
Content Strategy Manager
We all have experienced a foul smell in the toilets. The compounds that are responsible for that foul smell are the ammonia and the compounds of ammonia known as Amines. Amines have a pungent smell and they play an important role in Organic Chemistry. This article deals with the Physical Properties of Amines.
| Table of Content |
Key Terms: Organic Chemistry, Amine, Electrons, Hydrogen, Aniline, Ammonia, Amino acids, Trimethylamine, Organic compounds, Haloalkanes and Haloarenes
What are Amines?
[Click Here for Sample Questions]
Amines are natural organic compounds having functional groups which contain a nitrogen particle with a lone pair of electrons. They are the derivatives of alkali where one or more hydrogen molecules can be replaced by substituent groups like alkyl or aryl. Amino acids, trimethylamine, biogenic amine and aniline are examples of some important amines.
Also Read:
| Related Articles | ||
|---|---|---|
| Nucleophilic Substitution | Sandmeyer Reaction | Enantiomers |
| Wurtz Reaction | Uses of Amine | Mitsunobu Reaction |
Classification of Amines
[Click Here for Previous Year Questions]
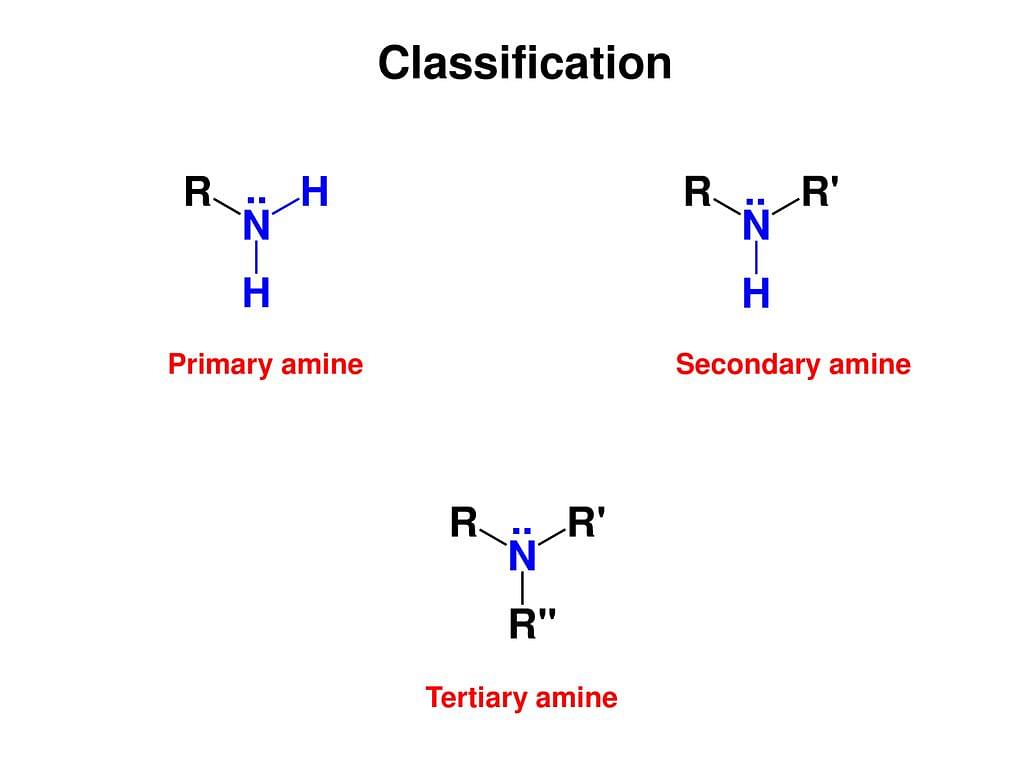
Amines are classified into four categories which are given below :
- Primary - These are formed when one of the three hydrogen particles is replaced by an alkyl or aryl group.
- Secondary - These are formed when two of the three hydrogen particles are replaced by an alkyl or aryl group.
- Tertiary - These are formed when all the three hydrogen particles are replaced by an alkyl or aryl group.
- Cyclic - Only secondary or tertiary amines can be cyclic. The three member ring aziridine is an important example of cyclic amine.
Cyclic amine
Physical Properties of Amines
[Click Here for Sample Questions]
- The lower aliphatic amines are gaseous in nature. They have a fishy smell
Example: Methylamine (CH3NH2) , Ethanamine (C2H5NH2)
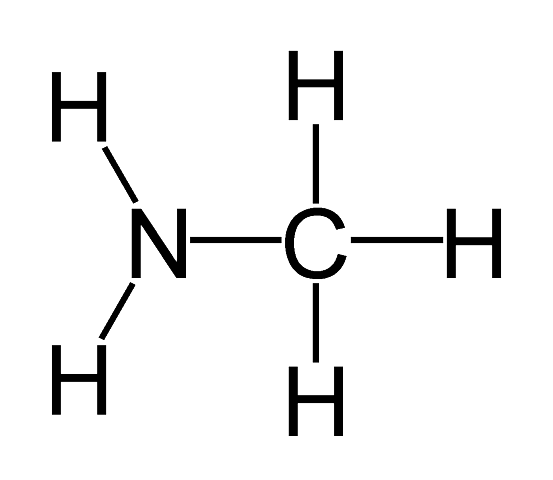
Methylamine

Ethanamine
- Primary amines with three or four carbon atoms are fluids at room temperature though higher ones are solids
Example: Propanamine (C3H7NH2) , Butanamine (C4H9NH2)


Butanamine
- Aniline and other arylamines are generally colourless. However, they get coloured when we store them in the open because of atmospheric oxidation.
Example: Aniline (C6H5NH2)

- Lower aliphatic amines can form hydrogen bonds with water molecules. These amines are soluble in water.
Example: Methylamine (CH3NH2) , Ethanamine (C2H5NH2)

- Increase in the size of the hydrophobic alkyl part increases the molar mass of amines. This normally decreases its solubility in water.
Example: Hexanamine (C6H13NH2) , Heptanamine (C7H15NH2)

- Higher amines are insoluble in water. Organic solvents like alcohol, benzene and ether easily dissolve amines in it. Aromatic amines are insoluble in water because the large hydrocarbon part (hydrophobic part) of aromatic amines retards the formation of H-bonding.
Example: Hexanamine (C6H13NH2) gets dissolved in Benzene (C6H12)
- Alcohols have higher polarity in comparison with amines due to which they form more grounded intermolecular hydrogen bonds.
Example: Propanamine (C3H7NH2) forms bond with Propanol (C3H7OH)
- Primary and Secondary amines frequently form intermolecular association because of hydrogen bonding between the nitrogen of one amine and hydrogen of the other amine.

Example: Ethanamine (C2H5NH2), Prop-1,2- amine
- This type of intermolecular bonding is more noticeable in primary amines as compared to secondary amines because of the availability of two hydrogen particles in primary amines.
- In tertiary amines, there is no intermolecular association because of the absence of free hydrogen atoms for bonding.
Example: Prop-2,2-amine
- The order of boiling point of amines is represented as:
Primary > Secondary > Tertiary.
Example: Methanamine (CH3NH2) > Ethanamine (C2H5NH2) > Propanamine (C3H7NH2)
Read More:-
Previous Year Questions
Ques: Why is an alkylamine more basic than ammonia? (Delhi 2009, 1 Mark)
Ans: Alkylamine is more basic than ammonia because of the electron releasing inductive effect (+1) of the alkyl group. Due to this the electron density on the nitrogen atom increases and it donates the lone pair of electrons more easily than ammonia.
Ques: Arrange the following compounds in an increasing order of basic strengths in their aqueous solutions : NH3, CH3NH2, (CH3)2NH, (CH3)3N (All India 2009, 1 Mark)
Ans: The basicity order of the aqueous solutions is due to stability of ammonium cation. The order of basicity -
(CH3)2 NH > CH3NH2 > (CH3)3 N > NH3
Ques: Give a chemical test to distinguish between ethylamine and aniline. (All India 2011, 2 Marks)
Ans: To distinguish between ethylamine and aniline Azo dye test is done.
Azo dye test: Azo dye test involves the reaction of any aromatic primary amine with HNO2(NaNO2 + dil. HCl) at 273-278 K. After that the product is treated with an alkaline solution of 2-naphthol and a yellow, orange or red coloured dye is obtained.

Ques: How are the following conversions carried out?
(i) CH3CH2Cl to CH3CH2CH2NH2
(ii) Benzene to aniline (Comptt. Delhi 2012, 3 Marks)
Ans: (i) With NaCN and reduction
(ii)


Ques: Give reasons :
(a) Aniline is a weaker base than cyclohexyl-amine.
(b) It is difficult to prepare pure amines by ammonolysis of alkyl halides. (Comptt. All India 2013, 3 Marks)
Ans: a) In aniline, the lone pair of electrons on the Nitrogen atom is delocalised over the benzene ring. Due to which the electron density of the nitrogen decreases.
Whereas in cyclohexylamine, the lone pair of electrons on the Nitrogen atom is easily available due to absence of the π-electrons.
This is the reason why aniline is a weaker base than cyclohexylamine.
(b) Because the primary amine formed by ammonolysis itself acts as a nucleophile and produces further 2° and 3° alkyl amine.
Ques: Give reasons :
(i) Electrophilic substitution in aromatic amines takes place more readily than benzene.
(ii) CH3CONH2 is a weaker base than CH3CH2NH2. (Comptt. All India 2013, 2 Marks)
Ans: (i) NH2 group has a strong activating effect, due to which aromatic amines undergo electrophilic substitution reactions more readily than benzene.
(ii) In case of acetamide due to resonance, the lone pair of electrons on the nitrogen atom is delocalized over keto group which decreases the density of the electron hence it is less basic while in ethylamine due to +1 effect of ethyl group the electron density increases on nitrogen atom and hence its basic character increases.
Ques: In the following cases rearrange the compounds as directed : (Delhi 2010, 3 Marks)
(i) In an increasing order of basic strength :
C6H5NH2, C6H5 N(CH3)2, (C2H5)2NH and CH3NH2
(ii) In a decreasing order of basic strength :
Aniline, p-nitroaniline and p-toluidine
(iii) In an increasing order of pKb values :
C2H5NH2, C6H5 NHCH3, (C2H5)2NH and C6H5NH2
Ans: (i) Increasing order of basic strength :
C6H5NH2 < C6H5 N(CH3)2 < CH3NH2 < (C2H5)2NH
(ii) Decreasing order of basic strength :
p-toluidine > Aniline > p-nitroaniline
(iii) Increasing order of pKb value
(C2H5)2NH < C2H5NH2 < C6H5NHCH3 < C6H5NH2
Since a stronger base has a lower pKb value therefore basic strength order will be-
(C2H5)2NH > C2H5NH2 > C6H5NHCH3 > C6H5NH2
Ques: (a) Explain why an alkylamine is more basic than ammonia?
(b) How would you convert
(i) Aniline to nitrobenzene (ii) Aniline to iodobenzene (Delhi 2011, 3 Marks)
Ans: (a) Due to electron releasing inductive effect (+1) of alkyl group, the electron density on the nitrogen atom increases. Since the electron density is more so it can easily donate the lone pair of electrons than the ammonia.
(b) (i) Aniline to nitrobenzene

(ii) Aniline to iodobenzene

Ques: State reasons for the following :
(i) pKb value for aniline is more than that for methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not soluble in water.
(iii) Primary amines have higher boiling points than tertiary amines. (All India 2011, 3 Marks)
Ans: (i) When the pKb value is higher, the basicity will be lower therefore aniline is less basic than methylamine because the lone pair of electrons on nitrogen atom gets delocalized over the benzene ring and are unavailable for protonation due to resonance in aniline which is absent in case of alkylamine.
(ii) Ethylamine can easily form Hydrogen bonds with water while aniline has a larger hydrocarbon part which tends to retard the formation of Hydrogen bonds. This is the reason why Ethylamine is more soluble in water than Aniline.
(iii) Due to the presence of two hydrogen atoms on the Nitrogen atom of primary amines, they undergo extensive intermolecular hydrogen bonding while tertiary amines due to the absence of a Hydrogen atom on the Nitrogen atom do not undergo Hydrogen bonding. As a result, primary amines have higher boiling points than 3° amines.
Ques: Account for the following :
(i) Primary amines (R-NH2) have higher boiling point than tertiary amines (R3N).
(ii) Aniline does not undergo Friedel – Crafts reaction.
(iii) (CH3)2NH is more basic than (CH3)3N in an aqueous solution. (All India 2014, 3 Marks)
Ans: (i) Due to presence of two H-atoms on N-atom of primary amines, they undergo extensive intermolecular H-bonding while tertiary amines due to the absence of a H-atom on the N-atom, do not undergo H- bonding. As a result, primary amines have higher boiling points than 3° amines.
(ii) Aniline being a Lewis base reacts with Lewis acid AlCl3 to form a salt.

As a result, N of aniline acquires positive charge and hence it acts as a strong deactivating group for electrophilic substitution reaction. Consequently, aniline does not undergo Friedel Craft reaction.
(iii) (CH3)3N has more steric hindrance due to which (CH3)3N is less basic than (CH3)2NH.
Ques: Give reasons for the following:
(i) Aniline does not undergo Friedel-Crafts reaction.
(ii) (CH3)2 NH is more basic than (CH3)3 N in an aqueous solution.
(iii) Primary amines have higher boiling point than tertiary amines. (All India 2016, Marks)
Ans: (i) Aniline being a Lewis base reacts with Lewis acid AlCl3 to form a salt.
As a result, N of aniline acquires positive charge and hence it acts as a strong deactivating group for electrophilic substitution reaction. Consequently, aniline does not undergo Freidel Crafts reaction.
(ii) In (CH3)N there is maximum steric hindrance and least solvation but in (CH3)2NH the solvation is more and the steric hindrance is less than in (CH3)3NH; although + I effect is less, since there are two methyl groups. This is why di-methyl amine is still a stronger base than tri-methyl.
(iii) Due to the presence of two H-atoms on N-atom of primary amines, they undergo extensive intermolecular H-bonding while due to the absence of a H-atom on the N-atom on tertiary amines, they do not undergo H- bonding. As a result, primary amines have higher boiling points than 3° amines.
Sample Questions
Ques: What is the shape of amine?(1 mark)
Ans: An amine molecule has the shape of a fairly straightened three-sided pyramid, with the nitrogen molecule at the peak. An unshared electron pair is limited over the nitrogen particle. In quaternary ammonium ions this region is involved by a substituent, framing an almost customary tetrahedron with the nitrogen molecule at its middle.
Ques: Which amines are more basic?(1 mark)
Ans: Because alkyl groups donate electrons to the more electronegative nitrogen. The inductive effect makes the electron density on the alkylamine's nitrogen atom higher than the nitrogen of ammonium ions. Due to which primary, secondary, and tertiary alkyl amines are more basic than ammonia molecules.
Ques: Why are amines soluble in HCL?(1 mark)
Ans: At the point when the amines get a H+ particle from the acid they become charged and can shape (solid) particle dipole associations with water atoms. Instantly, it turns into a salt and it breaks down easily like how table salt gets soluble in a glass of water.
Ques: What are the uses of amines?(1 mark)
Ans: Amines, beside narcotics and medications, are utilized in delivering azo-colors and nylon. They are generally utilized in the creation of harvest security, medication and water filtration synthetic compounds. They likewise discover use in close to home consideration merchandise. The most well known type of amine utilized in the worldwide market is ethanol amine.
Ques: Which amine is more soluble?(1 mark)
Ans: Higher amines are insoluble in water. Organic solvents like alcohol, benzene and ether easily dissolve the amines. For Example-
Hexanamine (C6H13NH2) gets dissolved in Benzene (C6H12)
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:





Comments