Muskan Shafi Education Content Expert
Education Content Expert
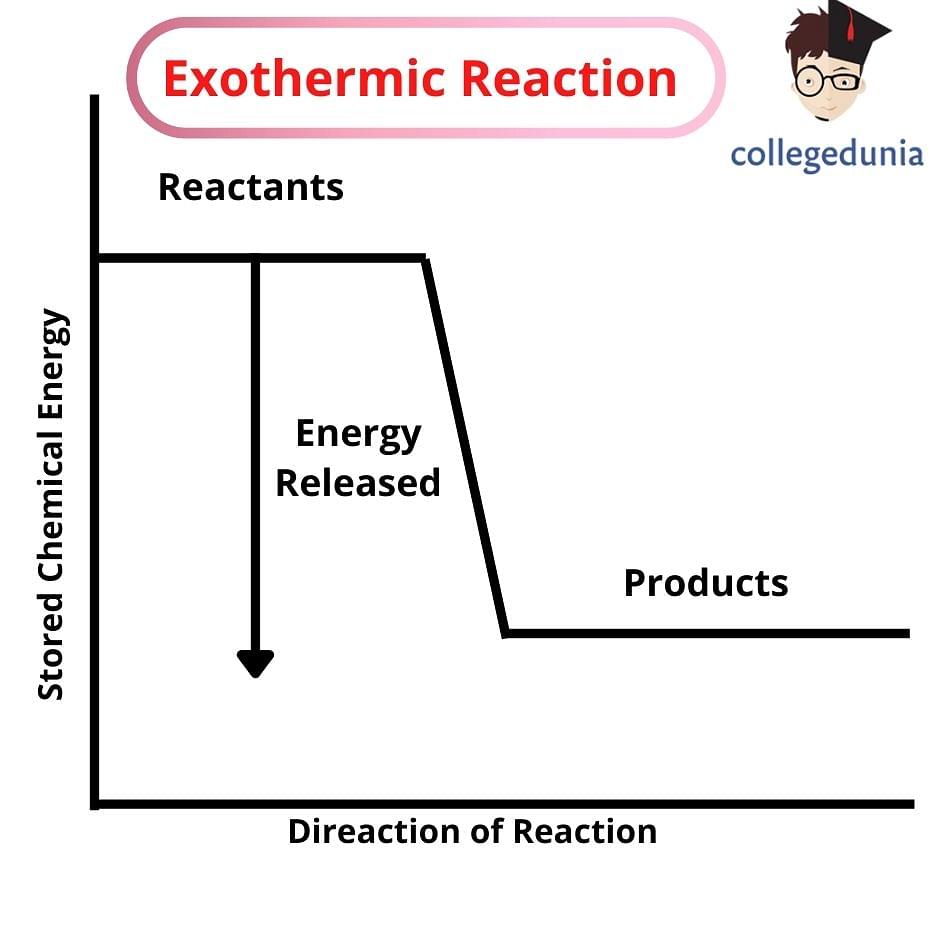
Exothermic Reaction is a chemical reaction that produces energy in the form of heat or light. In an exothermic reaction, the energy is transferred to the surroundings. Lighting a matchstick is one example of an exothermic reaction where the energy is released in the form of both heat and light.
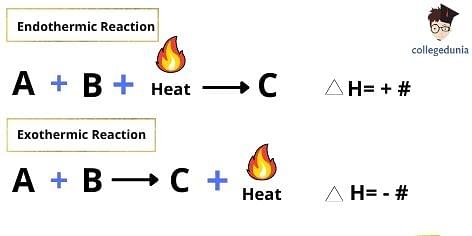
All chemical reactions involve the transfer of energy wherein, the reaction either releases energy to or absorbs energy from its surroundings. These two types of reactions in thermodynamics are classified as exothermic and endothermic reactions respectively. In endothermic reactions, energy is taken from the surroundings whereas, in exothermic reactions, energy is released to the surroundings.
Key Terms: Exothermic Reactions, Chemical Reaction, Reactants, Energy, Heat, Light, Combustion, Neutralization
What are Exothermic Reactions?
[Click Here for Sample Questions]
Exothermic Reactions are those chemical reactions in which energy is released in the form of heat and light. Exothermic reactions can be expressed as:
Reactants → Products + Energy
Exothermic reactions are opposite to endothermic reactions which involve the absorption of energy from the surrounding in the form of heat. Exothermic chemical reactions transfer the energy in the surrounding area.
Some examples of Exothermic Reactions are:
- Neutralization
- Respiration
- Burning a substance
- Deposition of dry ice
- Reactions of fuels
- Solution of sulfuric acid into water
| Related Topics | ||
|---|---|---|
| Elimination Reaction | Displacement Reactions | Standard Enthalpy of Formation |
| Bond Energy | Redox Reactions | Bond Enthalpy |
Thermochemical Equations for Exothermic Reactions
The net amount of energy needed to initiate an exothermic reaction is less than the net amount of energy released by the reaction.
- When a device (Calorimeter) is used to measure the heat released by a chemical reaction, the net amount of energy in the form of heat that flows through the device is equal to the negative of the total energy change of the system.
- It is extremely difficult to measure or calculate the absolute total energy in a given chemical reaction.
- This is the reason why the energy change or the enthalpy change (denoted by ΔH) is measured instead.
The relation between the value of Delta H and the bond energies of the reaction can be represented by the following equation:
ΔH = (Energy Used in Bond Formation) - (Energy Released after Reactant Bonds are Broken)
Thermochemical Equations for Exothermic Reactions
Thus, it can be understood that an exothermic reaction will always possess a negative value for the change in enthalpy, (i.e, ΔH < 0).
Read More: Substitution Reaction
Here are a few notable examples of exothermic reactions with a detailed explanation:
Combustion
- Combustion is an exothermic reaction in which fuel undergoes the process of reduction in the presence of an oxidizing agent. This oxidizing agent is generally the oxygen that is present in the atmosphere.
- In the process of combustion, the energy is released in the form of heat. However, harmful byproducts like smoke and ash are also formed.
- These harmful by-products are the major contributors to air pollution.
- An example of combustion is the burning of methane. The chemical reaction for the same is CH4 + O2 → CO2 + H2O + Heat
Neutralization Reaction
- A chemical reaction where a base and an acid react to form salt and water is called a neutralization reaction. Water is formed by a reaction of H+ ions and OH– ions.
- Acid + Base → Salt + Water
- When a strong acid and a strong base react, the pH value is equal to 7. When a strong acid and a weak base react, the pH value is less than 7. When a strong base and a weak acid react, the pH value is greater than 7.
- Some applications of a neutralization reaction are Titration methods, Wastewater treatment, Digestion process, Controlling soil pH, etc.

Respiration
- Respiration is an exothermic reaction as it involves the intake of oxygen. Oxygen further undergoes a reaction with glucose, which gives out carbon dioxide, water, and energy. This energy is used for performing lungs, heart, and other organ-related activities.
- The equation for respiration is Glucose + Oxygen → Carbon dioxide + Water

Detonation of Nitroglycerin
- Nitroglycerin is a combustible substance with extremely volatile physical properties. It is used in the production of explosives.
- It catches fire easily and this is the reason that it is the most sought-after element for explosives.
- When nitroglycerin is detonated, the fuel in the surrounding burns which creates a shockwave.
- Nitroglycerin decomposes itself during combustion which releases a large amount of energy. This is why it is a preferred element for explosives and dynamite.
Nuclear Fission of Uranium-235
- The fission of one atom of uranium-235 produces more than 2.5 million times the energy that is produced from the combustion of coal.
- The fission of one atom can form a chain reaction wherein the neutron from the fission of one uranium-235 atom can strike another atom that leads to its nuclear fission.
- The isotopes of uranium are used in nuclear power plants wherein the fission reaction is controlled with the help of control rods that are capable of absorbing neutrons.
Exothermic Reactions: Change in Enthalpy
[Click Here for Previous Years Questions]
Enthalpy is a thermodynamic quantity that measures energy change in a chemical reaction.
- Enthalpy refers to the difference between the energy that is required to break the bonds of the reactants and the energy released by the formation of new bonds (that is, the energy of the products reacting - energy released after the reaction)
- The change in enthalpy in exothermic reactions is negative. This means that the energy is higher than the combined energy of the reactants.
Things to Remember
- Exothermic reactions are chemical reactions that involve the release of energy in the form of heat or energy.
- Exothermic reactions can be expressed as Reactants → Products + Energy.
- Exothermic reactions are the opposite of endothermic reactions which absorb or take energy from the surroundings.
- The net amount of energy required to initiate an exothermic reaction is less than the net amount of energy released by the reaction.
- The change in enthalpy (ΔH) in an exothermic reaction will be always negative.
- Some examples of exothermic reactions are combustion, neutralization reactions, respiration, etc.
Previous Years’ Questions (PYQs)
- For an exothermic reaction, the value of… (BHU UET 2004)
- An exothermic chemical reaction proceeds by two stages. Reactants… (AMUEEE 2014)
- In which of the following exothermic reactions, the heat liberated…
- A certain reaction is non-spontaneous at 298K. The entropy change… (VITEEE 2017)
- Which of the following is not an endothermic reaction… (JKCET 2005)
- In a reversible reaction, the energy of activation of the forward… (NEET 1996)
- For an endothermic reaction, ΔH represents the enthalpy of the reaction…
- In which of the following reactions, standard entropy change… (BITSAT 2012)
- The enthalpy changes for the following processes are listed…
- The absolute enthalpy of neutralisation of the reaction…
Sample Questions
Ques: What is meant is an exothermic reaction? Give example to explain the same. (2 Marks)
Ans: Exothermic reactions are chemical reactions in which energy is released in the form of heat and light. For instance, the burning of fuel.
CH4(g) (Methane) + O2 → CO2 (g) + 2H2O(g) + Heat
The natural gas methane burns in the air to form carbon dioxide and water and releases a large amount of heat.
Ques: Distinguish between an exothermic and endothermic reaction. (2 Marks)
Ans: The difference between exothermic and endothermic reactions is as follows:
| Exothermic Reaction | Endothermic Reaction |
|---|---|
| The reaction in which energy is released in the form of heat or light is called an exothermic reaction. | The reaction in which the heat is absorbed from the surroundings is called an endothermic reaction. |
| For example, Neutralization reaction, burning of a substance, reactions of fuels, deposition of dry ice, and respiration. | For example, Photosynthesis, evaporating liquids, melting ice, alkanes cracking, and thermal decomposition. |
Ques: Give the reason why respiration is an Exothermic Reaction. (3 Marks)
Ans: The process of respiration involves the inhalation of oxygen. Then, the oxygen undergoes a reaction with glucose, and gives out carbon dioxide, water, and energy as the products. Water and carbon dioxide are by-products, and the energy is used by the lungs and heart to pump blood and other related activities of our body, thus, respiration is an exothermic reaction.
Ques: What is combustion? Is combustion an exothermic or endothermic reaction? (3 Marks)
Ans: Combustion is an exothermic reaction in which fuel undergoes the process of reduction in the presence of an oxidizing agent. This oxidizing agent is generally the oxygen that is present in the atmosphere. For instance, the combustion of methane.
In the process of combustion, the energy is released in the form of heat. However, harmful byproducts like smoke and ash are also formed. These harmful by-products are the major contributors to air pollution.
An example of combustion is the burning of methane. The chemical reaction for the same is CH4 + O2 → CO2 + H2O + Heat
Ques: Define a combination reaction. Give one example of a combination reaction which is also an exothermic reaction. (2 Marks)
Ans: A combination reaction is a reaction in which two elements are combined together to form a product.
CaO (Quick Lime) + H2O (Water) → Ca(OH)2 (Slaked Lime) + Heat
Ques: What are the examples of Exothermic reactions? Briefly explain them. (5 Marks)
Ans: Examples of Exothermic Reactions are:
- Combustion: Combustion is an exothermic reaction in which fuel undergoes the process of reduction in the presence of an oxidizing agent. This oxidizing agent is generally the oxygen that is present in the atmosphere.
- Neutralization: A chemical reaction where a base and an acid react to form salt and water is called a neutralization reaction. Water is formed by a reaction of H+ ions and OH– ions.
- Respiration: Respiration is an exothermic reaction as it involves the intake of oxygen. Oxygen further undergoes a reaction with glucose, which gives out carbon dioxide, water, and energy. This energy is used for performing lungs, heart, and other organ-related activities.
- Detonation of Nitroglycerin: Nitroglycerin is a combustible substance with extremely volatile physical properties. It is used in the production of explosives. It catches fire easily and this is the reason that it is the most sought-after element for explosives.
- Nuclear fission of Uranium-235: The fission of one atom of uranium-235 produces more than 2.5 million times the energy that is produced from the combustion of coal. The fission of one atom can form a chain reaction wherein the neutron from the fission of one uranium-235 atom can strike another atom leading to its nuclear fission.
Ques: What will be the sign of enthalpy change for an exothermic reaction? (2 Marks)
Ans: For an exothermic reaction, the net amount of energy required to initiate the reaction is less than the net amount of energy released by the reaction. Thus, the change in enthalpy (ΔH) will be negative.
Ques: What do you understand by the term enthalpy? (3 Marks)
Ans: Enthalpy is a thermodynamic quantity that is used to measure the change of energy in a given reaction. We know that it is not feasible to measure how much energy is dissipated in a reaction, thus, the change in energy is measured instead. Enthalpy is calculated by finding out the difference between the energy of the reactants and the energy released after the reaction
Check-Out:



Comments