Jasmine Grover Content Strategy Manager
Content Strategy Manager
Amines, the derivatives of ammonia, are classified as primary (1°), secondary (2°) and tertiary (3°) based on the number of hydrogen atoms replaced by alkyl or aryl groups in ammonia molecule. If one alkyl group has replaced one hydrogen atom of NH3, it is a primary amine. In the same way, if two hydrogens are replaced, it is a secondary amine and if three hydrogens are replaced, it is tertiary amine.
| Table of Content |
Key Terms: Nomenclature, amine, electrons, Alkyl Group, hydrogen, tertiary amine, primary amine, atoms, secondary amine, aryl groups, molecule
Define Amines
[Click Here for Sample Questions]
Amines are derivatives of Ammonia (NH3). Amines are obtained by the replacement of one or more Hydrogen atoms by the Alkyl or Aryl group. Amines are an important part of organic compounds and have many uses and are naturally present in Vitamins, Proteins, Hormones, and Alkaloids. Amines are also used in the making of various medical drugs. Some examples of Amines are Methyl Amine (CH3NH2), Aniline (C6H5NH2), Ethyl Amine (C2H5NH2), etc.
Also Read:
| Related Articles | ||
|---|---|---|
| Gabriel Phthalamide Synthesis Reaction | Sandmeyer Reaction | Enantiomers |
| Hofmann bromamide reaction | Uses of Amine | Nucleophilic Substitution |
Structure of Amines
[Click Here for Sample Questions]
Ammonia is slightly distorted tetrahedral in shape. There is a lone pair of electrons that occupies one of the tetrahedral positions. These Nitrogen atoms of amines are sp3 hybridized, with bulky lone pair compressing the H-N-H bond which is less than 109.5o.
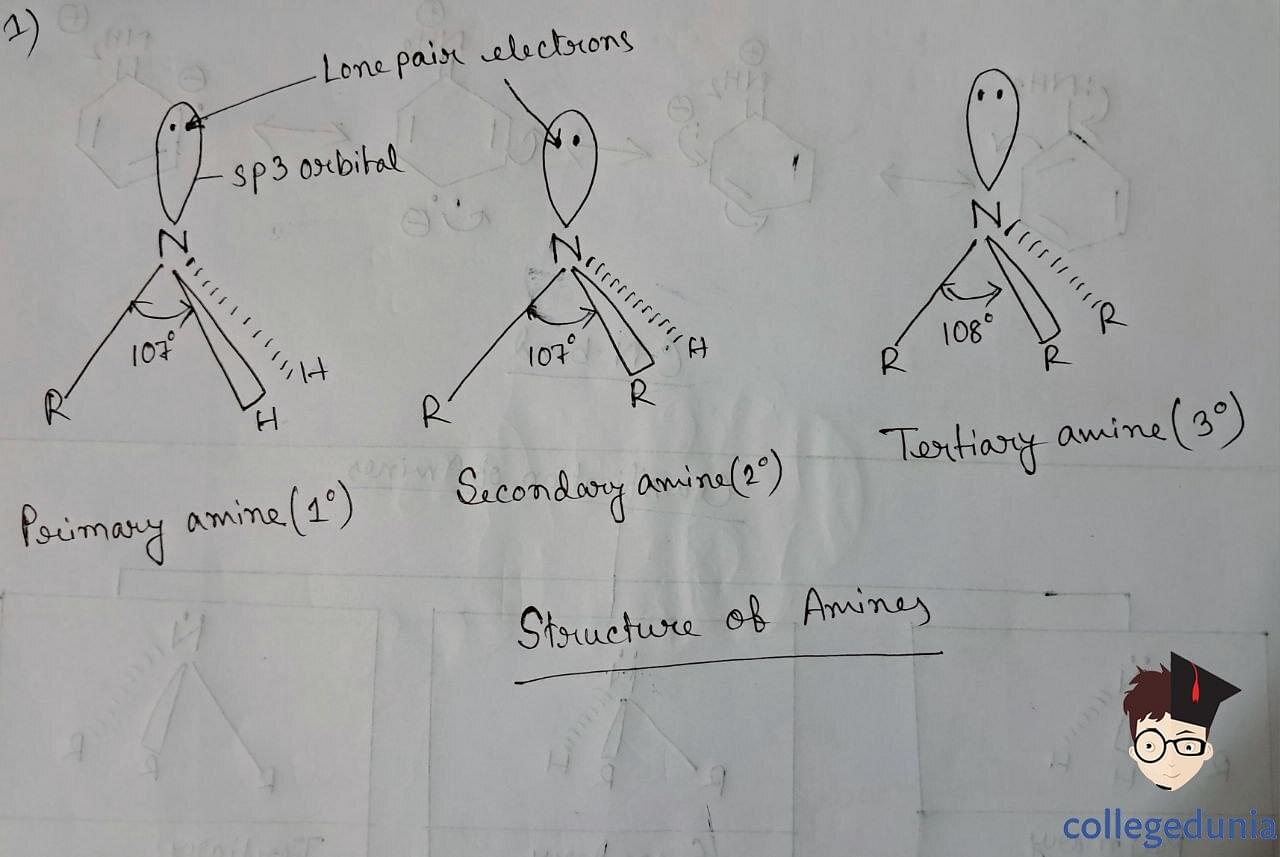
Structure of Amines
The C-N bond is slightly stronger in aromatic amines, due to the partial double-bonded character that arises due to the delocalization of lone pair of N with the benzene ring.

Classification of Amines
[Click Here for Sample Questions]
On the basis of the number of Hydrogen atoms replaced in NH3 molecules, Amines are classified as Primary (1o Amine), Secondary (2o Amine), and Tertiary (3o Amine).
- Primary (1o Amine): In 1o Amine a single hydrogen atom of NH3 is replaced by Aryl or Alkyl group.
For Example CH3NH2 (Methyl Amine).
- Secondary (2o Amine): Two hydrogen atoms of NH3 are replaced by Aryl or Alkyl Group in Secondary Amines.
For Example CH3NHCH3 (Dimethyl Amine)
- Tertiary (3o Amine): In 3o Amine three hydrogen atoms of NH3 are replaced by Aryl or Alkyl group. For example:

Classification of Amines
Primary and secondary amines are involved in an intermolecular association. This is due to the hydrogen bonding that exists between the nitrogen of one molecule and hydrogen of another.
Compared to secondary amines, this intermolecular association is more in primary amines. This is because, for the hydrogen bond formation in it, there are two hydrogen atoms available
Due to the absence of hydrogen atoms available for hydrogen bond formation, the tertiary amines do not have an intermolecular association.
The order of boiling points of Isomeric amines is as following:
Primary > Secondary > Tertiary
Nomenclature of Amines
[Click Here for Sample Questions]

- Aliphatic amines are named by adding a prefix ‘mine’ to the alkyl group.
For example:

Aliphatic amines
- Secondary or Tertiary amines are named by adding the prefix ‘di’ or ‘tri’ before the name of an alkyl group.
For example:

Secondary or Tertiary amines
- Aromatic Amines are named after parent member- aniline.
For example:

Aromatic Amines
Read More:-
Sample Questions
Ques. State the importance of Amines? (1 mark)
Ans. Amines are used in our day-to-day life. It is available in Vitamins, Proteins, Hormones, and Alkaloids. Amines are also used in the making of various medical drugs.
Ques. What is the basicity of amine? (1 mark)
Ans. The basicity of amine is 23.5. This is because of the lone pair of electrons of nitrogen atoms of amines.
Ques. What is the difference between Amide and Amine? (1 mark)
Ans. The basic difference between amide and amine is the presence of the carbonyl group. In Amines no carbonyl groups are attached to nitrogen whereas it is opposite in Amide i.e., a carbonyl group is attached to a nitrogen atom.
Ques. What is the order of boiling points of isomeric amines? (1 mark)
Ans. The order of boiling points of Isomeric amines is as following:
Primary > Secondary > Tertiary
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:



Comments