Anjali Mishra Content Writer-SME
Content Writer-SME
Hybridization is an important concept in chemistry that deals with intermixing of atomic orbitals of the same energy level to give rise to new hybridized orbitals. These hybridized orbitals will have equivalent energy levels to their parent orbitals. Both half-filled and completely filled orbitals take part in the process of hybridization.
- Chemist Linus Pauling was the first person to introduce the theory of Hybridization or Hybridisation in the year 1931.
- The process is carried out by atomic orbitals of the same energy level.
- The atomic orbitals explain the structure of simple molecules such as methane.
- Based on the parent orbitals involved, the hybridization can be classified as sp, sp2, sp3, sp3d, and sp3d2.
- It is used to create equivalent orbitals that have the most symmetry.
Related Links
What is Hybridization?
[Click Here for Sample Questions]
When two or more atomic orbitals of different shapes but identical energy levels re-distribute themselves to form hybrid orbitals with slightly different shapes, equivalent energy levels, and orientations, it is called hybridization or hybridisation. There is minimum repulsion between these hybridized orbitals.
Hybridization in chemistry is an extension of the valence bond theory that helps us understand the bond order, bond energies, and bond lengths. The concept of hybridization in chemistry can be best understood with the help of an example.
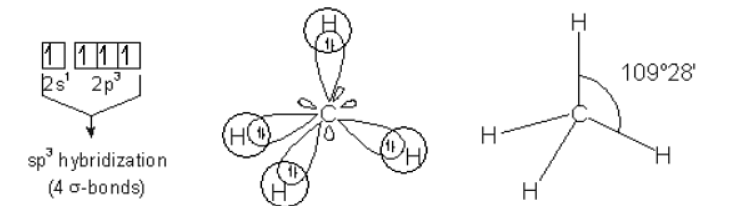
Now, let's take the example of a carbon atom which has four valence electrons. The bond is formed only between the three valence electrons that form sp3. The structure formed by the arrangement of different atoms is called tetrahedral structure of carbon.
Hybridization in Chemistry
This intermixing is primarily based on quantum mechanics. During the process of hybridization, the atomic orbitals of equivalent energy such as two 's' orbitals or two 'p' orbitals or 's' orbitals with a 'p' orbital or 's' orbital with a 'd' orbital, are intermixed to form hybridized orbitals.
- This theory helps explain covalent bonds in organic molecules.
- Due to presence of stronger bonds, hybridised orbitals are more stable than unhybridised orbitals.
- Four different types of hybridization exist in chemistry namely- sp, sp3, sp2, sp3d, sp3d2.
- Hybridization or hybridisation consists of orbitals that are identical with respect to energy and shape.
Also Check: Valence Electrons
Features of Hybridization
[Click Here for Previous Year Questions]
The positive sign is denoted for the bigger lobe of the hybrid orbital and the negative sign for the smaller lobe on the opposite side. Some of the important features of hybridization are listed below:
- Energy: Only atomic orbitals with equal energies undergo hybridization.
- Number of Orbitals: The number of atomic orbitals formed is equal to the number of atomic orbitals involved in mixing.
- Enthalpy: Atomic orbitals that are involved in hybridization have different enthalpies than those orbitals that are not involved in hybridization.
- Participation: Both half-filled or completely filled orbitals can participate in hybridization.
- Bond Formation: Hybridization occurs only during bond formation.
- Shape: Once the hybridization is known, the molecule shape can be easily predicted.
How to Determine the Type of Hybridization?
[Click Here for Previous Year Questions]
The rules an individual must follow to determine the type of hybridization in an atom or an ion are as follows:
- First and foremost, draw a lewis structure and calculate the total number of valence electrons in an atom.
- Now, determine the number of lone pairs that are attached to an atom.
- Depending upon the orbitals of the valence shell, mixing and redistribution of atomic orbitals of an atom take place.
- The number of orbitals is determined by adding the number of lone pairs of electrons and the number of duplexes or octets.
- The atomic orbitals taking part in hybridisation will have the same amount of energy, while the hybrid orbitals will degenerate.
Types of Hybridization
[Click Here for Sample Questions]
Based on the types of atomic orbitals involved in intermixing, hybridization can be classified as follows:
sp Hybridization
sp Hybridization is where when one s orbital and one p orbital intermix to form an sp hybridized orbital. Some key features are:
- The molecules formed are linear and have a bond angle of 1800.
- sp hybridized orbital has an equal distribution of s and p orbital characteristics (50%-50%).
- It is also called diagonal hybridization.
Examples of sp hybridization
|
sp2 Hybridization
sp2 Hybridization is where when one s orbital and two p orbitals intermix to form an sp2 hybridized orbital. Some key features are:
- The molecules formed are triangular planar in shape and thus have a bond angle of 1200.
- sp2 hybridized orbital has 33% of s orbital characteristics and 66% of p orbital characteristics.
- It is also called trigonal hybridization.
Examples of sp2 Hybridization
|
sp3 Hybridization
sp3 Hybridization is where when one s orbital and three p orbitals intermix to form an sp3 hybridized orbital. Some key features are:
- The molecules formed are tetrahedrons in fashion and thus have a bond angle of 109028’.
- sp3 hybridized orbital has 25% of s orbital characteristics and 75% of p orbital characteristics.
- It is also called tetrahedral hybridization.
Examples of sp3 hybridization
|
Download PDF Notes: sp, sp2, sp3 Hybridisation PDF
sp3d Hybridization
sp3d Hybridization is where when one s orbital, three p orbitals and one d orbital intermix to form 5 sp3d hybridized orbitals. Some key features are:
- The molecules formed are trigonal bipyramidal in fashion and thus have a bond angle of 1200 in the horizontal plane and 900 in the vertical plane.
- It is also called trigonal bipyramidal hybridization.
Examples of sp3d hybridizationPhosphorus Pentachloride (PCl5). |
sp3d2 Hybridization
sp3d2 Hybridization is where when one s orbital, three p orbitals and two d orbitals intermix to form 6 sp3d2 hybridized orbitals. Some key features are:
- The molecules formed are octahedrons in fashion and thus have a bond angle of 900.
- It is also called Octahedral hybridization.
Examples of sp3d2 hybridizationSulphur Hexafluoride (SF6) |
Shapes of Hybridization
[Click Here for Sample Questions]
Apart from atom-atom bonding, hybridization in chemistry is also helpful in determining the shape of molecules. Here are some variations of hybridization shapes which are listed below:
Linear
This is the formost shape of hybridization, which results in the formation of sp hybridization. It is formed when two electron groups interact with each other at an orbital angle of 180°.
Trigonal Planar
Trigonal Planar is formed by the interaction of three electron groups, which results in the formation of sp2 hybridization with the orbital angle of 120°.
Tetrahedral
Tetrahedral is formed by interaction of four electron groups which result in the formation of sp3 hybridization with orbital angle of 109.5°.
Trigonal Bipyramidal
Trigonal Bipyramidal is formed by the interaction of five electron groups, which results in the formation of sp3d hybridization with orbital angles of 90° and 120°.
Octahedral
Octahedral is formed by the interaction of six electron groups, which results in the formation of sp3d2 hybridization with the orbital angle of 90° apart.
Examples of Hybridization
Hybridization in different molecules is given below as examples:
MethaneBy the interactions of C-sp3 with an H-1s, 4 equivalent C-H σ bonds can be formed.
Hybridization in Methane Ethane6 C-H sigma σ bonds can be formed by the interaction of C-sp3 with an H-1s orbital and 1 C-C sigma bond can be made by the interaction of C-sp3 with another C-sp3 orbital.
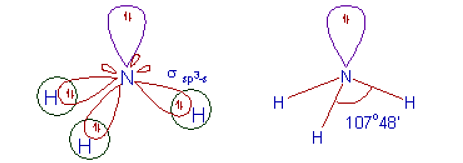
Hybridization in Ethane Formation of Ammonia (NH3) and Water (H2O)In the NH2 molecule, the nitrogen atom is sp3- hybridized and one hybrid orbital possesses two electrons. Now to form an NH3 molecule, three 1s- orbitals of three hydrogen atoms overlap with three sp3 orbitals.
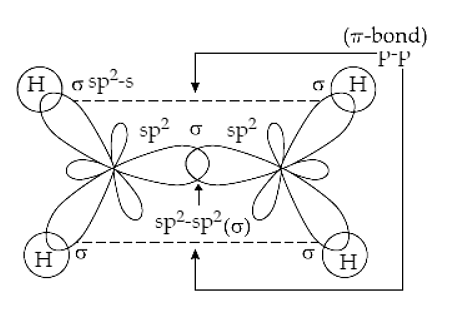
Hybridzation in Ammonia Formation of C2H4 and C2H2 moleculesIn the C2H4 molecule, carbon atoms are sp2- hybridized and one 2p- orbital remains out to hybridization which forms a p-bond. Whereas, sp2- hybrid orbitals form sigma bonds.
Hybridization in C2H4 |
Things to Remember
- Hybridizations is the process of merging two atomic orbitals to produce a new class of hybridized orbitals.
- sp hybridization consists of one s orbital and one p orbital intermix to form two sp hybridized orbitals.
- sp2 hybridization consists of one s orbital and two p orbitals intermix to form three sp2 hybridized orbitals.
- sp3 hybridization consists of one s orbital and three p orbitals intermix to form four sp3 hybridized orbitals.
- sp3d hybridization consists of one s orbital, three p orbitals, and one d orbital intermix to form five sp3d hybridized orbitals.
- sp3d2 hybridization consists of one s orbital, three p orbitals, and two d orbitals intermix to form six sp3d2 hybridized orbitals.
Previous Year Questions on Hybridization
- Hybridization of B in BCl3 is? [AP EAPCET 1998]
- Correct geometry and hybridization of XeF4 is? [NEET 2016]
- What is the hybridization of boron in diborane? [NEET 1999]
- The hybridization of oxygen in oxygen peroxide is? [KEAM]
- sp3d2 hybridization is not displayed by? [JEE Main 2017]
- Number of sp2 hybrid orbitals in benzene. [JEE Main 2020]
- Calulate the number of bonds made by Sulphur atoms. [JEE Main 2020]
- What is the structure of water? [JEE Main 2019]
- What is the hybridization of carbon in fullerene. [KEAM]
- Molecular Geometry of BF3 is? [KEAM]
- Find the incorrect statement n hybridization. [KEAM]
- State the hybridisation on 5 carbon atoms of the given molecule. [KEAM]
Sample Questions
Ques: What is meant by hybridization of atomic orbitals? (2 marks)
Ans: Hybridization is intermixing of atomic orbitals of various shapes and almost identical electricity to provide the same variety of hybrid orbitals of the identical shape, same electricity, and orientation such that there's minimum repulsion between these hybridized orbitals. This intermixing is primarily based on quantum mechanics.
Ques: Is there any change in the hybridization of B and N atoms as a result of the following reaction? BF3 + NH3 → F3 B.NH3 (2 marks)
Ans: In BF3, B atom gets sp2 hybridized and in NH3, N is sp3 hybridized.
After the reaction, the hybridization of B changes from sp2 to sp3.
Ques: Describe the change in hybridization (if any) of the Al atom in the following reaction: AlCl3 + Cl- → AlCl4-. (2 marks)
Ans: The electronic configuration of 13Al = 1s2 2s2 2p6 3s1 3px1 3py1
It is excited state which is why the hybridization will be sp2
In AlCl4-, the empty 3pz orbital is also involved. Thus, the hybridization is sp3 and the shape is tetrahedral.
Ques: Describe the shape of sp, sp2, sp3 hybrid orbitals? (3 marks)
Ans: Shapes of orbitals:
- sp Hybridization- When one s- and one p-orbital intermix, it is called hybridization. For instance, in BeF2, Be undergoes sp-hybridization which has a linear shape, and the bond angle is 180°.
- sp2 Hybridization- Here one s- and two p- orbitals get hybridized in order to form three equivalent hybrid orbitals. The three hybrid orbitals are directed towards the three corners of an equilateral triangle. Thus it is known as trigonal hybridization.
- sp3 Hybridization- Here one s- and three p- orbitals get hybridized to form four equivalent hybrid orbitals. These orbitals are directed towards the four corners of a regular tetrahedron.
Ques: Draw a diagram to illustrate the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules. (2 marks)
Ans:

Ques: Which hybrid orbitals are used by carbon atoms in the following molecules:
a) CH3-CH3
b) CH3-CH - CH2
c) CH3-CH2-OH
d) CH3-CHO
e) CH3-COOH (5 marks)
Ans:

Ques: Describe the hybridization in the case of PCl5. explain why are the axial bonds longer than the equatorial bonds? (5 marks)
Ans: The ground state E.C and the excited state E.C of phosphorous are represented as:

The one s-, three p- and one d-orbitals hybridize to form five sets of sp3 d hybrid orbitals which are directed towards the five corners of a trigonal bipyramidal as shown below:

As axial bonds suffer more repulsive interaction as compared to the equatorial bond pairs, thus axial bonds have been found to be a bit longer and hence slightly weaker than the equatorial bond.
Ques: Explain the hybridization of Iodine heptafluoride (IF7)? (3 marks)
Ans: The electronic configuration of the iodine atom in a ground state is: [Kr]4d105s25p5. As the formation of IF7 needs 7 unpaired electrons, the iodine atom promotes three of its electrons (one from the 5s orbital and two from the 5p orbitals) into empty 5d orbitals. This state is referred to as the third excited state.
- In the third excited state, the iodine atom undergoes sp3d3 hybridization in order to give 7 half-filled sp3d3 hybrid orbitals in pentagonal bipyramidal symmetry.
- These will form 7 σsp3d3-p bonds with fluorine atoms.
- Thus the shape of IF7 is pentagonal bipyramidal.
- The bond angle of ∠F-I-F in the pentagonal plane is equal to 72o, whereas two fluorine atoms are present perpendicularly to the pentagonal plane above and below by making 90o bond angle with the I-F bonds on the pentagonal plane.
Ques: What is the main difference between sp, sp2, and sp3? (3 marks)
Ans: The main difference between sp, sp2, and sp3 hybridization is that –
- Sp hybridization forms hybrid orbitals with 50% characteristics of s orbital.
- sp2 hybridization forms hybrid orbitals with 33% characteristics of s orbital.
- sp3 hybridization forms hybrid orbitals with 25% characteristics of s orbital.
Ques: Write the characteristics of the orbital formed by sp hybridization. (3 marks)
Ans: 2 sp hybridized orbitals are formed when one s-orbital and p-orbital undergo hybridization.
- One sp-orbital has 50% characteristics of s-orbital and 50% of p-orbital.
- They have a linear geometry.
- It is also known as diagonal hybridization.
Ques: What type of hybridization does a BCl3 molecule undergo? (2 marks)
Ans: In the BCl3 molecule, an s-orbital and two p-orbitals combine together to form three equivalent hybrid orbits. Therefore. it undergoes sp2 hybridization to form trigonal planar shape with B in the center and Cl in the 3 corners.
Ques: What is the bond angle of H-C-H in methane molecules? (2 marks)
Ans: The molecule of methane i.e. CH4 undergoes sp3 hybridization (1 s-orbital and 3 p-orbitals combine to form 4 sp3 orbitals). It shows tetrahedral geometry. In tetrahedral geometry, the angle between the bonds of the corner atoms and the central atom is 109.5°.
Ques: What is the number of sigma bonds in an ethene molecule? (2 marks)
Ans: The formula of the ethene molecule is C2H4. There is a sigma bond between two carbon atoms and 2 sigma bonds between each carbon and the hydrogen atoms. So in total, it’s five bonds (one pi-bond is present between the 2 carbon atoms).
Ques: Which hybridization renders octahedral geometry? (2 marks)
Ans: Octahedral geometry occurs when the atomic orbitals undergo sp3d2 or d2sp3 hydration only. So it involves one s-orbital, three p-orbitals, and two d-orbitals. For example – SF6.
Ques: Do orbitals that form after hybridization have equal energy? (2 marks)
Ans: Hybridization refers to the intermixing of different orbitals to form a new set of equivalent orbitals known as hybrid orbitals. All the hybrid orbitals that have undergone the same hybridization have the same amount of energy.
Ques: What shapes are associated with sp3d and sp3d2 hybrid orbitals, respectively? (1 mark)
Ans: sp3d hybrid orbitals have Pentagonal bipyramidal shape while sp3d2 hybridized orbitals have octahedral shape.
Ques: The molecule of CO2 has a 180° bond angle. Why? (2 marks)
Ans: In the formation of CO2 molecule of orbital, hybridization of orbitals of carbon occur to a limited extent involving just one x and ane p orbitals. There is sp hybridization of valence shell orbitals of the carbon atom that forms two sp hybrid orbitals.
Ques: Explain hybridization in acetylene? (2 marks)
Ans: Acetylene has sp hybridization it has a 50% S character that has greater electronegativity. The bond length of the Sp hybridized bond is less than Sp2, Sp3. Acetylene exists as a gas molecule.
Ques: What is the hybridization of Ni in Ni(CO)4? (2 marks)
Ans: The oxidation state of Ni is 0. The electronic configuration of the valence shell in the ground state of Ni is 3d84s2, but all the 10 electrons are pushed into 3d orbital due to the strong field CO ligands approaching the Ni atom, thereby forming sp3 hybridization.




Comments