
Content Writer
Electrolyte is a substance that gets separated from water, and is converted into charged particles, making an Ion or creating an Electric Current.
- In electrolytes, negatively charged ions are called Anions.
- Positively charged ions are called Cations.
- From a nutritional standpoint, it refers to vital Minerals that can be present in sweat, urine, and blood.
- Electrolytes are essential for many bodily functions, including preserving healthy nerve and muscle function, maintaining a balanced pH in the body, and keeping us hydrated.
| Table of Content |
Key Terms: Electrolyte, Cell, Current, Ions, Chemical, Conductor, Body, Electric Current, Solvation
What is Electrolysis?
[Click Here for Sample Questions]
Electrolysis is referred to as the process of breaking down a substance by running an Electric Current through it while it is liquid or molten.
- Electrolyte is a substance that conducts electricity when it is molten or when it is dissolved in water.
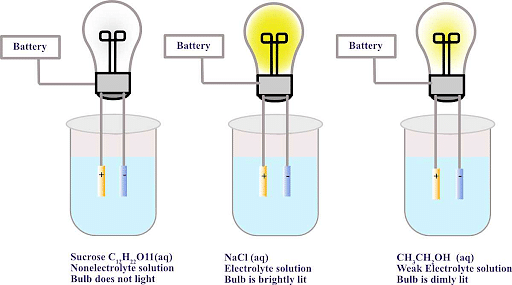
- Due to the lack of an electric charge, glucose, and urea do not dissociate in water. As a result, they referred to these substances as Non-Electrolytes.
Also Read:
| Relevant Concepts | ||
|---|---|---|
| Reduction Potential | Coulomb's Law | Quantitative Aspects Of Electrolysis |
| Electrode Potential | Nonelectrolyte | Concentration Cell |
What are Electrolytes?
[Click Here for Sample Questions]
An Electrolyte is referred to as an ionic compound that conducts Electricity such as sodium chloride when it is dissolved in water.
Following are some points expressing the importance of Electrolytes as part of the human body:
- Electrolytes are responsible for conducting electricity, which is crucial for the health of muscles and nerves of the body.
- Electrolytes create osmotic pressure, which keeps bodily fluids segregated.
- Electrolytes continue to work as before to prevent pH changes in body fluids.
- Numerous biological substances, such as Carbohydrates are not ionic and do not exhibit any electrical properties when dissolved in water.
- Other significant Electrolytes include Potassium, Calcium, Bicarbonates, and Phosphate, in addition to Sodium and Chloride.
Electrolytes
Formation of Electrolytes
[Click Here for Sample Questions]
Electrolyte solutions is typically created when salt is added to a solvent like water and the individual components dissociate due to the Thermodynamic Interactions between the solvent and solute molecules. This process is known as "Solvation”.
- As an example, when table salt (Sodium Chloride), NaCl, is dissolved in water, it forms its component ions.
![]()
- If There is an Electrolyte in a solution containing a lot of ions, it is said to be "Concentrated," and if it contains few ions, it is said to be "Dilute."
- The Electrolyte is strong if a significant portion of the solute dissociates to form free ions; it is weak if the majority of the solute does not dissociate.
- Electrolysis can be used to extract the solution's constituent Element and compounds by taking advantage of electrolytes' unique properties.
Types of Electrolytes
[Click Here for Sample Questions]
Electrolytes have been classified into Strong and Weak Electrolytes which are described as follows:
Strong Electrolytes
Strong electrolytes are those Electrolytes that completely get ionized in their Liquid or Molten state.
- They typically have ions as their main species in solution.
- Strong Electrolytes consist of Strong Acids, Strong Bases, and Salts.
- Examples: HCl, HClO3, NaOH, Ba (OH) 2, NaCl, and KBr.
Weak Electrolytes
Weak Electrolytes are those Electrolytes that ionize only partially in their liquid or molten state (usually in the range of 1–10%).
- The unionized compound is the primary species in solutions for weak Electrolytes.
- Examples: HF, HC2H3O2, NH3, and C5H5N.
List of Electrolytes
[Click Here for Sample Questions]
There is a different list of electrolytes that occurs in unique structures. They are as follows:
Major Electrolytes Inside the Cell
The most prevalent Electrolytes in a cell are Magnesium, Potassium, and Phosphate.
Phosphorus as a Fundamental Electrolyte
Phosphate salts is the major form of phosphorus found in the body. Scientists occasionally use phosphate salts in place of the word "phosphorus."
- Energy metabolism depends on phosphate.
- Phosphorus is essential for the mineralization of bones and teeth when combined with calcium. Additionally, it supports the preservation of the acid-base balance.
- The enzyme reactions are accelerated by magnesium. In addition to regulating neuromuscular contraction,
- It supports healthy Nervous and Cardiovascular systems, promotes protein synthesis, and facilitates sodium and potassium ion transport.
Potassium as an Essential Electrolyte
Potassium plays a vital role in
- Regulation of cell excitability.
- Transmission of nerve impulses.
- Potential of the resting membrane.
- Muscle contraction and responsiveness of the Myocardial Membrane.
- Osmolality regulation inside cells.
Major Electrolytes Outside the Cell
The two main Electrolytes in extracellular fluid, sodium, and chloride, work primarily outside of the cell. Sodium concentration influences the extracellular fluid volume and serum osmolality.
- Sodium also promotes communication between muscle and nerve cells.
- Chloride aids in preserving Osmotic Pressure.
- Gastric mucosal cells require chloride to make hydrochloric acid, which disintegrates food into components that can be absorbed.
- Two other electrolytes found in extracellular fluid are calcium and Bicarbonates The main cation involved in the formation and maintenance of bones and teeth is calcium.
Calcium is also essential for
- Transmitting nerve impulses by stabilizing the cell membrane and lowering sodium permeability.
- Tensing the muscles.
- Blood coagulation.
- Developing tooth and bone.
- Maintaining a proper Acids and Bases balance, in form of bicarbonate.
Electrochemical Cells
[Click Here for Sample Questions]
An Electrochemical cell is a structure that can produce electrical energy from chemical reactions taking place inside of it or use external electrical energy to speed up chemical reactions taking place inside of it.
- These gadgets can change chemical energy into electrical energy or the other way around.
- There are three major categories of Electrochemical cells:
- Concentration cells.
- Electrolytic and Galvanic Cells
There are also four fundamental parts to all such cells, which make them similar. They are
- The Electrolyte serves as the Conduct for Current Flow between the anode and the cathode.
- In an aqueous solution that typically has Homogeneous Mixtures, the concentration or type of dissolved chemicals in moist soil may vary locally.
- The Anode, which can conduct Electricity and is in contact with the electrolyte, corrodes when it combines with the chemicals in the electrolyte.
- In addition to the anode, the Cathode is another metal that comes into contact with the electrolyte; it is not corroded but is given anti-corrosion treatment.
- The conductor, which also completes the circuit necessary for current flow, joins the Anode and Cathode.
Movement of Electrolytes
[Click Here for Sample Questions]
In Electrolytes when contents leak into the Extracellular Space and disturb the Electrolyte balance. Cells appear to be dead.
- Plasma, for instance, contains significant amounts of intracellular Electrolytes.
- Electrolytes are not restricted, even though something typically concentrates them in a particular compartment.
- They move around like fluids to maintain Chemical Equilibrium and electroneutrality.
Understanding Electrolytes
[Click Here for Sample Questions]
Electrolytes play a role in controlling Nerve Impulse transmission, acid-base balance, and Water Management. Furthermore, they aid in Blood Coagulation and Energy production.
The main Electrolytes in the body are listed in this table with a brief description of each one's role.
Sodium (Na)
- Sodium is the Principal cation in the extracellular fluid (ECF).
- It keeps the acid-base balance balanced.
- It controls the normal ECF osmolality.
- It activates the muscle and nerve cells.
Chloride (Cl)
- Chloride is the Principal ECF anion that contributes significantly to preserving the acid base.
- It balance and, when combined with hydrogen ions, yields hydrochloric acid.
- It keeps the ECF osmolality in a normal range.
- It affects the pH of the body.
Calcium (Ca)
- Calcium helps in coagulation.
- ICF and ECF contain roughly equal amounts of a major cation found in teeth and bones.
- It acts as an Enzyme Activator within cells
- It can be found in cell Membranes.
- It aids in cell adhesion and shape maintenance.
- It affects the permeability of cell membranes and firing rate.
Potassium (K)
- Potassium is the major cation of the intracellular fluid (ICF).
- It controls cellular excitability.
- It penetrates cell membranes and thus has an impact on the electrical status of the cell.
- It aids in regulating ICF osmolality, which in turn regulates ICF osmotic pressure.
Magnesium (Mg)
- Magnesium is a leading cation of ICF.
- It contributes to a variety of metabolic and enzymatic processes, especially protein synthesis.
- It changes the transmission of Nerve Impulses and the response of skeletal muscles.
Phosphorus (P)
- Phosphorus is the Primary ICF anion.
- It encourages Metabolism and serves as a buffer for hydrogen.
Importance of Electrolytes
[Click Here for Sample Questions]
Electrolytes are important because of the following reasons:
- Electrolytes, signals are transmitted from the Central Nervous System to various body parts through nerve cells.
- The electrical charge of the membrane of the nerve cell produces these signals, which are referred to as Nerve Impulses.
- Electrolytes, especially Sodium, move in and out of the membrane, causing these changes.
- This starts a series of events that helps in the Signal Transmission down the length of the nerve cells.
- The electrolyte required for Muscle contraction is Calcium ions as it causes muscle fibers to slide past and contract against one another, which causes muscle contraction and relaxation.
- Each cell in our body must have an appropriate balance of fluids both inside and outside. Correct water levels must always be maintained.
- Through Osmosis, Electrolytes, particularly Sodium, aid in preserving fluid balance.
- A substance's pH, which is controlled by buffers, weak Acids, and Bases.
- It is simply a measurement of how basic or acidic it is.
- The body must have a pH of about 7.5 to function properly.
- Your body's pH level is kept at the proper level by having the proper electrolyte balance.
Things to Remember
- Electrolytes are substances that undergo physical or chemical change resulting in ions in solution belonging to a significant class.
- Electrolytes can either be Ionic Compounds that break down into their component cations.
- They can be anions or covalent compounds that chemically react with water to produce ions (such as acids and bases)When dissolved in water.
- Electrolytic cells are a class of Electrochemical cells that use electric currents to facilitate the cell reaction.
- Strong Electrolytes are soluble ionic substances and strong acids that completely ionize, whereas weak acids and bases that only partially ionize are known as weak Electrolytes.
- Most carbon compounds are not Electrolytes. The majority of fats, sugars, and alcohol are not Electrolytes.
- Since the solution of an Electrolyte, as a whole, is electrically neutral, the total charge on the cations is equal to the total charge on the anions.
- In addition to sodium and chloride, other significant Electrolytes include Potassium, Calcium, Bicarbonate, and Phosphate.
Also Read:
Sample Questions
Ques. What is an Electrolyte? (1 Mark)
Ans. A medium that contains ions and is electrically conducting due to the movement of those ions but does not conduct electrons is called an electrolyte.
Ques. Are there Electrolytes in the Human body? (2 Marks)
Ans. Yes. Numerous electrolytic processes can be found inside the human body. The common electrolytes—sodium, calcium, potassium, chloride, phosphate, and magnesium—are all obtained from food and liquids you drink. Your body's electrolyte levels can fluctuate between too low and too high. This may occur if the body's water balance changes.
Ques. How can Electrolytes conduct Electricity? (2 Marks)
Ans. Due to the dissociation of the molecules into ions, the electrolyte can conduct electricity. These fragmented molecules enable electrical conduction in the solution when the electrolyte fuses or dissolves in the solvent.
Ques. Why are Electrolytes so important? (1 Mark)
Ans: Body fluids contain electrolytes that form electrically charged particles, or ions. These ions contain the necessary electrical energy for a variety of processes, such as nerve impulse transmission and muscle contraction. Electrolytes are essential for many bodily processes.
Ques. Is normal water an Electrolyte? (1 Mark)
Ans. Simple water cannot conduct electricity because it contains very few ions. A solvent is referred to as an "electrolyte" when it dissociates to create ions in water because the resulting solution is a potent electrical conductor.
Ques. What is an example of a non-Electrolytic substance? (2 Marks)
Ans. One typical example of a nonelectrolyte is glucose or C6H12O6. Glucose (sugar) dissolves easily in water, but because it does not separate into ions in solution, it is referred to as a nonelectrolyte, and solutions containing it do not conduct electricity.
Ques. What are Electrolytic cells? (2 Marks)
Ans. Electrolytic cells are a subset of electrochemical cells that use electric currents to speed up the cell reaction. Electrolysis is the name given to the chemical process that takes place within these cells. Bauxite can be broken down into aluminum and other components using electrolytic cells. These cells can also be used to electrolyze water to produce hydrogen and oxygen.
Ques. Difference between an Electrolytic cell and a Galvanic cell? (5 Marks)
Ans. The difference between an Electrolytic and a Galvanic cell is as follows:
| Electrolytic Cell | Galvanic Cell |
|---|---|
| In these cells, electrical energy is converted to chemical energy. | In these electrochemical cells, chemical energy is converted to electrical energy. |
| The redox reactions in these cells are not spontaneous; rather, they require an input of energy to proceed. | The redox reactions that occur in these cells are essentially spontaneous. |
| These cells have a cathode that is negatively charged and an anode that is positively charged. | These electrochemical cells have a positively charged cathode and a negatively charged anode. |
| Electrons come from an outside source, like a battery. | The species that experience oxidation provides the electrons. |
Ques. What are the Key Differences between Cathode and Anode? (2 Marks)
Ans. The location where reduction takes place in an electrochemical cell is the cathode. Usually, a positive (+) sign is used to indicate it. The anode emits electrons that travel to the cathode.
The anode is the electrode in electrochemical cells where oxidation takes place. It is generally indicated by a negative (-) sign.
Ques. Determine the difference between strong electrolytes and weak electrolytes. (5 Marks)
Ans. The difference between strong and weak electrolytes are:
| Strong Electrolytes | Weak Electrolytes |
|---|---|
| Strong electrolytes are electrolytes that are fully ionized. | Weak electrolytes are electrolytes that are partially ionized. |
| They have a high conductivity of electricity. | They have a low conductivity of electricity. |
| In a solution or molten form, strong electrolytes are totally ionized. Which results that the compounds only containing ions in a liquid or molten form. | In a solution or molten state, these chemicals are partly ionized. Ions and un-dissociated molecules which are present in these electrolytes. |
| At moderate quantities, strong electrolytes are totally dissociated. | At moderate concentrations, weak electrolytes do not completely dissociate. |
| At high concentrations, there are strong interionic interactions. | At higher concentrations, interionic interactions are weak. |
| Acids, alkalis, and salts like sodium chloride, potassium chloride, sodium hydroxide, potassium hydroxide, sodium nitrate, and lead sulfate are all examples of strong electrolytes. | Acids, alkalis, and salts like oxalic acid, formic acid, acetic acid, ammonium hydroxide, and calcium hydroxide are all examples of weak electrolytes. |
Ques. (a) Define conductivity and molar conductivity for the solution of an electrolyte.
(b) Why do electrochemical cells stop working after some time? (5 Marks)
Ans. (a) Conductivity: The conductance of the solution of an electrolyte enclosed in a cell between two electrodes of a unit area of cross-section separated by 1 cm. They represented it as K with unit ohm-1 cm-1
Molar conductivity: It is the conductance of volume V of the solution containing one mole of electrolyte kept between two electrodes with the area of cross-section A and distance of unit length.
Molar conductivity increases with a decrease in the concentration of solute for both weak and strong electrolytes.
(b) Electrochemical cells will stop working after some time because,
- When one compound in the anode of the electrochemical cell is oxidized, the electron of that compound serves to reduce the compound on the cathode side.
- When the material at the anode does not have electrons to lose, hence the reaction stops and the cell stops working.
Hence, the electrode potential of the electrodes becomes equal.
Ques. Explain the Working of Electrolytes. (3 Marks)
Ans. Electrolytes work by allowing electrically charged particles, called ions, to move through a solution.
- When an Electrolyte is dissolved in a solvent, such as water, it dissociates into its constituent ions.
- For example, when table salt (sodium chloride) is dissolved in water, it dissociates into sodium ions (Na+) and chloride ions (Cl-).
- The movement of these ions in the solution is what allows the solution to conduct electricity. This movement of ions from positively charged electrons to negatively charged electrons is called Electrolysis.
- The ability of an Electrolyte to conduct electricity depends on several factors, including the concentration of the dissolved ions, the size, and charge of the ions, of the solvent.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:





Comments