Jasmine Grover Study Abroad Expert
Study Abroad Expert
A neutron is a neutral subatomic particle. As we know, atoms are small and so are their interior entities. Neutron is one such entity with no charge. In this topic, we will discuss neutrons, the rest mass of neutrons, the mass of one neutron, relative charge, and relative mass of neutrons.
| Table of Contents |
Keyterms: Neutron, Atoms, subatomic particle, proton, electron, photons, radiation, velocity, Neutron Mass, Atomic mass, light
What is a Neutron?
[Click Here for Sample Questions]
A neutron is a subatomic particle that has no electrical charge i.e. it is neutral. The mass of a neutron is almost equal to a proton. An atom consists of three entities: a proton (positive), an electron (negative), and a neutron (neutral).
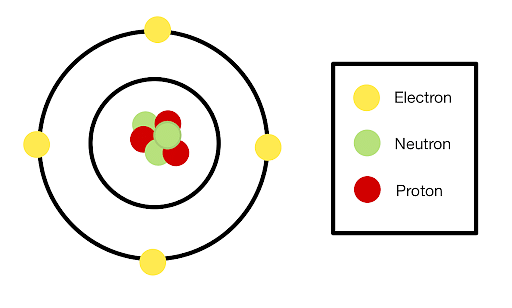
Structure of an atom
Discovery of a Neutron
- A physicist named Ernest Rutherford performed an experiment to investigate the structure of atoms in 1906. He concluded that in an atom, most of the mass and positive charge are concentrated at the centre.
- James Chadwick in 1932 performed an experiment by bombarding beryllium with the alpha particles. He noticed the emission of neutral radiation during this experiment.
- At that time, the only neutral Radiation known was that of photons. But the neutral radiations emitted during the experiment contained much less energy than photons. He named these new neutral particles “Neutrons”.
Also Read:
| Related Articles | ||
|---|---|---|
| Nuclear Physics | Size of the Nucleus | Nucleon |
| Mass Energy Equivalence | Atoms and Nuclei Important Questions | Nuclear Binding Energy |
Neutron Mass
[Click Here for Sample Questions]
- The mass of a neutron is equal to 1.6726231 × 10-27 kg. This number is marginally lesser than that of a proton.
- But it is nearly 1,839 times the mass of electrons.
Neutron Mass in grams
Since 1kg = 103 grams
mn = 1.67493 × 10-27 × 103
mn = 1.67493 × 10-24 gm
Neutron Mass in atomic mass unit(amu)
Since, 1 kg = 6.0229552894949E + 26 amu
Therefore,
1.67493 × 10-27 kg = 1.67493 × 10-27× 6.0229552894949E + 26 amu
mn = 1.008664904(14) amu
Rest Mass of a Neutron
[Click Here for Sample Questions]
- There is also a concept of the rest mass of neutrons in physics.
- We know that mass is a constant quantity for an object.
- However, according to Einstein's theory of relativity, the energy and mass of a body are interchangeable.
- With this concept, we can get to the point that the mass of a body increases with the increased velocity of the body relative to the observer.
- So we can conclude that the energy of the body gets affected with the increase in mass of the body.
- Therefore, the minimum mass of a body is when the body is at rest or stationary position.
- Rest Mass of the body is defined as the mass of the body which is at rest relative to the observer and given by
m = m0/ √(1- v2/c2)
where mo = rest mass,
v = velocity,
c = speed of light,
Now putting the value of m = 1.6749286 ×10-27 kg , v = 2.19 km/s or 2190 m/s and c = 3 × 108 m/s
1.6749286 ×10-27 = m0 / √1- (2190)2/(3 × 108)2
= m0 / √(3 × 108 )2 - (2190)2/(3 × 108)2
= m0 × (3 × 108 /√9 ×1016) - (4796100)
= m0 × (3 × 108 /√9 ×1016)
= m0 × (3 × 108/3 × 108 )
After cancelling the common terms,
1.6749286 × 10-27 = m0 × 1
So, the value of the rest mass of a neutron is
m0 = 1.6749286 × 10-27 kg
Mass of One Neutron
[Click Here for Sample Questions]
Mass of a free neutron = 1.67493 × 10-27 kg
Now we will change it to electron volts,
We know that, 1 eV = 1.6 × 10-19 J
and Speed of light, c = 3 × 108 m/s
Since, 1 kg = 5.6095883571872E + 35eV
Therefore,
1.67493 × 10-27 kg = (5.6095883571872E + 35eV )× 1.67493 × 10-27
mn = 939,565,413.3 eV
Also, in Mega electron Volts (MeV),
mn = 939.565346 MeV/C2

Mass of neutron
Definition of amu and eV
- Atomic Mass Unit, also known as amu, is defined as 1/12 of the Carbon-12 atom’s mass. Carbon-12 atom has 6 neutrons and 6 protons in its nucleus.
- Electron Volt(eV) is nothing but the energy gained by an electron when it travels with 1 volt of potential.
Relative Mass of Neutron
[Click Here for Sample Questions]
- As we all know, atoms consist of three subatomic particles, namely, protons, electrons, and neutrons.
- Protons and neutrons are found in the nucleus which is at the centre of an atom.
- Protons are as massive as neutrons; we can say approximately 99.86 % and electrons are 0.054% heavier than neutrons.
- The relative mass of each particle is measured in kilograms.
- Therefore, a neutron has the relative mass as 1.

Relative Mass of Neutron
Relative charge on a Neutron
[Click Here for Sample Questions]
- Neutrons are neutral entities meaning they do not possess any charge.
- When James Chadwick conducted his experiment, he found out that the neutral subatomic particle didn’t get deflected by electric or magnetic fields.
- Hence, we conclude that the relative charge on a neutron is 0.
Things to Remember
- A neutron is a subatomic particle that has no electrical charge i.e. it is neutral.
- The mass of a neutron is almost equal to a proton. An atom consists of three entities: a proton (positive), an electron (negative), and a neutron (neutral).
- Mass of neutron in different units:
mn= 1.67493 × 10-24 gm
mn = 1.008664904(14) amu
- Rest mass of a neutron is equal to 1.67493 × 10-27 kg
- Mass of one neutron = 939,565,413.3 eV
- The relative mass of a neutron is 1
- The discovery of a neutral particle or neutron by James Chadwick led to the present concept that the nucleus consists of protons and neutrons
- The relative charge on a neutron is 0
Also Read:
Sample Questions
Ques. What is the mass of one neutron? (1 mark)
Ans. Mass of one neutron = 939,565,413.3 eV
Ques. An element having 14 as an atomic mass number and 6 as an atomic number 6 has how many neutrons? (2 mark)
Ans. The number of neutrons = Atomic mass no. - Atomic no.
= 14 - 6 = 8
Ques. What is the relative charge on a neutron? (1 mark)
Ans. The relative charge on a neutron is 0
Ques. What can you say about the relative mass of a neutron? (1 mark)
Ans. The relative mass of a neutron is 1. The mass of a neutron is nearly equal to protons and heavier than electrons.
Ques. How is the rest mass different from the mass of a neutron? (1 mark)
Ans. According to Einstein's theory of relativity, the mass of a body increases with the increased velocity of the body relative to the observer. So, the minimum mass of a body is when the body is at rest or in a stationary position.
Ques. Which observation of Chadwick led to the discovery of neutrons? (2 mark)
Ans. James Chadwick in 1932 noticed the emission of neutral radiation during this experiment. At that time, the only neutral radiation known was of photons. But the neutral radiations emitted during the experiment contained much less energy than photons. He also found out that the neutral subatomic particle didn’t get deflected by electric or magnetic fields He named these new neutral particles “Neutrons”.
Ques. What is the rest mass of a neutron? (3 marks)
Ans. Rest Mass of the body is defined as the mass of the body which is at rest relative to the observer and given by
m = m0/ √(1- v2/c2)
where m0 = rest mass,
v = velocity,
c = speed of light,
Putting all the values in the given equation, we get
So, the value of the rest mass of a neutron is
m0 = 1.6749286 ×10-27 kg
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Also check:




Comments