Jasmine Grover Study Abroad Expert
Study Abroad Expert
Electrochemistry is the study of the production of electricity by the energy that is released during spontaneous chemical reactions and the usage of that electrical energy to create any non-spontaneous chemical transformation. There are a large number of chemicals like sodium hydroxide, chlorine, fluorine, etc. They are formed through electrochemical methods. Batteries and fuel cells work on the principle of electrolysis. Other than that, there are a variety of fields where electrochemistry plays a huge role.
| Table of Content |
Keyterms: Electrochemistry, Electrolysis, Fluorine, Galvanic Cells, Faraday’s law, Electrolytic Cell, Electrolysis Products
Electrolysis
[Click Here for Sample Questions]
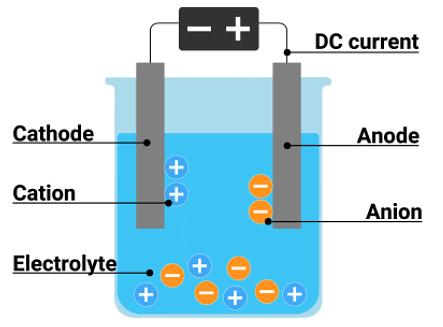
Electrolysis is the process by which an element is decomposed and undergoes some chemical change under the influence of any electric current. The first-ever electrolysis was executed out by Sir Humphrey Davey in the year 1808. Electrolysis can occur in both Galvanic cells and Electrolytic cells. In this article, we will discuss particularly electrolytic cells.
Also Read:
| Related Articles | ||
|---|---|---|
| Electrochemistry Important Questions | Variations Molar Conductivity | Electrochemistry Ncert Solutions |
| Electrochemical Cells | Nernst Equation | Batteries |
Electrolytic Cell
[Click Here for Sample Questions]
An electrolytic cell is an electrochemical device that uses electrochemical energy to promote any non-spontaneous redox reaction. In simple words, an electrolytic cell uses two electrodes (anode and cathode) in an electrolyte solution to process a redox reaction between the two electrodes. An external battery is used for the supply of electrons. An electrolytic tank is made of glass or any sort of bad conductor.
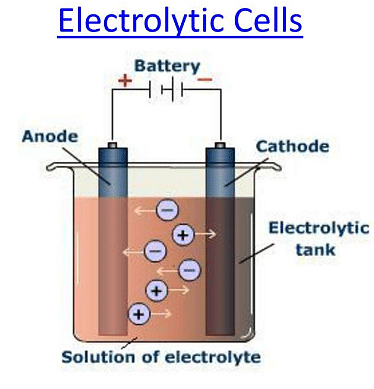
Electrolytic cells are quite similar to Galvanic cells, but there are some major Differences between Electrolytic Cells and Galvanic Cells them as listed below:
| Electrolytic Cell | Galvanic Cell |
|---|---|
| All the cell reactions are nonspontaneous. Contains positively charged anodes and negatively charged cathodes. Consumes electrical energy to drive nonspontaneous redox reactions. | All cell reactions are spontaneous. Contains positively charged cathodes and negatively charged anodes. Converts chemical energy into electrical energy |

The product of the electrolysis depends upon the material of the electrodes and the nature of the material that is being electrolyzed. If the electrode is made up of inert materials like gold and platinum, they are not going to take part in the reaction and are going to act only as a sink or source of electrons. But, if the electrodes are reactive, they are going to take part in the chemical reactions occurring within the electrolyte.
Let us take an example of a molten NaCl electrolytic cell:

Example of a molten NaCl electrolytic cell
Here, two inert electrodes are dipped in molten sodium chloride, which contains dissociated Na+ and Cl- anions.
NaCl→ Na+ + Cl-
When an electric current is passed through the cell, the cathode becomes electron-rich and becomes negatively charged. The sodium cations that are positively charged are now attracted towards the cathode. This, in turn, results in the formation of metallic sodium at the cathode.
At Cathode: Na+ + e-→Na (reduction)
Simultaneously, the chlorine atoms are attracted to the positively charged anode that results in the production of chlorine gas in the anode
At Anode: Cl-→ Cl2 + e-
Here, the two main end products of the electrolysis are Sodium metal and Chlorine gas.
Let us take another example of electrolysis of Aqueous Sodium Chloride:

Here, the two inert electrodes are dipped in an aqueous solution of sodium chloride. In water, NaCl ionizes as:
NaCl ![]() Na+ (aq) + Cl- (aq)
Na+ (aq) + Cl- (aq)
But, here water also dissociates to a certain extent:
H2O (Liq) ![]() H+ (aq) + OH- (aq)
H+ (aq) + OH- (aq)
Thus, unlike the case of molten NaCl solution as an electrolyte, an aqueous solution of NaCl contains Na+, Cl-, H+, and OH- ions. When an electric current is passed through the solution, the ion with more electrode potential will end up going to the oppositely charged electrode.
As H+ has higher electrode potential than Na+, the cathode reaction is going to be:
H2O (l) + e-→ H2 (g) + OH- (aq)
And, the anode reaction is going to be:
Cl- (aq)→ Cl2 (g) + e-
Hence, the two main end products of this electrolysis are chlorine gas and hydrogen gas.
Let us take another example with active electrodes:
Here, two copper rods are dipped in an electrolytic solution of aqueous copper sulphate. When an electric current is passed through the two electrodes, Cu2+ ions get discharged at the negatively charged cathode, and solid copper metal is deposited in the electrode.
Cu2+ + aq + 2e- → Cu (s)
At the anode, copper is converted to Cu2+ ions.
Cu (s) → Cu2+ (aq) + e-
In a way, within the electrolytic cell, copper is getting oxidized at the anode and reduced at the cathode. This process is done with many other metals such as Na, Mg, Al, etc to produce them on a large scale for the production of pure metals.
Faraday's Law Of Electrolysis
[Click Here for Sample Questions]

Faraday's Law Of Electrolysis
Faraday’s first law of electrolysis
The first law states that the amount of any substance deposited or liberated at an electrode is directly proportional to the quantity of electricity passed through the electrolytic solution. Thus, if on passing Q Coulombs of electricity, w gram of the substance is deposited, then:
w ∝ Q
w= ZQ
Here Z is a constant of proportionality, known as Electrochemical equivalent.
Faraday’s second law of Electrolysis
The second law states that when the same quantity of electricity is passed through various different electrolytic solutions connected in a series, the weights of the substances produced at the electrodes are directly proportional to their chemical equivalent weights.
Applications of Electrolysis
[Click Here for Sample Questions]
- Electrolytic cells are used for the production of Hydrogen and Oxygen gas from water.
- They are used for the large-scale production of pure metals like sodium, copper, magnesium, etc.
- They are used to extract Aluminium from Bauxite.
- Electrolytic cells are used for electroplating, a process by which a thin protective layer of one metal is formed over another metal, mostly for protection.
- Electrolysis is used for the electrorefining of non-ferrous metals.
- Electrolytic cells are used in electrowinning processes.
Also Read:
Sample Questions
Ques. Why is electrolysis an expensive way of extracting metal from its ore? (2 marks)
Ans. The processes that are used to extract metal from its ore are quite complex and expensive. The costs involved during electrolysis are:
- The cost of getting the metal in the suitable solution
- The cost of electricity
Due to the worldwide mass production of metals like aluminum, which are only extracted through electrolysis, the process turns expensive.
Ques. How do electrolytic cells work? (2017)
Ans. When an external current is passed through the electrolytic cell, the negative charge in the cathode attracts the dissociated positive ions in the electrolyte. This results in the deposition of positively charged ions in the cathode. At the same time, the positive charge of the anode attracts the dissociated negative ions.
Ques. During electrolysis of water, what are the end products? (Delhi, 2010)
Ans. During the electrolysis of water, oxygen will evolve from the anode and hydrogen will evolve from the cathode. It is because oxidation always occurs in an anode.
Ques. What are the three primary components of an electrolytic cell? (All India, 2015)
Ans. The three main components of an electrolytic cell are the cathode, anode, and electrolyte. Other than that, an external DC source is also used to provide energy.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:



Comments