JEE Main 2025 April 3 Chemistry Question Paper is available for download. NTA conducted JEE Main 2025 Shift 1 B.Tech Exam on 3rd April 2025 from 9:00 AM to 12:00 PM and for JEE Main 2025 B.Tech Shift 2 appearing candidates from 3:00 PM to 6:00 PM. The JEE Main 2025 3rd April B.Tech Question Paper was Moderate to Tough.
Also Check: JEE Main 2025 Question Paper with Solution PDF Download
JEE Main 2025 April 3 Shift 1 Chemistry Question Paper with Solutions
| JEE Main 2025 April 3 Shift 1 Chemistry Question Paper Pdf | Download PDF | View Solution |

JEE Main 2025 Chemistry Questions with Solutions
Question 1:
Which of the following postulate of Bohr's model of hydrogen atom in not in agreement with quantum mechanical model of an atom ?
Given below are two statements:
Statement I : The N-N single bond is weaker and longer than that of P-P single bond
Statement II : Compounds of group 15 elements in +3 oxidation states readily undergo disproportionation reactions.
In the light of above statements, choose the correct answer from the options given below
Given below are two statements:
Statement I: A catalyst cannot alter the equilibrium constant (\( K_c \)) of the reaction, temperature remaining constant.
Statement II: A homogeneous catalyst can change the equilibrium composition of a system, temperature remaining constant.
In the light of the above statements, choose the correct answer from the options given below.
The metal ions that have the calculated spin only magnetic moment value of 4.9 B.M. are
A. \( Cr^{2+} \)
B. \( Fe^{2+} \)
C. \( Fe^{3+} \)
D. \( Co^{2+} \)
E. \( Mn^{2+} \)
Choose the correct answer from the options given below
In a reaction A + B → C, initial concentrations of A and B are related as \( [A]_0 = 8[B]_0 \). The half lives of A and B are 10 min and 40 min, respectively. If they start to disappear at the same time, both following first order kinetics, after how much time will the concentration of both the reactants be same?
Which of the following is the correct structure of L-fructose?
Identify the correct statements from the following
A. 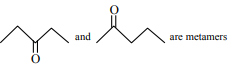
B. ![]()
C. ![]()
D. ![]()
Choose the correct answer from the options given below
Among \( 10^{-10} \) g (each) of the following elements, which one will have the highest number of atoms?
Element : Pb, Po, Pr and Pt
Which of the following statements are correct?
A. The process of the addition an electron to a neutral gaseous atom is always exothermic
B. The process of removing an electron from an isolated gaseous atom is always endothermic
C. The 1st ionization energy of the boron is less than that of the beryllium
D. The electronegativity of C is 2.5 in \( CH_4 \) and \( CCl_4 \)
E. Li is the most electropositive among elements of group 1
Choose the correct answer from the options given below
Which of the following properties will change when system containing solution 1 will become solution 2 ?
Number of molecules from below which cannot give iodoform reaction is:
Ethanol, Isopropyl alcohol, Bromoacetone, 2-Butanol, 2-Butanone, Butanal, 2-Pentanone, 3-Pentanone, Pentanal and 3-Pentanol
Identify [A], [B], and [C], respectively in the following reaction sequence :
In the following reactions, which one is NOT correct?
The correct order of the complexes
[5pt] \([Co(NH_3)_5(H_2O)]^{3+}\) (A),
[3pt] \([Co(NH_3)_6]^{3+}\) (B),
[3pt] \([Co(CN)_6]^{3-}\) (C),
[3pt] \([CoCl(NH_3)_5]^{2+}\) (D)
[5pt]
in terms of wavelength of light absorbed is:
In the following system, \( PCl_5(g) \rightleftharpoons PCl_3(g) + Cl_2(g) \) at equilibrium, upon addition of xenon gas at constant T and p, the concentration of
2 moles each of ethylene glycol and glucose are dissolved in 500 g of water. The boiling point of the resulting solution is: (Given: Ebullioscopic constant of water = \( 0.52 \, K kg mol^{-1} \))
Which compound would give 3-methyl-6-oxoheptanal upon ozonolysis ?
Match the LIST-I with LIST-II
LIST-I LIST-II
A. \( PF_5 \) I. \( dsp^2 \)
B. \( SF_6 \) II. \( sp^3d \)
C. \( Ni(CO)_4 \) III. \( sp^3d^2 \)
D. \( [PtCl_4]^{2-} \) IV. \( sp^3 \)
Choose the correct answer from the options given below :
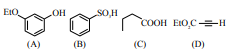
The least acidic compound, among the following is
Correct order of limiting molar conductivity for cations in water at 298 K is :
During estimation of Nitrogen by Dumas' method of compound X (0.42 g) :
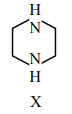
mL of \( N_2 \) gas will be liberated at STP. (nearest integer)
(Given molar mass in g \(mol^{-1}\) : C : 12, H : 1, N : 14)
0.5 g of an organic compound on combustion gave 1.46 g of \( CO_2 \) and 0.9 g of \( H_2O \). The percentage of carbon in the compound is ______ (Nearest integer)
(Given : Molar mass (in g \(mol^{-1}\)) C : 12, H : 1, O : 16)
The number of optical isomers exhibited by the iron complex (A) obtained from the following reaction is ______ \( FeCl_3 + KOH + H_2C_2O_4 \rightarrow A \)
Given: \( \Delta H_f^0 [C(graphite)] = 710 \) kJ mol⁻¹ \( \Delta_c H^0 = 414 \) kJ mol⁻¹ \( \Delta_{H-H}^0 = 436 \) kJ mol⁻¹ \( \Delta_{C-H}^0 = 611 \) kJ mol⁻¹
The \(\Delta\) \(H_{C=C}^0\) for \(CH_2=CH_2\) is ______ kJ \(mol^{-1}\) (nearest integer value)
Consider the following reactions \( A + HCl + H_2SO_4 \rightarrow CrO_2Cl_2 + Side Products \)
Little amount \( CrO_2Cl_2(vapour) + NaOH \rightarrow B + NaCl + H_2O \) \( B + H^+ \rightarrow C + H_2O \)
The number of terminal 'O' present in the compound 'C' is ______









JEE Main 2025 April 3 Shift 1 Chemistry Exam Analysis
| Aspect | Details |
|---|---|
| Overall Difficulty | The Chemistry section of the JEE Main 2025 April 3 Shift 1 exam was considered easy to moderate. Students found the questions straightforward, with a focus on fundamental concepts and NCERT-based topics. |
| Time Consumption | The Chemistry section was time-efficient for most students. Many candidates were able to complete it in about 40 minutes, allowing ample time for revision. |
| Topic Distribution |
|
| Question Types | The questions included a mixture of multiple-choice questions (MCQs) and numerical value-based questions, testing both conceptual understanding and application of chemical principles. |
| Student Feedback | Students reported that the Chemistry section was balanced, with clear and NCERT-aligned questions. Organic Chemistry was the most prominent topic, and many students found the section well within their preparation range. |
Also Check JEE Main 2025 April 2 Shift 1 Question Paper With Video Solutions
Get the JEE Main 2025 April 2 Shift 1 Question Paper with detailed video solutions. Compare answers with the official answer key and understand the difficulty level for upcoming shifts..







Comments