Arpita Srivastava Content Writer
Content Writer
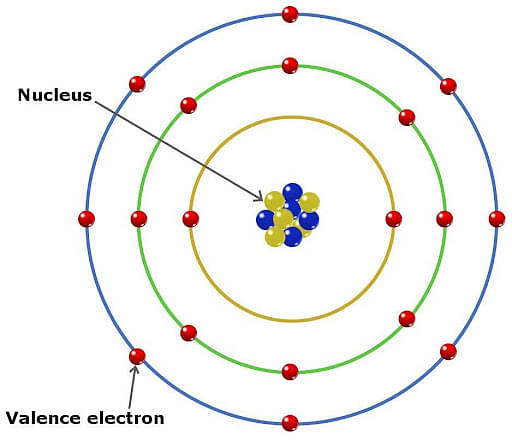
Valence Electrons are the electrons found in an electron's outermost shell. The atom loosely holds these electrons as they lie farthest from the nucleus. These electrons are responsible for determining the chemical change in atoms and molecules.
- Valence electrons help in determining the reactivity of the atom.
- It is a value that can tell us about the combining capacity of any element.
- Since it is a value, it would always be a whole number and cannot have a minus or plus sign in it.
- The outermost shell of the electron is filled with s and p orbitals.
- Atoms are made up of various shells that can hold some electrons.
- Electrons in the inner shell are well-protected and are not involved in the process of combining elements.
- In the case of transition metal, valence electrons are found in the innermost shell.
Read More: Variable Valency
Key Terms: Valence Electron, Atoms, Molecules, Electron, Valency, Compound, Orbitals, Covalent Bond, Outer Shell, Inner Shell, Inert Gas, Noble Gas
What are Valence Electrons?
[Click Here for Sample Questions]
Valence electrons are defined as an electron found in the outermost shell and not filled completely so that they do not attain inert gas configuration. An element can lose or gain some electrons to attain stability, known as valency.
- Valence electrons help in determining the chemical properties of an element.
- It helps calculate how many atoms can take part in the bond formation.
- The process determines the number of unpaired electrons.
- It is the number of electrons that are prepared at the time of formation of a molecule.
- The chemical formula of a compound is known as valency.
- A valence electron has the power to release and absorb energy like a photon.
- In valency, atoms of an element get mixed with atoms of another element to form another element.
- Electrons of the outer shell are more powerful than the elements of the inner shell.
- The formation of bonds is done by valence electrons.
Read More: Reverberatory Furnace
Characteristics of Valence Electrons
[Click Here for Sample Questions]
The characteristics of Valence Electrons are as follows:
- Valence electrons can be shared and transferred within atoms.
- It can satisfy the octet rule to attain noble gas configuration.
- The number of valence electrons in an atom can be determined with the help of the periodic table.
- An atom will be chemically alert if it consists of a closed shell having valence electrons in it.
- The electrical conductivity of any element can be specified with the help of these electrons.
- It helps determine whether an atom is metal, non-metal, or a metalloid.
- The number of electrons helps determine the bonding behaviour of an atom.
- Elements that have the same number of valence electrons are grouped together in a periodic table.
Read More:
Determination of Valence Electrons
[Click Here for Sample Questions]
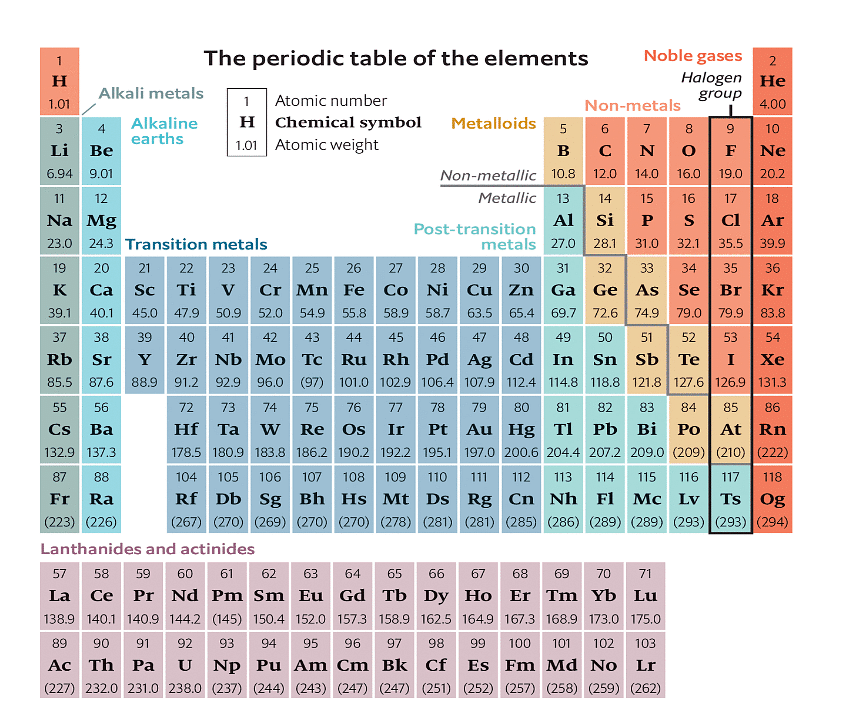
Valence electrons can easily be determined with the help of the periodic table. It can be found in all elements, even if they are from Group 3 to Group 12. The electrons of these groups can be determined easily, but they are difficult to predict. The structure of these elements could be much better.
- Valence electrons can be determined by checking the vertical column of the periodic table.
- With the help of group numbers, we can easily calculate the number of valence electrons of an element.
- Another way to calculate the valence electron is by determining the electronic configuration of an element.
For Electronic configuration, we have to check the outermost shell of an electron in the following ways:
- If there are 1, 2, or 3 electrons in the outermost shell, it will lose these electrons to complete its octet.
- If there are 5, 6, or 7 electrons in the outermost shell, it will gain some electrons to complete its octet.
- If there are 4 electrons in the outermost shell, it can lose or gain 4 electrons to complete its octet.
- If there are 8 electrons in the outermost shell, it means that the octet is completed already, and there is no need to lose or gain electrons.
The periodic table of all the elements is mentioned below:
Read More:
Valence Chart of Elements
[Click Here for Sample Questions]
The valency chart of the first 30 elements of a periodic table is as follows:
| Atomic Number | Element | Valency |
|---|---|---|
| 1 | Hydrogen | 1 |
| 2 | Helium | 0 |
| 3 | lithium | 1 |
| 4 | Beryllium | 2 |
| 5 | Boron | 3 |
| 6 | Carbon | 4 |
| 7 | Nitrogen | 3 |
| 8 | Oxygen | 2 |
| 9 | Fluorine | 1 |
| 10 | Neon | 0 |
| 11 | Sodium | 1 |
| 12 | Magnesium | 2 |
| 13 | Aluminum | 3 |
| 14 | Silicon | 4 |
| 15 | Phosphorus | 3 |
| 16 | Sulphur | 2 |
| 17 | Chlorine | 1 |
| 18 | Argon | 0 |
| 19 | Potassium | 1 |
| 20 | Calcium | 2 |
| 21 | Scandium | 3 |
| 22 | Titanium | 2,3,4 |
| 23 | Vanadium | 2,3,5,4 |
| 24 | Chromium | 2,3,4,5,6 |
| 25 | Manganese | 2,3,4,5,6,7 |
| 26 | Iron | 2,3,4,6 |
| 27 | Cobalt | 2,3,4 |
| 28 | Nickel | 3,2 |
| 29 | Copper | 2,1 |
| 30 | Zinc | 2 |
Read More: Tetravalency
Valence Chart of Electron of Elements
[Click Here for Sample Questions]
Atoms are divided into different groups of elements. This can help to find the number of valence electrons present in any element. The valence chart of electrons of elements is given below:
| Periodic Table Group | Valence Electrons |
|---|---|
| Group 1 (Alkali metals) | 1 |
| Group 2 (Alkaline earth metals) | 2 |
| Group 13 (Boron group) | 3 |
| Group 14 (Carbon group) | 4 |
| Group 15 (Nitrogen group) | 5 |
| Group 16 (Oxygen group) | 6 |
| Group 17 (Halogens) | 7 |
| Group 18 (Noble gases) | 8 |
Read More: Polytetrafluoroethene (Teflon)
Formulas of Valence Electrons
[Click Here for Sample Questions]
Here are the formulas of Valence Electrons for some elements:
Sodium
- The atomic number of sodium is 11.
- Its electronic configuration is 2, 8, 1.
- Sodium will lose 1 electron to complete its octet.
- Hence, its valency is 1.
Helium
- The atomic number of helium is 2.
- Its electronic configuration is 2.
- The outermost shell is already filled, so it will not lose electrons.
- Hence, its valency is 0.
Copper
- The atomic number of copper is 29.
- It is a transition element, and most of these elements have variable valences.
- It shows two valences: 1 (or Cu(I)), known as cuprous, and 2 (or Cu(II)), known as Cupric.
Phosphorus
- The atomic number of phosphorus is 15.
- Its electronic configuration is 2, 8, 5.
- It will gain 3 more electrons to complete its octet.
- Hence, the valency of phosphorus is 3.
Vanadium
- The atomic number of vanadium is 23.
- It is a transition element and most of these show variable valences.
- Its electronic configuration is Ar 3d3 4s2
- Hence, the valency of vanadium are 2,3,4 and 5.
Read More: Diatomic
Formulas of Compounds by Valence Electrons
[Click Here for Sample Questions]
Some formulas through which we can make compounds by knowing the valency of their elements are as follows:
Hydrogen Chloride
- Its symbols are H and Cl.
- The valency of the compound is 1 and 1.

Hence, the formula of the compound is HCl
Hydrogen Sulphide
- Its symbols are H and S.
- The valency of the compound is 1 and 2.

Hence, the formula of the compound is H2S
Diazonium Sulfate
- Its symbols are NH4 and SO4
- The valency of the compound is 1 and 2.

Hence, the formula of the compound is (NH4)2SO4
Magnesium Chloride
- Its symbols are Mg and Cl.
- The valency of the compound is 2 and 1.

Hence, the formula of the compound is MgCl2
Read More: Hydrides
Things to Remember
- Valence Electrons are associated with an atom and found in the outermost shell of an electron.
- It helps determine the type of bond an atom shares with another atom.
- These electrons have the ability to absorb and release energy.
- Valence electrons lie farthest in the nucleus.
- The number of electrons can be determined by the use of the periodic table and electronic configuration.
- These types of electrons take part in the formation of chemical bonds.
Sample Questions
Ques. How can we find the valency of an element with atomic number 51? (2 marks)
Ans. The element having atomic number 51 is Antimony(Sb). Its valency can be found out as follows:
- Atomic Number is 51.
- Electronic Configuration is 2, 8, 18, 18, 5.
- Outermost shell has 5 electrons.
- Hence, Valency is 5.
Ques. Aluminum has a proton number of 13. What will be its electron configuration? (2 marks)
Ans. The Proton number of Aluminum is 13 which means its atomic number is 13 and it has 13 elements. A neutral aluminum atom will have the following electronic configuration in its ground state: 2,8,3
Ques. Why do most elements not have full electron shells? (2 marks)
Ans. Most elements do not have full electron shells so that they can bond with other elements. They have to complete its octet by bonding with another element. For this, we have to know its valency.
Ques. What is the atomic number and mass number of Magnesium if it has 12 protons and 12 neutrons? (2 marks)
Ans. Atomic Number = Number of protons = 12
Hence, Mass Number = Number of protons + Number of neutrons = 12+12 = 24.
Ques. What is the chemical formula of the compound Carbon Tetrachloride? (3 marks)
Ans. The formula of Carbon Tetrachloride can be produced by using the valency of these elements.
- Its symbols are C and Cl.
- The valency of the compound is 4 and 1.

Hence, the formula of Compound Carbon Tetrachloride is CCl4.
Ques. Determine the valency of sulphur? (3 marks)
Ans. In the case of valence electrons less than or equal to 4, then, in that case, the valency of a required element is equal to the number of electrons present in the outermost shell of an electron.
- Similarly, in the case of valence electrons more than 4 then in that case the valency is determined by subtracting the valence electrons from 8.
- The electronic configuration of sulphur is calculated to be 2,8,6.
- This shows it has six valence electrons.
- So, the valency of sulphur would be equal to 8 – the number of valence electrons.
- Valency of sulphur= 8 – Number of valence electrons
- Valency of sulphur = 8 – 6
- Valency of sulphur = 2.
Hence, the valency of sulphur is two.
Ques. Determine the valency of oxygen? (3 marks)
Ans. In the case of valence electrons less than or equal to 4, then, in that case, the valency of a required element is equal to the number of electrons present in the outermost shell of an electron.
- Similarly, in the case of valence electrons more than 4 then in that case the valency is determined by subtracting the valence electrons from 8.
- The electronic configuration of oxygen is calculated to be 2,6.
- This shows it has six valence electrons.
- So, the valency of oxygen would be equal to 8 – the number of valence electrons.
- Valency of = 8 – Number of valence electrons
- Valency of oxygen = 8 – 6
- Valency of oxygen = 2.
Ques. What is the difference between valency and covalency? (3 marks)
Ans. The difference between valency and covalency are as follows:
| Valency | Covalency |
|---|---|
| Valency is defined as number of electron an atom gain or lose to become completely stabilised. | Covalency is defined as the number of bond an atom shares with empty orbitals. |
| It forms both ionic and covalent bond. | It only form covalent bond. |
| It does not depend upon the number of valence electrons. | It depends upon the number of valence electrons. |
Ques. What is the difference between the ionic bond and covalent bond? (3 marks)
Ans. The difference between ionic bond and covalent bond are as follows:
| Ionic Bond | Covalent Bond |
|---|---|
| Ionic Bond are formed when one atom donates electron to other atom. | Covalent Bond are formed when atoms are formed by sharing of electrons. |
| It forms non-directional bond. | It forms directional bond. |
| Ionic bond has higher melting and boiling point. | Covalent bond has lower melting and boiling point. |
Ques. What is the electronic configuration of phosphorus and manganese? (2 marks)
Ans.
Electronic configuration is defined as the distribution of the required number of electrons in an atom or molecule present in a compound. In this, the electron moves independently of the orbitals. It helps in understanding the type of chemical bond an atom shares with another atom.- Electronic Configuration of Phosphorus: 1s2 2s2 2p6 3s2 3p3
- Electronic Configuration of Manganese: 1s2 2s2 2p6 3s2 3p6 4s2 3d5
Ques. Explain (A) Periodic Table (B) Electrical Conductivity (C) Valency (3 marks)
Ans. (A) Periodic Table: A periodic Table is defined as the arrangement of elements in order of increasing number of atomic masses. It represents the total number of protons found in a nucleus.
(B) Electrical Conductivity: Electrical Conductivity, also known as specific conductance, is defined as the reciprocal of resistivity. It determines the required capacity of the material to conduct electric current.
(C) Valency: Valency is defined as the number of hydrogen atoms that combine directly and indirectly with another atom of an element to form the required molecule.
Ques. How many valence electrons are found in CH3CH2Cl? (3 marks)
Ans. Atomic number of carbon is calculated to be 6
- The electronic configuration of Carbon is 2s2 2p2
- Number of valence electrons in required compound is 4
- The number of valence electrons in Hydrogen (H) and Chlorine (Cl) are 1 and 7 respectively.
- In CH3CH2Cl, we have 2 atoms of carbon, 5 atoms of hydrogen and one atom of chlorine.
- Hence, number of valence electrons in CH3CH2Cl = 4 × 2 +1×5 + 7= 20
Ques. What is the reason for the valency of oxygen being 2? (3 marks)
Ans.
Valency is defined as the number of atoms of a particular element and is used to measure the combining capacity of an atom or molecule with another atom. It depends upon the type of chemical bond an atom shares with another atom. The number of electrons in oxygen is calculated to be eight. It has an electronic configuration of 2 and 6, thus requiring only two electrons to achieve eight valence electrons in an outermost shell. Hence, the valency of oxygen is calculated to be two.Ques. Determine the number of valence electrons of nitrogen? (3 marks)
Ans.Valence Electrons are those electron that are located in the outermost shell of an electron. In the case of valence electrons less than or equal to 4, then, in that case, the valency of a required element is equal to the number of electrons present in the outermost shell of an electron.
- Similarly, in the case of valence electrons more than 4 then in that case the valency is determined by subtracting the valence electrons from 8.
- The electronic configuration of sulphur is calculated to be 2,5.
- This shows it has five valence electrons.
Ques. Determine the outermost orbit of an atom along with the number of valence electrons if the number of electrons in an atom is found to be 20? (3 marks)
Ans. Since the number of electron in an atom is found to be 20 then in such case the electronic configuration is estimated to be 2, 8, 8, 2.
- The number of electron in each shell is found to be:
- K Shell will have following number of electron: 2 × 12 = 2
- L Shell will have following number of electron: 2 × 22 = 8
- M Shell will have following number of electron: 2 × 32 =18
- N Shell will have following number of electron: 2 × 42= 32
- So the outermost orbit of an atom along with the number of valence electrons is determined to be N and 2 respectively.
Also Check:



Comments