Jasmine Grover Study Abroad Expert
Study Abroad Expert
Heinrich Kayser, the German physicist was the first to coin the term adsorption which can be explained as a surface phenomenon where particles remain attached on the top of a material. Generally, it comprises the molecules, atoms, liquid, solid in a dissolved stage, even the ions of a gas that are attached to the surface. Much to our surprise, the consequence of surface energy i.e, adsorption is present in biological, physical, chemical, and natural systems and are used in many industrial applications.
| Table of Content |
Key Terms: Adsorption, adsorbent, mechanism, adsorption isotherm, desorption, Freundlich isotherm.
Define Adsorption
[Click Here for Sample Questions]
Adsorption is the phenomenon wherein the molecules (Liquid or gaseous) from the environment of an object (solid or liquid) begin to settle on its surface, without penetrating into the bulk matter that is present throughout the object.
- The molecules that settle are known as adsorbate and the ones adsorbing are called adsorbent.
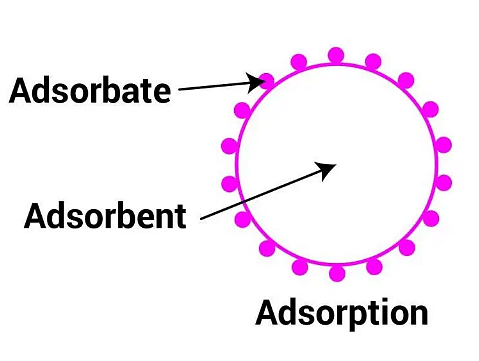
Adsorption
- We know that any substance has a chemical structure embedded in it, may it be solid, liquid or gas. Hence, not only their inner mass, but the surface has its unique chemistry as well.
- In scientific terms, we can define adsorption as the accumulation of molecular species at the surface rather than in the bulk of a solid or liquid.
- The process of removing an adsorbed substance from a surface on which it is adsorbed is called desorption.
So far, we have been learning about the phenomenon of absorption by substances, but adsorption differ with absorption as shown in the following table:
| Adsorption | Absorption |
|---|---|
| It is the phenomenon where the particles of gas or liquid do not penetrate inside the solid, rather it settles on the surface of the solid. | It is the phenomenon where the particles of gas or liquid are uniformly distributed throughout the body of the solid. |
| There is almost negligible concentration of the substance found inside the solid. | The concentration of the particles is the same throughout the solid. |
| Adsorption is very rapid in the beginning as it is directly exposed to the surface of the solid. And with time, the rate decreases. | Absorption occurs at a uniform rate |
| For example, if we dip chalk in blue ink, the colour in the ink would get settled on the surface of the chalk, while it would not be seen through the section of a chalk. | For example, if we dip chalk in blue ink, the solvent in which the colour is dissolved is absorbed by the chalk and is seen throughout the chalk. |

Difference between adsorption and absorption
Also Read:
| Related Articles | ||
|---|---|---|
| Catalysis | Colloids | Classification of Colloids |
| Emulsions | Properties Of Colloids | Colloidal Solutions |
| Haber Process | Structure of Zeolites | Preparation of Colloidal Solutions |
Mechanism
[Click Here for Sample Questions]
When the object is exposed to a certain liquid or gaseous environment, the first thing that happens is the molecules from the environment get settled on the surface of the object, but as the bulk material inside the object is not exposed to the environment, it has lower or negligible concentrations of those molecules.
- Adsorption increases with increasing surface area.
- The molecules from the environment tend to form chemical bonds or physical bonding with the surface of the object, where the chemical interactions are possible due to atoms that are not involved in bulk formation.
- There is a release in heat from the surface due to bond formations and hence, this is an exothermic reaction.
- Hence, we have a system with the following thermodynamic properties:
- ΔH is negative
- ΔS is negative (as the freedom of movement of the molecule in adsorption becomes difficult)
- ΔG can be negative only when the ΔH is sufficiently negative to fit in the following formula = ΔG = ΔH – TΔS
- The combination of these two factors are very important for the value of the ΔG.
- As the adsorption moves forward, ΔH moves towards being positive, finally ΔH becomes equal to TΔS and ΔG becomes zero. At this stage, the equilibrium is attained.
Types of Adsorption
[Click Here for Sample Questions]
| Chemisorption | Physisorption |
|---|---|
| It is caused by chemical bond formation on the surface of the object. | It arises because of van der Waals’ forces, which are physical in nature. |
| It is highly specific in nature. | It is not specific in nature. |
| It is an irreversible process | It is a reversible process |
| It also depends on the nature of gas. Gases which can react with the adsorbent show chemisorption. | It depends on the nature of gas. More easily liquefiable gases are adsorbed readily. |
| Enthalpy of adsorption is high | Enthalpy of adsorption is low |
| High temperature is favorable for adsorption. i.e. Adsorption increases with increase in temperature. | Low temperature is favorable for adsorption. i.e. Adsorption increases with decrease in temperature. |
| High activation energy is at times needed for chemisorption. | No activation energy is needed for physisorption. |
| It also increases with an increase of surface area. | It increases if there is an increase of surface area. |

Types of Adsorption
Adsorption Isotherm
[Click Here for Sample Questions]
This was given by scientist Freundlich, in 1909. It says that, empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature.
This isotherm is a graph wherein we plot, the pressure on the x-axis, while the ratio of mass of adsorbate and mass of adsorbent is plotted with respect to the temperatures at that time. The final graph for every adsorption process results as follows:
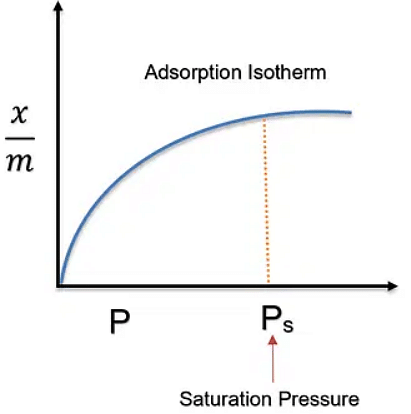
Adsorption Isotherm
Here,
T3 > T2 > T1 are the temperatures
- x = mass of adsorbate
- m = mass of adsorbent
- p = Pressure
We can observe the following things from the graph:
These curves indicate that at a fixed pressure, there is a decrease in physical adsorption while the temperature increases.
These curves tend to approach saturation at high pressure.
In mathematical terms,
xm = k.p 1/ n (n > 1)
where, k is constant
after taking logarithm on both the sides of the equation, it becomes:
log xm = log k + 1/n . log p
If the plot is a straight line the Freundlich isotherm is valid, otherwise, it is not.
When, 1/n = 0,
xm = constant,
The adsorption is independent of the pressure
When 1/n= 1
xm = k.p
i.e. xm ∝ p
The adsorption varies directly with the pressure.
Both conditions are proven experimentally.
The experimental isotherms were seen to approach saturation at high pressure. This was not explained by Freundlich isotherm.
Hence, it tends to fail under high pressure.
Examples of Adsorption
- Charcoal adsorbs the blue color from a solution having methylene blue dye in it, leaving the solution colorless.
- If a gas like O2, H2, CO, Cl2, NH3 or SO2 are taken in a closed beaker containing activated charcoal. Here, the gas molecules concentrate at the surface of the charcoal meaning the gases are adsorbed on the surface of the charcoal, resulting in decreasing pressure, inside the beaker.
- The air becomes dry in the presence of silica or aluminum gel as the water molecules or vapor molecules get adsorbed on the surface of the gel.

Example of Adsorption
Application of Adsorption
- A number of drugs are used to kill germs by getting adsorbed on them. Hence, diseases can be cured.
- Helps in the removal of coloring matter from the industrial solutions.
- Silica and Aluminum gels are used to control humidity in the air.
- The gas mask is usually used for breathing in coal mines to adsorb poisonous gases, as it contains activated charcoal or other mixtures of adsorbents.
- The remaining traces of air can be adsorbed by charcoal from a column evacuated by a vacuum pump to increase vacuum in the column.
Also Read:
Sample Questions
Ques. What can we achieve with the help of adsorption? (1 mark)
Ans. Adsorption is effective for purifications, e.g. extracting a contaminant. In addition, adsorption is good for bulk matter separations. Adsorption is also used for preventing pollution, purifying materials that will react.
Ques. Why do the adsorbate not get adsorbed by the bulk matter, despite having the same chemistry throughout the bulk, even on surface? (1 mark)
Ans. It is true that the bulk matter has similar structure chemically, throughout the bulk, but as there are no layers of molecules of the bulk interacting on the surface of it, the surface remains open to other layers of molecules like adsorbate.
Ques. Why does the cosmetic industry use activated charcoal? (1 mark)
Ans. The activated charcoal is the best kind of adsorbent. Hence, it helps in extraction of the dirt from the surface of our skin.
Ques. Which gas shows maximum adsorption out of these two, CH4 and CO2? (1 mark)
Ans. CO2 exhibits more adsorption.
Ques. Where is adsorption used in the field of biological research? (1 mark)
Ans. Adsorption chromatography is a technique used to separate out the biological chemicals.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:



Comments