholic solution of NaOH, primary amines can be prepared.

Ques: Give chemical tests to differentiate between the following pairs of compounds:
(A) Aniline and Ethylamine
(B) Ethylamine and Dimethylamine? (3 marks)
Ans: (A) Aniline and Ethylamine
By Azo dye test, it involves the reaction of any aromatic primary amine with HNO2(NaNO2 + dil. HCl) at 273 – 278 K which is followed by a treatment with an alkaline solution of 2-naphthol when a brilliant yellow, orange and red coloured dye is obtained.

(B) Ethylamine and Dimethylamine can be differentiated by the carbylamine test where, Aliphatic and aromatic amines on heating with chloroform and ethanolic potassium hydroxide form foul smelling isocyanides or carbylamines. Ethylamine, being an aliphatic primary amine, gives a positive carbylamine test but dimethylamine does not.

Ques: Give the structure of A, B and C in the given reactions? (3 marks)

Ans:

Ques: How will you convert the following:
(i) Nitrobenzene into aniline
(ii) Ethanoic acid into methanamine
(iii) Aniline into N-phenylethanamide. Also write the chemical equations involved. (3 marks)
Ans: (i) Nitrobenzene into aniline


Ques: Write the structures of A, B and C in the following equations? (3 marks)

Ans:

Ques: Give reactions that are used in preparation of alkanes using halogenoalkanes? (2 marks)
Ans: The reactions that are used in preparation of alkanes using halogenoalkanes are as follows:
CH3CH2Br + NH3 -----------→ CH3CH2NH3+Br-
CH3CH2NH3+Br- + NH3 -----------→ CH3CH2NH2 + NH4+Br-
Ques: What is the difference between amine and amide? (3 marks)
Ans: The difference between amine and amide are as follows:
| Amine | Amide |
|---|---|
| Amines are formed with one or more nitrogen atoms bonded with alkyl groups. | Amide are formed with ammonium group with an acyl group. |
| It is basic in nature. | It is acidic in nature. |
| Amine has low boiling point. | Amide has high boiling point. |
| It has no carbonyl group attached to carbon. | It has carbonyl group attached to carbon. |
Ques: What are different types of amines? (3 marks)
Ans: There are three types of amines which are as follows:
- Primary Amines :When one hydrogen atom in ammonia is substituted by an alkyl or aromatic group then primary amines are formed. Amino acids and methyl amine are some examples of primary alkyl amines why aromatic amines include aniline.
- Secondary Amines: Amines that have two organic substitutes either alkyl or aryl ones or both and are bound to the nitrogen together with one hydrogen are termed as secondary amines. Dimethylamine is a secondary amine.
- Tertiary Amines: Amines where the nitrogen consists of three organic substitutes are tertiary amines. Trimethylamine and EDTA are the examples of tertiary amines.
Ques: What is the difference between amine and imine? (3 marks)
Ans: The difference between amine and imide are as follows:
| Amine | Imine |
|---|---|
| Amines are formed with one or more nitrogen atoms bonded with alkyl groups. | Imine are formed with carbon-nitrogen double bond. |
| It is basic in nature. | It is less basic in nature. |
| Amine has fishy kind of smell. | Amide has roasted smell. |
Ques: Mention the physical properties of amines? (3 marks)
Ans: The physical properties of amine are as follows:
- Amine compounds can easily formed with the help of hydrogen bonds.
- They are soluble in water, whose solubility decreases with an increase in carbon atoms.
- Aromatic compounds are less soluble in water.
- The lower class of amines are gaseous in nature with a fish like odour.
Ques: Explain the common name nomenclature of amine? (2 marks)
Ans. In the common name system, an aliphatic amine is named by prefixing the alkyl group to amine, i.e., alkylamine, as one word. The criteria are different in the case of secondary and tertiary amines.In secondary and tertiary amines, the alkyl groups are added in alphabetical order, which is followed by the word amine.The prefix tri or di is appended before the name of the alkyl group in secondary and tertiary amines.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:




Comments