Jasmine Grover Study Abroad Expert
Study Abroad Expert
Beta decay can be referred to as one of the types of reactions that take place during the nuclear reactions. In beta decay, a neutron decomposes into an electron and a proton. The process of radioactivity is very useful but it is a dangerous phenomenon.
| Table of Contents |
Keyterms: Radioactivity, Ci or curie, Bq (Becquerel), Alpha Decay, Beta Decay, Gamma Deca, Beta minus decay, Beta plus decay, Neutron, Proton, Electron
Beta Decay Definition
[Click Here for Sample Questions]
In radioactivity, beta decay is a process in which a proton is transformed into a neutron or vice versa. This reaction takes place inside the nucleus of a radioactive sample. Both the alpha decay & beta decay let the nucleus get as possible of the optimum proton or neutron. While this, a beta particle is emitted by the nucleus that can either be a positron or an electron. What we need to remember is that the positron generated basically to obey the law of conservation of charge. The proton may turn to a neutron or vice-versa. Beta-decay is a process that occurs via weak interaction. It is of two types – Beta minus decay (\(\beta\)-) and beta plus decay(\(\beta\)+).

Beta Decay
In short beta decay can be elaborated as a property of various elements which are naturally available along with their isotopes to produce artificial isotopes of those elements.
There are three ways for differentiating this phenomenon which are- neutron decay, proton decay and electron decay. In every decay, the atomic number keeps on changing continuously for the formation of parents atoms & daughters atoms are formed of different elements.
Read More:
| Related Articles | ||
|---|---|---|
| Nuclear Physics | Nuclear reactor based on nuclear fission | Nucleon |
| Mass Energy Equivalence | Atoms and Nuclei Important Questions | Nuclear Binding Energy |
Beta Decay Procedure
[Click Here for Sample Questions]
In the process of Beta decay, the proton present inside the nucleus turns into a neutron or the exact opposite thing takes place. If a neutron gets converted to proton, the process is called (\(\beta\)-) decay and if the proton is converted to neutron, it is known as (\(\beta\)+) decay. Beta particles are emitted due to the change in the nucleus. And these particles are used to cure various health conditions like –bone cancer, eye cancer. Beta particles are often used as tracers
Example of Beta Decay
[Click Here for Sample Questions]
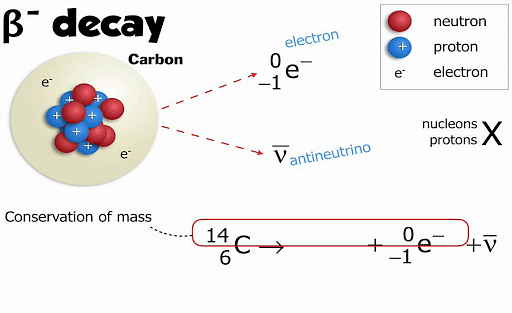
(\(\beta\)-) Decay is an example of Beta decay of carbon atoms. The neutron of a carbon atom is converted into proton and emits beta particle which is an electron. An example of (\(\beta\)-) decay of C-14 into Nitrogen-14:
146C → 147N + e- + ve
This form of decay is also known as nuclear transmutation. The decaying element is known as parent nuclide and the resulting element is called daughter nuclide.
Beta Decay Types
[Click Here for Sample Questions]
The radioactivity beta decay involves two types-
Radioactivity beta decay Types
1. Beta- Minus Decay
- In Beta Minus decay a proton is transformed which causes an increase in the atomic number of the atom.
- During the process, the nucleus also produces an electron & an antineutrino to balance the conservation of charge.
- The antimatter counterpart of neutrinos is known as antineutrino. These are neutral particles with very negligible mass. The interactions of these particles with other matter are so weak that the entire earth remains undisturbed.
- The atomic configuration in beta-minus decay is:
AZX → AZ+1 Y +e- +v
N = P + e- + v-
2. Beta- Plus Decay

Beta plus decay
- Beta plus decay results in the transformation of a proton into a neutron and that decreases the atomic number of the radioactive element. Hence, a loss of proton and gain of a neutron is experienced inside the nucleus.
- The beta decay process too produces a positron & a neutrino to maintain the law of conservation of charges. The Positron consists of a positive charge.
- The behaviour of neutrino is similar to that of the antineutrinos.
- The equation of its atomic configuration is:
AZX → AZ-1Y + e+ + v
P = N+ e+ + v
Beta Decay: Beta Emission
[Click Here for Sample Questions]
The beta particles which are emitted by some radioactive nuclei consist of high energy & high speed electrons like for potassium – 40. The penetrations of the beta particles are greater than the alpha particles but still there is a lack of strength to the beta-gamma rays. The beta particles which are emitted are in the ionizing radiation form and also known as beta emission or beta rays.
Beta Decay: Fermi’s Theory
According to Enrico Fermi’s proposed theory, the 4 fermions interact directly with one another at one vertex. This type of interaction takes place by coupling an electron with a neutron, a neutrino and also a proton. This theory was formulated by Fermi in 1933.
Read More:
Things to Remember
- Beta Decay is a part of CBSE class 12 physics syllabus and is taken from chapter 13, Nuclei.
- It carries 1 to 2 marks and the overall chapter carries 4-5 marks in CBSE Class 12 examinations.
- Radioactive atoms consist of energy and it also produces electromagnetic waves, the type of emission is called radiation.
- Half-life is known as the rate of radioactive element decays, i.e. the total time needed for one half of a given quantity of isotope.
- Beta decay of elements is generally the production of artificial isotopes of the elements.
- There are two types of beta decay- Beta plus & beta minus.
Sample Questions
Ques. Give an example of beta plus decay. (1 mark)
Ans. An example can be given of Beta-plus decay of Magnesium-23 into sodium -23:
2312Mg → 2311Na + e+ + ve
Beta decay results in reduction of atomic number and nuclear transmutation.
Ques. What do you mean by beta decay? (1 mark)
Ans. Beta decay can be said as decay of a nucleus by emitting an electron or a positron. Beta particles emitted in the process are fast moving electrons of nuclear origin.
Ques. What is the usage of beta decay? (1 mark)
Ans. Beta decay is very much beneficial in medical science to cure various health problems:
- To treat eye & bone cancer
- used in form of tracers
Ques. What is the law of Conservation in Beta decay? (1 mark)
Ans. In radioactivity beta decay, law of conservation takes place. To maintain balance in the charges inside a nucleus, when a parent nucleus decays it forms a daughter nucleus to follow the law of conservation.
Ques. What do you mean by Beta particles in Beta decay? (1 mark)
Ans. Beta particles are the emitted charged particles which flow from the nucleus of a certain radioactive element during the beta decay procedure which has mass equal to 1/1837 in comparison to the mass of protons. These particles carry a single negative (electron) or a single positive (positron) charge.
Ques. (a) Write the basic process involved in nuclei responsible for: \(\beta\)- and \(\beta\)+ decay
(b) Why is it found experimentally difficult to detect neutrinos? (2015)
Ans. (a) \(\beta\)- decay = 10n11P+0+1 \(\beta\)+v
\(\beta\)+ decay = 11P10n+0+1\(\beta\)+v
(b) It is difficult to detect neutrinos in nuclear \(\beta\)-decay because they are considered chargeless containing very little mass particles that rarely interact with matter.
Ques. Why is it found experimentally difficult to detect neutrinos in nuclear \(\beta\)-decay? (2014)
Ans. It is difficult to detect neutrinos in nuclear \(\beta\)-decay because they are considered chargeless containing very little mass particles that rarely interact with matter.
Ques. In both \(\beta\)- and \(\beta\)+ decay processes the mass number of a nucleus remains same whereas the atomic number Z increases by one in\(\beta\)- decay and decreases by one in \(\beta\)+ decay. Explain giving reasons. (2014)
Ans. On case of \(\beta\)-decay , a \(\beta\) having zero mass and -1 charge is exerted. The decay process can be observed through:![]()
Since the \(\beta\)-particle has negligible small mass, there is no change in the mass number of the nucleus and there is an increase in the atomic number by 1 as a result of the loss of 1 negative charge.
In the same manner for a \(\beta\)-decay, a \(\beta\) particle exerts an extremely small and +1 charge. The decay process can be expressed as: ![]()
There is no change in the mass number but in this case there is a decrease in the atomic number by 1 as a result of the loss of 1 positive charge.
Ques. A nucleus undergoes \(\beta\)--decay. How does its (i) mass number (ii) atomic number change? (2011)
Ans. When a nucleus undergoes \(\beta\)--decay:
(i) there is no change in the mass number.
(ii) There is an increase in the atomic number by one unit.
Ques. A nucleus 2310Neundergoes \(\beta\)-decay and becomes 2311Na. Calculate the maximum kinetic energy of emitted electrons assuming that the daughter nucleus and antineutrino carry negligible kinetic energy. (2008)
(a) Mass of 2310Ne = 22.994466 u
(b) Mass of 2311Na = 22.989770 u
© Lu = 931 MeV/c²
Ans. The equation that represents \(\beta\)- decay of 2310Ne is 2310Ne 2311Na+\(\beta\)-+v+Q
Where Q is considered as the kinetic energy shared by 2310Ne and 2311Na ignoring the other part of the mass of antineutrino (v) and electron.
Mass defect \(\Delta\)m = m(2310Ne) - m(2311Na) - m(\(\beta\)-) = (22.994466 - 22.989770) = 0.004696 u
Therefore Q = 0.004696*931 MeV = 4.372 MeV
Hence K.E. of \(\beta\)- = 4.372 MeV when (v) carries the energy and is 0.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:




Comments