Jasmine Grover Study Abroad Expert
Study Abroad Expert
Radioactive decay (or nuclear decay) is the process caused by radiation to emit the energy of an unstable atomic nucleus. The three most common types of decay are alpha decay (\(\alpha\)-decay), beta decay (\(\beta\)-decay), and gamma decay (\(\gamma\)-decay). Alpha and gamma decay are ruled by the usual electromagnetic and strong forces, where beta decay is governed by the weak force.
| Table of Content |
Keyterms: Radioactivity, Unit, Ci or curie, Bq (Becquerel), Alpha Decay, Beta Decay, Gamma Decay, Radiation, Electromagnetic force, nuclear decay
What is Radioactive Decay?
[Click Here for Sample Questions]
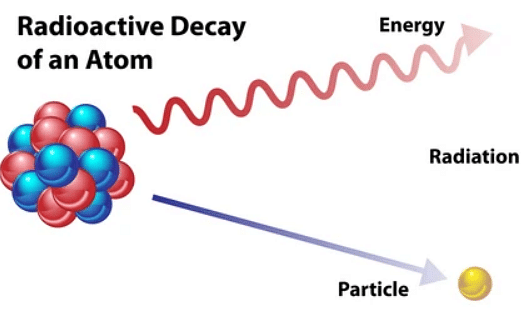
Radioactivity refers to the behaviour projected by the atomic nuclei because of nuclear instability. The nucleus of the atom begins to drain energy in the form of emitted radiation.
This phenomenon was confirmed through an experiment. A drawer was lined with photographic plates. A small amount of Uranium compound, wrapped in black paper was placed in the drawer. The plates were carefully examined after a while. They showed a clear exposure to radiation. This phenomenon came to be known as Radioactive Decay.

The presence of an unstable nucleus in the element’s radioisotope leeches out all the energy. This prevents the atom particles from being bounded. The isotopes present are in a state of constant decay in order to stabilise themselves. This causes a major release of energy in the form of radiation.
Also Read:
| Related Articles | ||
|---|---|---|
| Nuclear Force | Binding Energy | Nuclear Energy |
| Nuclear Physics | Nuclear reactor based on nuclear fission | Nucleon |
Types of Radioactive Decay
[Click Here for Sample Questions]
There are three types i.e Alpha Decay, Beta Decay and Gamma Decay.

Alpha Decay or α-decay
[Click Here for Sample Questions]
Alpha decay is a process where a nucleus emits an alpha particle (helium nucleus) into a different atomic nucleus. The following is the formula of alpha decay:
E = (mi − mf − mp) c2
Where,
mi stands for the initial mass of the nucleus
mf stands for the mass of the nucleus after alpha particle emission
mp stands for the mass of the emitted alpha particle

Example of Alpha Decay:
238U92 → 234Th90 + 4He2
When 238U92 goes through alpha decay, it transforms into Th23490 and He42 (containing two protons and two neutrons).
Beta Decay or β-decay
[Click Here for Sample Questions]
Beta decay is a process where a beta particle (electron/positron) is emitted from an atomic nucleus. The following is the process of beta decay:
234Th90 → 234Pa91 + 0e-1

Gamma Decay or γ-decay
[Click Here for Sample Questions]
Just like atoms, the nucleus also has energy levels. When the nucleus' high energy level shifts into a lower energy level, there is an emission of photons having MeV energy, hence, called gamma-ray.

Radioactive Decay Law
[Click Here for Sample Questions]
The radioactive decay law states when the number of radioactive nuclei undergoing the decay (α, β or γ-decay), per unit time, is proportionate to the total number of nuclei in the sample material.
Assuming, N is the number of nuclei in a sample and ΔN is the number of radioactive decays per unit time- Δt, then,
ΔN/Δt ∝ N
Or,
ΔN/Δt = λN
Where,
Λ is the proportionality constant or radioactive decay constant
Also,
ΔN is the decrease in the total number of nuclei present in the sample
∂N∂t=−λN
After rearranging, it should be
∂NN=−λ∂t
Uniting both will result in:
∫NN0∂NN=−λ∫tt0∂tInN−InN0=−λ(t−t0)
Here,
N0 means the initial number of nuclei present in the sample at a time
Applying t0 which is t=0 in the equation results in:
InNN0=−λt
Leading it to:
Nt=Ne−λt0
The above is an example of exponential type of decay.
Rate of Decay
[Click Here for Sample Questions]
From the above results, it is clear to focus on the rates, not on the number. The rate stands for the change per time.
Calculation of the rate of decay:
R=−∂N∂t
Replacing Nt in the equation
Nt=Ne−λt0
Result as
R=−∂N∂t=λNe−λt0R=Re−λt0R0
The decay rate at the time is t=0
Replacing the original equation:
ΔN Δt = λN
Results in
R = λN
Law Of Radioactive Decay Derivation
[Click Here for Sample Questions]
The radioactive decay law is:
ΔN/Δt ∝ N
or
ΔN/Δt = λN
Where,
λ is the radioactive decay constant
The change in the sample along with the number of nuclei is:
dN/dt = −λN (dN/N) = −λdt ∫NN0 dN/N = λ ∫tt0 dt
lnN − lnN0 = −λ (t−t0)
Where,
N0 is the number of radioactive nuclei
t0 is the arbitrary time
Hence, the law of radioactive decay:
ln (N/N0) = −λt N(t) = N0e−λt
Things to Remember
- Radioactive decay is the energy released in the form of radiation from an unstable atomic nucleus.
- Radioactive Decay is of three types: Alpha, Beta and Gamma Radiation
- Alpha decay is a process where a nucleus emits an alpha particle (helium nucleus) into a different atomic nucleus.
- Beta decay is a process where a beta particle (electron/positron) is emitted from an atomic nucleus.
- When the nucleus' high energy level shifts into a lower energy level, there is an emission of photons having MeV energy, called gamma-ray.
- The radioactive decay law states when the number of radioactive nuclei undergoing the decay (α, β or γ-decay), per unit time, is proportionate to the total number of nuclei in the sample material.
Also Read:
Sample Questions
Ques: When a nucleus in an atom undergoes radioactive decay, what are the electronic energy levels of the atom? (2 marks)
Ans: When a nucleus in an atom undergoes radioactive decay, the electronic energy levels of an alpha particle is 2 units of positive charge, beta particles are 1 unit positive charge and gamma particles have no charge.
Ques: Mx and My denote the atomic masses of the parent and the daughter nuclei respectively in radioactive decay. The Q-value for a β– decay is Q1 and that for a β+ decay is Q2. If me denotes the mass of an electron, then what will be the value? (3 marks)
Ans: Let’s assume ZXA to be the nucleus, according to ß+ decay will form as:
zXA → Z+1YA + +1e0 + v + Q2
Therefore, Q2 = [mN (zXA) - mN (Z-1YA) - me] c2
= [mN (ZXA) + Zme - mn (Z-1YA) - (z-1) me- 2me] c2
= [mN (ZXA) - m (Z-1YA) - 2me] c2
= (Mx -My - 2me) c2
ß- decay will form as:
zXA → Z+1AY + -1e0 + v1 + Q1
Therefore, Q1 = [mN (zXA) - mN (z + 1YA) - me] c2 = [mN (zXA) - m (z + 1YA)] c2 = (Mx –My) c2
Ques: Heavy stable nuclei have more neutrons than protons. The reason behind it is? (1 mark)
Ans: Heavy stable nuclei have more neutrons than protons because the electrostatic force between protons is repulsive which results in reducing the nucleus stability.
Ques: Tritium is an isotope of hydrogen whose nucleus Triton contains 2 neutrons and 1 proton. Free neutrons decay into p + ? + ν. If one of the neutrons in Triton decays, it would transform into an He3 nucleus. This does not happen because? (2 marks)
Ans: The nucleus of Tritium (1H3) contains 1 proton and 2 neutrons. A neutron decays as n → p + ? + ν, the nucleus may have 2 protons and 1 neutron, therefore, tritium will transform into 2He3.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Do Check Out:




Comments