
Content Curator
Glycosaminoglycans are negatively charged polysaccharide compounds.
- These are also known as mucopolysaccharides.
- Glycosaminoglycans consist of repeating disaccharide units that are found in the tissues of every mammal.
- The repeating disaccharide units are formed of either sulfated or non-sulfated monosaccharides.
- The molecular size and sulfation pattern of glycosaminoglycans vary depending on the tissue type and state.
- This variation plays an important role in both pathological and physiological conditions.
| Table of Content |
Key Terms: Glycosaminoglycans, Carbohydrates, polysaccharides, Cellulose, Disaccharides, Glucose, Fructose, Lactose, Monomer, Glycans
What are Carbohydrates?
[Click Here for Sample Questions]
Carbohydrates are bio-polymers that are made up of monomer units. These monomer units are called monosaccharides. Depending on the number of monomers present in the carbohydrate, they are divided into various types. These are
- Monosaccharides
- Disaccharides
- Polysaccharides
Monosaccharides
They are the simplest form of carbohydrates, consisting of a single sugar unit.
- Examples of monosaccharides include glucose, fructose, and galactose.
- These simple sugars are the building blocks of more complex carbohydrates.
Disaccharides
A disaccharide is a sugar, formed when two monosaccharides are joined by glycosidic linkage.
- It is also known as double sugar.
- Three common examples of disaccharides are sucrose, lactose, and maltose.
Polysaccharides
Polysaccharides correspond to the complex carbohydrates consisting of long chains of monosaccharides.
- These can chain together and remain attached through glycosidic linkages.
- These linkages are the reason why monosaccharides are also called glycans.
- The molecules of a polysaccharide contain a specific number of sugar molecules.
- When these single polysaccharides combine, they form a larger molecule.
- The general formula of polysaccharides is Cn(H2O)n-1, where n can be any number between 200 and 2500.
Types of Polysaccharides
Different types of Polysaccharides are
- Homopolysaccharides: They contain the same type of monosaccharide
- Heteropolysaccharides: They contain different types of monosaccharides

Also check:
| Related Concepts | ||
|---|---|---|
| Dehydration of Alcohols | Structure of Glucose and Fructose | Single Cell Protein |
| Amino Acids | Maltose | Nucleic Acid |
What are Glycosaminoglycans?
[Click Here for Sample Questions]
Glycosaminoglycans (GAGs) are long, unbranched polysaccharides composed of repeating disaccharide units.
- These polysaccharides are also called mucopolysaccharides because of their lubricating and viscous properties.
- In contrast to GAGs, both glycogen and starch are polysaccharides that consist of only glucose units.
- Plants store energy as starch, while animals store energy as glycogen.
- Starch is insoluble in water and is digested by the enzyme amylase.
- The primary structural component in plants is cellulose.
- They are polysaccharides that cannot be digested by the human digestive system.
- Some polysaccharides, like xanthan gum found in bacterial capsules, have protein cores.
- These cores are synthesized in the endoplasmic reticulum.
- Then they are modified by the Golgi apparatus in a post-translational process.
- GAGs are covalently bonded to these protein cores, forming structures called proteoglycans.
- Proteoglycans are essential components of connective tissues and play a vital role in various biological processes.
- GAG chains within proteoglycans can bind to other proteins, including chemokines, cytokines, morphogens, growth factors, and enzyme adhesion molecules.
Glycosaminoglycans Structure
[Click Here for Sample Questions]
Glycosaminoglycans (GAGs) are negatively charged, linear polysaccharides. They can be either sulfated or non-sulfated and typically have molecular weights ranging from 10 to 100 kilodaltons.
GAGs can be further classified into two groups based on the types of linkages between their disaccharide units and their specific structural elements. They are listed below-
- Sulfated GAGs: Chondroitin Sulfate (CS), Dermatan Sulfate (DS), Heparan Sulfate (HS), Keratan Sulfate (KS), and Heparin.
- Non-sulfated GAGs: Hyaluronic Acid (HA).
Glycosaminoglycan chains are built from repeating disaccharide units. Each unit contains an uronic acid (like D-glucuronic or L-iduronic acid) and an amino sugar (like D-galactosamine or D-glucosamine). The specific type of hexosamine, hexuronic acid, and the geometry of the glycosidic linkage all contribute to the variations seen in different glycosaminoglycans.
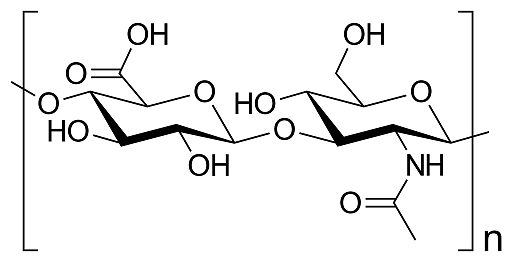
Glycosaminoglycans structure
For example, both dermatan sulfate and chondroitin sulfate contain galactosamine within their glycosaminoglycan units. Heparin sulfate and heparin, on the other hand, are built from glycosaminoglycan units themselves.
- Chondroitin sulfate contains beta-D-glucuronic acid linked to N-acetylgalactosamine-4-sulfate.
- Heparin, a mixture of linear anticoagulant polysaccharides, has varying sulfation patterns.
- Glycosaminoglycans (GAGs) have amino sugars that can be sulfated at different positions.
- This variation in sulfation creates a vast array of structures, even in small GAG chains.
- Within each glycosaminoglycan type, the repeating unit has varying potential sulfation sites.
- The amino sugar can have 2-3 positions sulfated, while the uronic acid can have 1-2.
- The variable sulfation of these sites creates a high degree of diversity.
- This allows a single glycosaminoglycan type to have 16 to 48 possible disaccharide variations.
Functions of Glycosaminoglycans
[Click Here for Sample Questions]
The following are the functions of Glycosaminoglycans
- Glycosaminoglycans (GAGs) participate in many biological processes by regulating their various protein partners called proteoglycans.
- The large structural diversity of GAGs makes them valuable tools in biochemistry, structural biology, and molecular modeling, contributing to new drug discovery.
- Its conformational flexibility and sulfation patterns underlie the complexity of GAG-protein interactions.
- Four major types of negatively charged molecules found in animal cells are glycosaminoglycans (GAGs), phospholipids, and nucleic acids (ribonucleic acid [RNA] and deoxyribonucleic acid [DNA]).
- Negatively charged GAGs blanket animal cell surfaces, interacting with hundreds of extracellular signaling molecules.
- Their complex structure suggests they may be the most information-dense biopolymers in nature.
- This hypothesis is supported by compelling evidence from transgenic and knockout animal studies conducted in the past decade.
Applications of Glycosaminoglycans
[Click Here for Sample Questions]
In different parts of the world, scientists have been researching and making discoveries about different applications of glycosaminoglycans. The work is still going on, and there are a lot of new discoveries scientists have been working on.
Some of the applications of glycosaminoglycans are-
- GAGs help in the regulation of FGF/FGFR signaling
- Glycosaminoglycan-based Activators and Inhibitors in FGF/FGFR Signaling
- GAG Basis of Joint Specificity in Rheumatoid Arthritis
- Serum GAGs and Proteoglycans as Biomarkers for Lung Cancer
Hyaluronic acid plays a significant role in various processes, including:
- Signaling activity during embryonic morphogenesis, pulmonary and vascular healing.
- Lubrication of synovial joints, facilitating smooth movement.
- Acting as a space filler, wetting agent, and flow barrier within the synovium (joint lining).
- Influencing cancer progression and protecting cartilage surfaces.
Health Effects of Glycosaminoglycans
[Click Here for Sample Questions]
There are a lot of things to discuss regarding the health effects of glycosaminoglycans. Being an important part of the body that regulates prominent functions in the body, it has various health effects.
Effect of Glycosaminoglycans on Leukocyte Movement
CD44, a cell-surface glycoprotein expressed on virtually all stem cells, including cancer stem cells, acts as the main receptor for hyaluronic acid.
- The interaction between hyaluronic acid and CD44 can mediate leukocyte rolling and extravasation in some tissues.
- Heparin, another glycosaminoglycan, was first discovered in 1917 and is mainly used for anticoagulation.
Effect of Glycosaminoglycans on Blood Coagulation
Sulfated glycosaminoglycans (GAGs) exhibit anticoagulant properties due to their ability to prolong blood clotting.
- This effect is achieved through GAGs potentiating the activity of antithrombin III (AT-III), a natural thrombin inhibitor.
- However, only about one-third of all heparin chains possess the structural features necessary for binding to AT-III.
Effects of Glycosaminoglycans on Cellular Communication and Development
Glycosaminoglycans (GAGs) play an essential role in various processes, including cell signaling and development, angiogenesis (blood vessel formation), anti-coagulation, tumor progression, axonal growth, and metastasis.
- They also contribute to cell proliferation by acting as co-receptors for growth factors of the fibroblast growth factor family.
- These growth factors require interaction with both a heparin/HS chain and their high-affinity receptor to fully activate their signaling potential.
Also check:
Things to Remember
- Carbohydrates are bio-polymers that are made up of monomer units.
- Monosaccharides are the simplest form of carbohydrates, consisting of a single sugar unit.
- A disaccharide is a sugar, formed when two monosaccharides are joined by glycosidic linkage.
- Polysaccharides correspond to the complex carbohydrates consisting of long chains of monosaccharides.
- Homopolysaccharides and Heteropolysaccharides are the types of Polysaccharides.
- Glycosaminoglycans are long, unbranched polysaccharides composed of repeating disaccharide units.
Sample Questions
Ques. What are Glycosaminoglycans? (2 Marks)
Ans. Glycosaminoglycans are defined by long unbranched polysaccharides that consist of repeating disaccharides. In short, they are called GAGs and because of their property of being lubricant and vicious, they are also called mucopolysaccharides.
Ques. What is the importance of Glycosaminoglycans? (5 Marks)
Ans. The importance of Glycosaminoglycans are
- Glycosaminoglycans have to take into account several intracellular and extracellular functions.
- Heparin in our body is a glycosaminoglycan that contains the highest net negative charge of the disaccharides and acts as a natural anticoagulant.
- GAGs can make a strong bond with a protein called antithrombin III which terminates the process of clotting.
- Glycosaminoglycans are important components of the vitreous humor in the eye and of synovial fluid which is a lubricant fluid of joints in the body.
- Keratan sulfate and chondroitin are also examples of glycosaminoglycans found in connective tissue like cartilage and tendons.
Ques. State two functions of Glycosaminoglycans. (2 Marks)
Ans. Two main functions of Glycosaminoglycans are-
- Glycosaminoglycans also known as GAGs take accountability for several biological processes and help in the regulation of various protein patterns called proteoglycan.
- GAGs have conformational flexibility and underlying sulfation patterns which are accountable for the complex nature of the GAG and protein interaction.
Ques. What are the different types of Carbohydrates? (3 Marks)
Ans. Based on the number of monomers present in the carbohydrate, carbohydrates are mainly divided into the following types-
- Monosaccharides
They have only one carbohydrate molecule.
- Disaccharides
They have two carbohydrate molecules.
- Oligosaccharides
They have two to ten carbohydrate molecules.
- Polysaccharides
They have ten to hundred carbohydrate molecules.
Ques. Explain the synthesis of Glycosaminoglycans. (2 Marks)
Ans. Glycosaminoglycans possess great variation in the construction of disaccharides, molecular mass, and sulfation. The reason behind this behavior is that the synthesis of GAG is not template-driven like nucleic acids or proteins but they are constantly altered by the processing enzymes.
Ques. Why are glycosaminoglycans negatively charged? (2 Marks)
Ans. Glycosaminoglycans are polysaccharides in linear shape that contain repeating disaccharide units of hexuronic acid linked to a hexosamine. GAGs are negative because of the various sulfate groups.
Ques. Are Heparin glycosaminoglycans? (3 Marks)
Ans. Heparin including Heparan sulfate (HS) and dermatan sulfate (DS) is considered a natural glycosaminoglycan (GAG). They are linear polysaccharides and heterogeneous in both length and sequence. GAGs are accountable for several functions in the body and they influence numerous physiological processes.
Ques. What are the components of glycosaminoglycans? (3 Marks)
Ans. Glycosaminoglycans also known as GAGs consist of disaccharide units, and each of the disaccharide units contains any of the three molecules- firstly, an acetamido sugar which could be either N-acetyl-d-glucosamine or N-acetyl-d-galactosamine, and secondly, a uronic acid which could be either d-glucuronic or l-iduronic acid. Lastly, it is d-galactose units that the disaccharide could be composed of.
Ques. Define Heparin or Heparan Sulfate. (3 Marks)
Ans. Heparan sulfate or heparin also termed HF and Hep respectively have repeating disaccharide units of hexuronic acid residues and N-acetylglucosamine. The hexuronic acid residue glucuronic acid has been found in the heparan sulfate, whereas the iduronic acid is found in heparin. Sulfation of the several hydroxyl groups or the amino group present in the glucosamine compound of Heparan Sulfate or Heparin determines the ability to interact with various proteins, growth factors, cytokines, and ultimately to the bioactive function.
Ques. What is Hyaluronic Acid? (2 Marks)
Ans. Hyaluronic acid (in short, HA) possesses the simplest structure among all the other GAGs, and unlike the other GAGs, it does not need to have additional sulfation of functional groups in the Golgi apparatus. The structure of Hyaluronic consists of sequentially bound glucuronic acid and N-acetylglucosamine residue.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Also Read:





Comments