Collegedunia Team Content Curator
Content Curator
Thermosetting polymer, often known as a thermoset or thermosetting plastic, is a polymer made up of highly branched molecules or a cross-linked structure. The thermosetting polymers harden throughout the moulding process and cannot be re-softened with heat. Bakelite and urea-formaldehyde resins are examples of thermosetting polymers.
| Table of Content |
Also Read: Polyamides
Structure of Thermosetting plastic
One of the reactants in the thermosetting polymer is a monomer, which creates the polymer's final chain. A cross-linker or comonomer, which serves as a crosslinking material, is the second component. The cross-linker aids in the joining of two or more monomer strands.
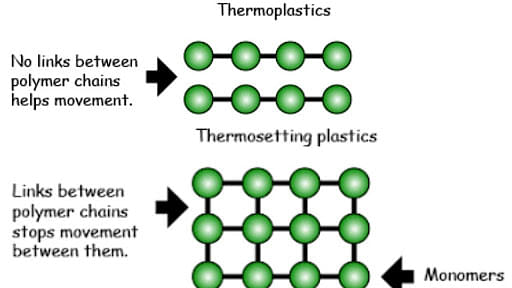
Difference between thermosetting polymer and thermoplastics
The important differences between thermoplastic and thermosetting plastic are tabulated below.
| Parameter | Thermoplastic | Thermosetting Plastic |
|---|---|---|
| Synthesis | The method of addition polymerization is used to make thermoplastics. | The method of condensation polymerization is used to make thermosetting polymers. |
| Tensile strength | They have a low tensile strength | Their tensile strength is high |
| Melting Points | The melting points of thermoplastics are low | The melting points of thermosetting plastics are high |
| Bonds | Secondary bonding between molecular chains is involved. | Primary bonds exist between the molecular chains that are linked together by strong cross-links. |
| Molecular weight | Their molecular weight is smaller than that of thermosetting polymers. | Molecular weight is high for thermosetting polymers |
| Processing | The thermoplastic process is done by the thermoforming process, rotational moulding, injection moulding, blow moulding, and extrusion process | The processing of thermosetting plastic is done by reaction injection moulding and compression moulding |
When it comes to polymers, one way to classify them is whether they are thermoplastic or thermosetting. Thermoplastics are reversible and reproducible in that they soften when heated and stiffen when cooled.

Thermoplastics include most linear polymers and branched structural polymers with flexible chains. Thermosetting polymers, on the other hand, do not soften when heated due to strong covalent crosslinks. Thermoset polymers are more rigid, stronger, and have greater dimensional stability than thermoplastics.

One of three types of polymer materials is thermosetting polymers. Thermosetting characteristics have a wide range of applications in engineering and everyday life.
Also Read: Condensation Polymer
Process Of Thermosetting Materials
The cross-linked molecular structure is a key feature of thermosetting polymers. The cross-linked structure is created in a variety of ways for thermosetting.
- Polymer is produced by combining two or more chemicals in the thermosetting process. These compounds form a thermosetting polymer by cross-linking with one another. Heat is commonly utilized to speed up chemical reactions. Epoxies are one of the most well-known members of this category.

- The second type of thermosetting polymer is created by using catalysts to speed up the formation of cross-linked structures in liquid form. These thermosetting polymers are stable for long periods of time without the need for catalysts.
- Heat is used to melt the initial granular thermosetting material in thermosetting shaping operations. The form is then achieved by high-temperature moulding. Cross-linkings are formed during the solidification process.
Characteristics Of Thermosetting Materials
- Because of their cross-linked molecular structure, thermosetting materials are less soluble in common solvents.
- It can be used at considerably higher temperatures.
- Thermosetting materials cannot be re-melted because of their cross-linked structures.

- Thermosets have a brittle structure and lack the ductile qualities of thermoplastics.
- Because of their cross-linked molecular structure, thermosets have more stiffness and superior mechanical properties than thermoplastics. The modulus of elasticity of thermosetting materials is typically 2-3 times that of thermoplastics.
Also Read: Properties of Matter

Many plastics that are used in daily life are thermosetting plastics. Examples include:
Bakelite (phenolic) (C6-H6-O.C-H2-O), Cyanate esters, Duroplast, Epoxy resin, Fiberglass (a fibre-reinforced thermoset), Melamine, Polyester resin, Polyurethane, Silicone resin, Vinyl esters, Vulcanized rubber.
Thermosetting Polymers Examples
Advantages of Thermoset Polymers
- Thermoset materials increase chemical resistance, heat resistance, and structural integrity, as well as the mechanical characteristics of the material.
- Due to their resistance to deformation, thermoset polymers are utilized for sealed goods.
- Thermoset plastics are more resistant to high temperatures than thermoplastics.
- They have a highly flexible design, can be made with thick or thin walls, have a good aesthetic appearance, have high dimensional stability, and are cost-effective.
- There is no reversible changing behaviour in the thermoset.

- The thermoset polymers create connections or chemical linkages between neighbouring chains during polymerization (curing). As a result, the three-dimensional network is significantly stiffer than the two-dimensional (linear) thermoplastic construction.
- When heat is applied and the thermoset is set into a permanent hard shape, the interconnected chains are not free to move.
- Low crosslink density thermosets can be softened by heating to high temperatures, but they do not melt (like thermoplastics) and retain their original shape.
- Thermosets, such as phenolic and epoxy resins, have a long history as circuit board and packaging materials.
- Thermosets are preferred for many wet-paste applications because they have low solvent content and can be accommodated without difficulty in wet-paste processes for bonding fiat surfaces large in area.
Disadvantages of Thermoset Polymers
- The thermoset plastics can’t be recycled.
- It is difficult to provide a smooth surface finish to thermosetting polymers.
- It can’t be remoulded or reshaped.
Things to Remember
- Thermosetting polymer, often known as a thermoset or thermosetting plastic, is a polymer made up of highly branched molecules or a cross-linked structure.
- The thermosetting polymers harden throughout the moulding process and cannot be re-softened with heat.
- Bakelite and urea-formaldehyde resins are examples of thermosetting polymers.
- Because of their cross-linked molecular structure, thermosetting materials are less soluble in common solvents.
- Thermoset materials increase chemical resistance, heat resistance, and structural integrity, as well as the mechanical characteristics of the material.
- The thermoset plastics can’t be recycled.
Sample Questions
Ques: State the differences between thermoplastics and thermosetting plastics. (2 marks)
Ans: Thermoplastic polymers are long-chain polymers with a linear (slightly branching) structure that can be softened and hardened again when heated. As a result, they can be changed over and over again. Polythene and polystyrene are two examples.
Thermosetting polymers are extensively branched or cross-linked polymers that harden during the moulding process. Heating will not soften these polymers again. Bakelite and urea-formaldehyde resins are examples of thermosetting polymers.
Ques: Why are the following made of thermosetting plastics? Explain. (2 marks)
- Saucepan handles
- Electric plugs/ switches/ switchboards
Ans:
- A saucepan's handles are constructed of thermosetting plastic, which is a poor heat conductor and does not heat up when cooking.
- Because thermosetting plastic is a poor conductor of electricity, electric plugs, switches, and plug boards are built with it. Such polymers do not allow an electric current to flow through them.
Ques: Write the monomers used for creating the following polymers. (2 marks)
- Polyvinyl Chloride
- Teflon
- Bakelite
Ans:
- CHCl vinyl chloride is the monomer unit of Polyvinyl Chloride.
- CF2 tetrafluoroethylene is the monomer unit of Teflon.
- HCHO formaldehyde and C6H5OH phenol is the monomer unit of bakelite.
Ques: Define thermosetting polymer with two examples. (2 marks)
Ans: Thermosetting polymers are called polymers which are cross-linked or strongly branched polymers and are hardened during the moulding process. Through heating, they cannot be softened yet again.
Eg: urea-formaldehyde resins, bakelite.
Also Read: Quantitative Analysis
Ques: Determine the groups where the polymers are graded according to molecular forces? (2 marks)
Ans: Polymers are classified into groups given below based on the intermolecular magnitude of forces present in polymers:
- Fibres
- Elastomers
- Thermosetting polymers
- Thermoplastic polymers
Ques: Why Bakelite cannot be remoulded? Explain. (2 marks)
Ans: Thermosetting plastics are rigid and heavily cross-linked polymers. They cannot be remoulded once they are set. Bakelite is a thermosetting phenol-formaldehyde resin.
Ques: Give the characteristic properties and important uses of the following: (5 marks)
- polythene
- polystyrene
- teflon
- bakelite
Ans:
- Polythene's characteristics and applications are as follows:
Properties:
- It is resistant to water.
- It's strong but flexible, and it can be rolled into sheets and moulded into a variety of forms.
Uses:
Polythene sheets are commonly used to package liquids such as milk. Liquids such as oil and water are transported via polythene pipes. Liquids are stored in polythene containers.
Also Read: Micelle
- Polystyrene's characteristics and applications are as follows:
It has the following characteristics:
- It is easily moldable.
- It is chemically vulnerable.
Uses:
It is used to package fragile products like electrical devices. Thermocol is made from it. It's also utilized to insulate the refrigerator's hollow walls.
- Teflon's qualities and applications are as follows:
It has the following properties:
- It is slick and unaffected by heat.
- It has no chemical reactions with other compounds.
Uses:
It's used to make a non-stick coating for pans and other cooking equipment. Among other things, it's used to manufacture gaskets, pump packings, valves, and seals.
- Bakelite's qualities and applications are as follows:
- It is an excellent electrical insulator.
- It is more difficult to break than other polymers.
Uses:
It's used to manufacture combs, fountain pens, buttons, plugs, and switches, among other things.
Ques: What are polymers? (2 marks)
Ans: Polymers are high molecular mass substances (103 — 107u) consisting of a very large number of simple repeating structural units joined together through covalent bonds in a linear fashion. They are also called macromolecules. Ex: polythene, nylon 6,6, bakelite, rubber, etc.
Ques: What are natural and synthetic polymers? Give two examples of each. (3 marks)
Ans:
- Natural polymers: The polymers which occur in nature mostly in plants and animals are called natural polymers. A few common examples are starch, cellulose, proteins, rubber nucleic acids, etc. Among them, starch and cellulose are the polymers of glucose molecules. Proteins are formed from amino acids which may be linked in different ways. These have been discussed in detail in unit 15 on biomolecules. Natural rubber is yet another useful polymer which is obtained from the latex of the rubber tree. The monomer units are of the unsaturated hydrocarbon 2-methyl-i, 3-butadiene, also called isoprene.
Examples of natural polymers: Natural rubber, cellulose, nucleic acids, proteins etc.
- Synthetic polymers: The polymers which are prepared in the laboratory are called synthetic polymers. These are also called man-made polymers and have been developed in the present century to meet the ever-increasing demand of modern civilization.
Examples of synthetic polymers: Dacron (or terylene), Bakelite, PVC, Nylon-66, Nylon-6 etc.
Ques: How do you explain the functionality of a monomer? (2 marks)
Ans: The functionality of a monomer implies the number of bonding sites present in it. For example, monomers like propene, styrene, acrylonitrile have the functionality of one which means that they have one bonding site.
Monomers such as ethylene glycol, hexamethylenediamine, adipic acid have the functionality of two which means that they have two bonding sites.
Also Read:
Ques: What are the different types of linear copolymers? Explain them in brief. (5 Marks)
Ans: Periodic, Gradient, Block, Statistical and Alternating copolymers.
Alternating Copolymers
These copolymers have a regular, repetitive and alternating arrangement of two monomeric species, eg A and B.The general formula can be given as -(-A-B-)n- or -A-B-A-B-A-B-A-B-A-B-.
Examples Nylon -6-6-
Statistical Copolymers
When the sequence of monomeric units follows a certain statistical rule it is called a statistical copolymer. Moreover, if the probability of finding a given monomer at a particular point in a chain is equal to its mole fraction in the chain then it can be called a random polymer.
Examples Rubbers made from styrene-butadiene copolymers, resins from styrene acrylic etc.
Block Copolymers
When two or more homopolymer chains are joined by covalent bonds the resultant polymeric chain is called a Block copolymer.The intermediate unit where they join is called a junction block.They can be diblock or triblock copolymers.
Examples: Acrylonitrile butadiene styrene (SBS rubber).
Ques: What is the Condensation reaction? Give an example. (2 Marks)
Ans: Condensation polymerization reaction, also known as step-by-step growth polymerization, that is involved in either method of copolymerization, happens between two different monomeric species (either bi-functional or tri-functional). Substances like water, alcohol, and ammonia are eliminated in the final product due to repeated condensation reactions.
Example: Nylon 6,6 which is obtained from hexamethylene diamine is condensed repeatedly with adipic acid. The pressure and temperature required for the condition to succeed are quite high.
Check Also:




Comments