Jasmine Grover Study Abroad Expert
Study Abroad Expert
Colloids, which are also known as colloidal solutions or colloidal systems are mixtures in which micro-insoluble particles of one substance are suspended in another substance. Suspended particles in a colloid can range from 1 to 1000 nanometers that is 10–9 metre in size. Colloids usually contain substances that are evenly scattered in another. In such a mixture, the material that is dispersed is called dispersed state whereas the material through which it is dispersed is called continuous phase.
| Table of Content |
Key Terms: Colloids, colloidal solutions, colloidal systems, emulsions, purification of colloids, continuous phase
Colloids Meaning and Definition
[Click Here for Sample Questions]
In simple words, we can define colloids as a mixture where the same substance is divided into very fine particles which are spread to other substances. The Tiny particles are known as colloidal particles.
Alternatively, we can also say that the colloid is basically a solution of the size of solute particles ranging from 1nm - 1000 nm.
Preparation of Colloids
Also Read:
Classification of Colloids
[Click Here for Sample Questions]
Colloids are classified into several types.
Classification based on physical condition
(A) Solid Solution- Solids and dispersion medium in this scattered phase. Such as: Gemstone.
(B) Aerosol- These colloids contain air as the dispersion medium.
Example 1: Cloud- It consists of air as the dispersion medium and water droplets as the dispersion phase.
Example 2: Dust-. It consists of air dispersion medium and dent particles as dispersion phases.
Example 3: Smoke-It contains carbon particles in the air.
(C) Gels- In gels, the dispersion medium is solid and the dispersed phase is liquid. Eg. Butter, cheese
(D) Emulsions- In emulsions, both dispersed phase and medium are liquids. They are further of 2 types: oil in water type , eg. Milk and water in oil type, eg. vanishing cream.
Classification based on forces of attraction
On the basis of forces of attraction between the dispersion medium and dispersed phase, the colloids can be classified as lyophobic and lyophilic colloids. The characteristics of these colloids are summarised in the table below:
| Property | Lyophilic | Lyophobic |
|---|---|---|
| Definition | They are solvent loving colloids | They are solvent hating colloids |
| Stability | More stable due to strong forces of attraction between dispersed phase and dispersion medium | Less stable due to less forces of attraction between the dispersed phase and dispersion medium |
| Preparation | Readily formed by warming and mixing the dispersed phase with the dispersion medium | Preparation is difficult and involves long procedures such as oxidation, reduction, hydrolysis, double decomposition, exchange of solvent, bredig’s arc method, peptisation,excessive cooling, etc. |
| Coagulation | For coagulation of lyophilic colloids, addition of both solvent as well as electrolyte is required, since it is very stable. | Addition of a small amount of electrolyte leads to coagulation of the lyophobic colloidal solution. |
| Reversibility | These colloids are reversible in nature | These colloids are irreversible in nature |
| Examples | Metallic sols such as Ag, gold etc. | Starch, gum, gelatin,proteins,rubber etc |
Classification of colloids based on size of molecules
Based on the size of constituent molecules, the colloids can be classified as multimolecular, macromolecular and associated colloids.
Preparation of Colloid Solution
[Click Here for Sample Questions]
Stable colloids are also known as lyophilic sols, strong forces of interaction exist between the dispersion phase . These are prepared by the following appropriate methods.
Compaction method
In this method, small solute particles are condensed as dispersed phase particles.
- Chemical Methods
A) By Oxidation: Colloidal sulfur can be obtained by passing oxygen gas from the hydrogen sulfide solution. In this method any oxidizing agent such as HNO3, H3Br2 can be used.
2H2S + O2 → 2H2O + 2S (Sulfur Sol)
B) By Double Decomposition: In this method a solution of arsenic sulfide is obtained. In this process hydrogen sulfide is passed through an Arsenic oxide cold solution in water.
AS2O3 + 3H2S → AS2S3 + 3H2O Arsenic Sulfide (Sol)
C) By Cutting: Aqueous solutions of these salts are obtained by reacting with suitable reducing agents such as formaldehyde, phenylhydrazine, hydrogen peroxide, stainless chloride, and many metals such as gold, silver, and platinum.
Sncl2+AuCl3 -> SnCl4+Au (Gold sol)
AuCl3 + HCHO + H2O → Au + HCOOH + HCl
Gold prepared in the reduction of gold chloride solution is purple in sol and is called violet of cassius.
D) Extremely Cold: In this process, the colloidal sol of ice is obtained. In an organic solvent like chloroform ether we take ice. The ice sol is obtained by freezing the water solution in a solvent. Water molecules are no longer in separate solutions to separate colloidal-shaped particles.
E) By Exchange of Solvent: In this process, the colloidal sols of some substances, such as sulfur, phosphorus, which are soluble in alcohol, but insoluble in water, can be prepared by mixing their alcohol solution with water. When added to water, a milky colloidal solution of sulfur is given for a sufficient alcoholic solution of sulfur.
(i) By Change of Physical State: The vapor of a substance such as mercury and sulfur is prepared by passing through a stable stabilizer containing cold water containing salt of ammonia.
Methods of Dispersal
[Click Here for Sample Questions]

Example of Colloids
In these methods, larger particles of a substance (suspension) are broken into smaller particles.
They are as follows:
a) mechanical dispersion: In this method, the substance is first prepared for coarse particles. It is then mixed with a dispersion medium to obtain a suspension. The suspension is then grinded into a colloidal mill.
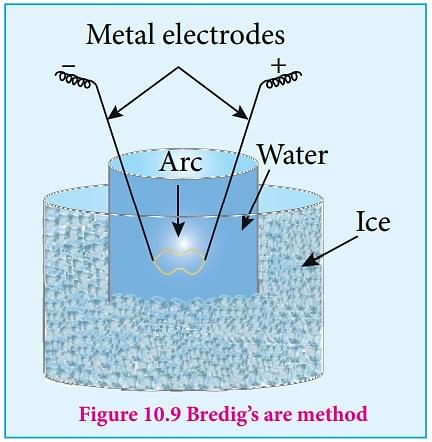
b) Bredig’s arc method or electroplating: This method is used to prepare platinum, silver copper, or gold sol. The metal of which the sol is to be prepared is made as a two-electrode which is immersed in a dispersion medium such as water etc.The dispersion medium is kept cool by ice. An electric arc is placed between the electrodes. The tremendous heat generated by and give colloidal solute. Electrolytes are used for this process for stabilization and cooling.
c) peptization: Peptization is the process of converting a freshly prepared precipitate into a colloidal solution. In this method a small amount of electrolyte is added which is known as peptization agent or peptizing agent.
A stable sol of stannic oxide is obtained by adding a small amount of HCl to the stenyl oxide, in the same way that the colloidal solution of Al(OH)3 and Aglox is prepared in the same way as Hydrochloric acid . By compatible treatment prepared from the slurry.
Purification of Colloids
[Click Here for Sample Questions]
Colloids contain ionic impurities and other categories of impurities that reduce the quality of colloids used in various applications.
The following are the methods of purifying colloids.
1) Dialysis
Dialysis is the method of separating ionic materials from colloidal solutions by means of a flow through a suitable membrane. Distilled water in the container where a bag sink must be replaced frequently to prevent accumulation on the bags of crystal.
2) Electro dialysis
Dialysis is a slow process and it takes so long to remove impurities. The process is rectified by an applied electric force.
3) Ultrafiltration
Normal filter paper cannot be used to filter out impurities of colloids as impure particles will be filtered due to large size pores. The size of the hole is reduced by applying paper to the Colderious solution, which is 4 - s. Calculate the nitrate solution in alcohol - a mixture of ether and dried with acetaldehyde. This is known as ultrafiltration and such papers are known as ultrafiltration paper.
Examples of colloids are
1) Blood: A respiratory pigment that contains albumin protein in water. The pigment part contains albumin which acts as the dispersion phase and the dispersion medium is water. It is a hydrosol.
2) Cloud: It consists of air which is the medium of dispersion and droplets of water as a medium of dispersion.
3) Sol of Gold: It is a metal sol in which gold particles are scattered in water.

Purification of Colloids
Application of Colloids
[Click Here for Sample Questions]
Colloids are widely useful in industries, medical and household applications.
As to foods: syrups, puddings, soups belong to a colloidal type system.
Medicine: Colloidal silver, named Argyroles, acts as an antiseptic for eye infections.
Air purification by Cottrell precipitator: This process involves deposition of a solution particle. Dust or smoke is passed through the inlet of an electrified chamber, which consists of a central electrical plate that is provided with a dental inverse charge of the accumulation of dust particles when dust particles accumulate and air. Pass through another outlet.
Leather tanning: The skin of the animal is very soft when immersed in a tannin solution, which has the opposite charge of hardening the animal's skin, coating particles, and skin
Delta Construction: It involves the deposition of soil particles of the river along with the electrolyte of seawater.
Properties of Colloids
[Click Here for Sample Questions]
The following are some characteristic properties, which are unique to colloids :
Brownian motion : The colloidal particles of the solution move in a typical zig zag motion. This motion is known as brownian motion. This motion is independent of the character of the colloid but depends on the scale of the particles and solution viscosity. Smaller the dimensions and lesser the viscosity, faster is that the motion. The Brownian movement has been explained to ensue to the unbalanced bombardment of the particles by the molecules of the dispersing phase. The Brownian movement includes a stirring effect which doesn't permit the particles to settle and thus, is liable for the steadiness of sols.
Tyndall effect : When light is passed through a colloidal solution, its path is visible. This is known as tyndall effect. This phenomenon is observed because colloidal particles are large enough to cause scattering of light. Tyndall effect is used to differentiate between colloidal and other solutions. A good example of this effect can be seen in cinema halls, when the path of light is visible through the colloidal solution of air.
Colligative properties : Since colloidal particles are larger in size than true solutions, they are lesser in number as compared to true solutions. As a result, the colligative property values such as osmotic pressure, lowering in vapour pressure, depression in freezing point and elevation in boiling point are lower in value as compared to true solutions.
Charge on colloidal particles : Colloidal particles can be negatively or positively charged. Negatively charged colloids are known as negative sols and positively charged particles are known as positive sols. The presence of charge on colloidal particles is confirmed by the electrophoresis experiment. Electrophoresis is defined as the movement of colloidal particles under the influence of an external electric potential.
Also Read:
Sample Questions
Ques. What happens when and why:
(i) To a hydrated ferric oxide sol in water, an electrolyte is added.
(ii) Through a colloidal solution, a beam of light is passed.
(iii) Through a colloidal solution, an electric current is passed. (All India 2009)(3 marks)
Ans. (i) When this happens, the process of coagulation of the colloidal solution happens. This is because the sol has charged particles in itself which get separated when an electrolyte is added.
(ii) The path of the beam of light becomes visible. This is because colloidal particles have a large enough size to scatter the beam of light. This is known as tyndall effect.
(iii) Passing electric current through a colloidal solution leads to the positively charged particles moving toward the cathode and negatively charged particles moving towards the anode. This leads to coagulation. The process is known as electrophoresis.
Ques. Differentiate between multimolecular, macromolecular and associated colloids. Give an example of each. (All India 2009, Delhi 2010)(3 marks)
Ans. The difference between the three is tabulated below:
| Multimolecular Colloids | Macromolecular Colloids | Associated Colloids |
|---|---|---|
| They are formed by aggregation of molecules of smaller size (<1 mm) | They are formed by aggregation of molecules of larger size | They are formed by the aggregation of ions |
| They have lower molecular mass | They have high molecular mass | They have high molecular mass |
| Examples include sol of gold, sulphur | Examples include polymers such as nylon | Examples include soap sol. |
Ques. Define emulsions. (Delhi 2010)(1 mark)
Ans. Emulsion is a type of colloid in which both dispersed phase and dispersion medium are liquids. Eg. Vanishing cream
Ques. What is electrophoresis ? (Delhi 2011)(1 mark)
Ans. Electrophoresis is defined as the movement of colloidal particles under the influence of an external electric potential.
Ques. Differentiate between lyophilic and lyophobic sols. (Compartment Delhi 2014)(3 marks)
Ans.
| Property | Lyophilic | Lyophobic |
|---|---|---|
| Definition | They are solvent loving colloids | They are solvent hating colloids |
| Stability | More stable due to strong forces of attraction between dispersed phase and dispersion medium | Less stable due to less forces of attraction between the dispersed phase and dispersion medium |
| Preparation | Readily formed by warming and mixing the dispersed phase with the dispersion medium | Preparation is difficult and involves long procedures such as oxidation, reduction, hydrolysis, double decomposition, exchange of solvent, bredig’s arc method, peptisation,excessive cooling, etc. |
| Coagulation | For coagulation of lyophilic colloids, addition of both solvent as well as electrolyte is required, since it is very stable. | Addition of a small amount of electrolyte leads to coagulation of the lyophobic colloidal solution. |
| Reversibility | These colloids are reversible in nature | These colloids are irreversible in nature |
| Examples | Metallic sols such as Ag, gold etc. | Starch, gum, gelatin,proteins,rubber etc |
Ques. How can a colloidal solution and true solution of the same colour be distinguished from each other? (Comptt. Delhi 2012)(1 mark)
Ans. A colloidal solution exhibits tyndall effect, i.e. it scatters a beam of light if passed through it, whereas a true solution does not exhibit tyndall effect.
Ques. What is meant by coagulation of a colloidal solution? Name any method by which coagulation of lyophobic sols can be carried out. (All India 2010)(2 marks)
Ans. When the particles of a colloidal solution settle down, it is known as coagulation.
Addition of a small amount of electrolyte leads to coagulation of the lyophobic colloids.
Ques. Write the dispersed phase and dispersion medium of the following colloidal systems:
(i) Smoke
(ii) Milk (Delhi 2013)(2 marks)
Ans. For smoke: dispersed phase is carbon particles and dispersion medium is the air. For milk : dispersed phase is fat and dispersion medium is water.
Ques. How are the following colloidal solutions prepared?
(a) Sulphur in water
(b) Gold in water (Compartment, Delhi 2013)(5 marks)
Ans. (a)Colloidal sulfur can be obtained by passing oxygen gas from the hydrogen sulfide solution. In this method any oxidizing agent such as HNO3, H3Br2 can be used.
2H2S + O2 → 2H2O + 2S (Sulfur Sol)
(b) Aqueous solutions of these salts are obtained by reacting with suitable reducing agents such as formaldehyde, phenylhydrazine, hydrogen peroxide, stainless chloride, and many metals such as gold, silver, and platinum.
SnCl2 + AuCl3 -> SnCl4 + Au (Gold sol)
AuCl3 + HCHO + H2O → Au + HCOOH + HCl
Gold prepared in the reduction of gold chloride solution is purple in sol and is called violet of cassius.Le Chatelier's Principle
Ques. Define (a)peptisation (b) Reversible sols (All India 2010)(2 marks)
Ans. (a) Peptization is the process of converting a freshly prepared precipitate into a colloidal solution. In this method a small amount of electrolyte is added which is known as peptization agent or peptizing agent.
(b) Reversible sols (Lyophilic sols) are those sols which attract the solvent. They are formed very readily by mixing the dispersed phase in a warm dispersion medium.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:



Comments