JEE Main 2023 Chemistry Question Paper Feb 1 Shift 2 is updated here after the conclusion of the exam. Candidates can download JEE Main 2023 Chemistry Question Paper PDF with Answer Key for Feb 1 Shift 2 using the link below. JEE Main Chemistry Question Paper is divided into two sections, Section A with 20 MCQs and Section B with 10 numerical type questions. Candidates are required to answer all questions from Section A and any 5 questions from section B.
Related Links:
- Download JEE Main Previous Year Question Papers PDF with Solutions
- Download JEE Main 2025 Question Paper for all Shifts
JEE Main 2023 Chemistry Question Paper Feb 1 Shift 2- Download PDF
| JEE Main 2023 Feb 1 Shift 2 Chemistry Question Paper with Solution PDF | Check Solution |

JEE Main 2023 Feb 1 Shift 2 Chemistry Question Paper with Solution
Question 1 :
In a reaction,
reagents 'X' and 'Y' respectively are :
(1) (CH\(_{3}\)CO)\(_{2}\)O/H\(+ \) and CH\(_{3}\)OH/H\(+ \), \(\Delta\)
View Solution
The correct order of bond enthalpy (kJ mol\(^{-1}\)) is:
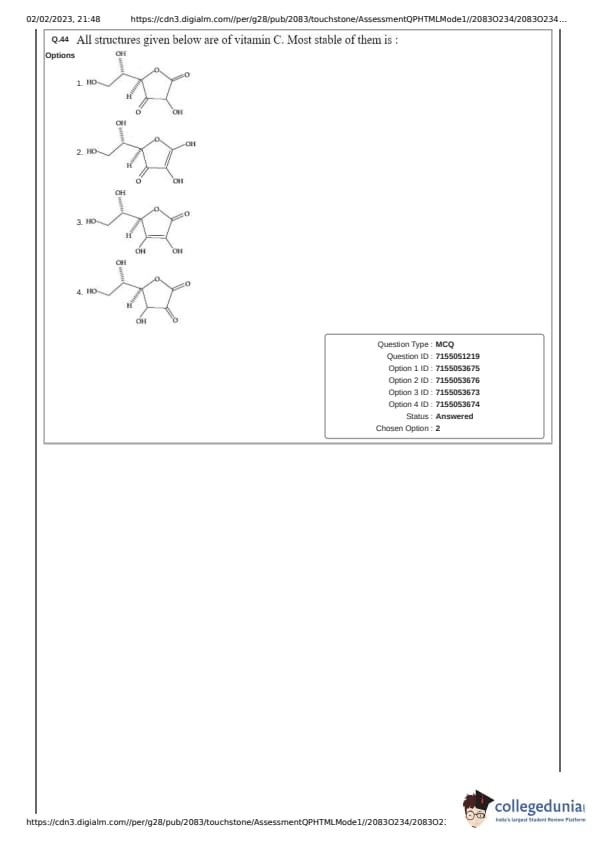
All structures given below are of vitamin C. Most stable of them is :
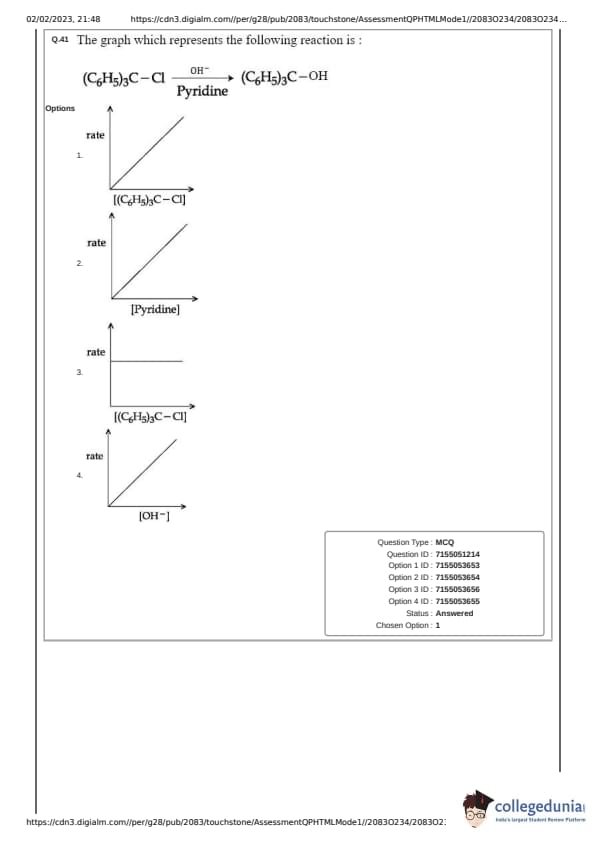
The graph which represents the following reaction is:
\[ (C_6H_5)_3C-Cl \xrightarrow[Pyridine]{OH^-} (C_6H_5)_3C-OH \]
(1)
View Solution
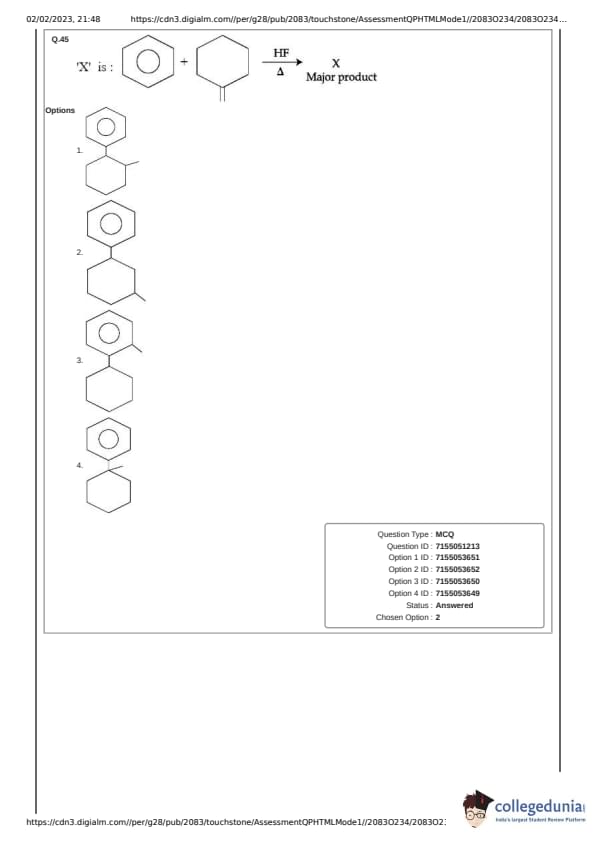
Reactants
The reactants are tetrahydrofuran (THF) and 2-methylpropene. The reaction is catalyzed by HF and takes place under heat.
Reaction Mechanism
This reaction is an electrophilic addition of THF to the alkene. HF protonates the alkene to form a carbocation. The oxygen in THF acts as a nucleophile and attacks the carbocation. Finally, deprotonation occurs to yield the product.
Major Product
The major product is determined by Markovnikov's rule, which states that the proton adds to the carbon of the double bond with more hydrogens. In this case, the carbocation will form on the more substituted carbon of 2-methylpropene, leading to product (1).
Conclusion: The major product 'X' is represented by structure (1). Quick Tip: Remember Markovnikov's rule for electrophilic addition reactions. The most stable carbocation intermediate leads to the major product.
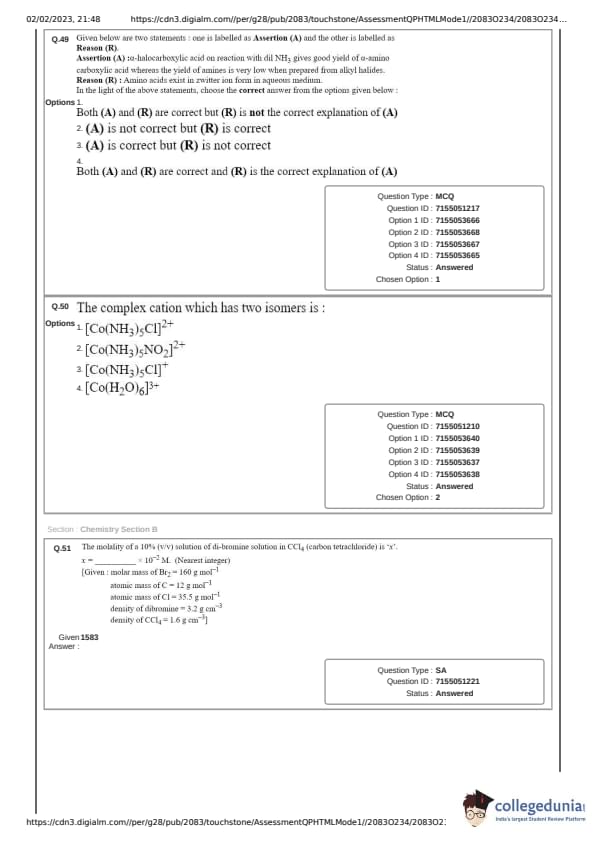
The complex cation which has two isomers is:
Given below are two statements :
Statement I : Sulphanilic acid gives esterification test for carboxyl group.
Statement II : Sulphanilic acid gives red colour in Lassaigne's test for extra element detection.
In the light of the above statements, choose the most appropriate answer from the options given below :
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Gypsum is used for making fireproof wall boards.
Reason (R) : Gypsum is unstable at high temperatures.
In the light of the above statements, choose the correct answer from the options given below :
(4) Both (A) and (R) are correct and (R) is the correct explanation of (A).
View Solution
Which element is not present in Nessler's reagent ?
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : \(\alpha\)-halocarboxylic acid on reaction with dil. NH\(_{3}\) gives good yield of \(\alpha\)-amino carboxylic acid whereas the yield of amines is very low when prepared from alkyl halides.
Reason (R) : Amino acids exist in zwitter ion form in aqueous medium.
In the light of the above statements, choose the correct answer from the options given below :
(2) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
View Solution
The industrial activity held least responsible for global warming is :
The structures of major products A, B and C in the following reaction are sequence.

Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Cu\(^{2+}\) in water is more stable than Cu\(+ \).
Reason (R) : Enthalpy of hydration for Cu\(^{2+}\) is much less than that of Cu\(+ \).
In the light of the above statements, choose the correct answer from the options given below :
(1) Both (A) and (R) are correct and (R) is the correct explanation of (A).
View Solution
The starting material for convenient preparation of deuterated hydrogen peroxide (D\(_{2}\)O\(_{2}\)) in laboratory is:
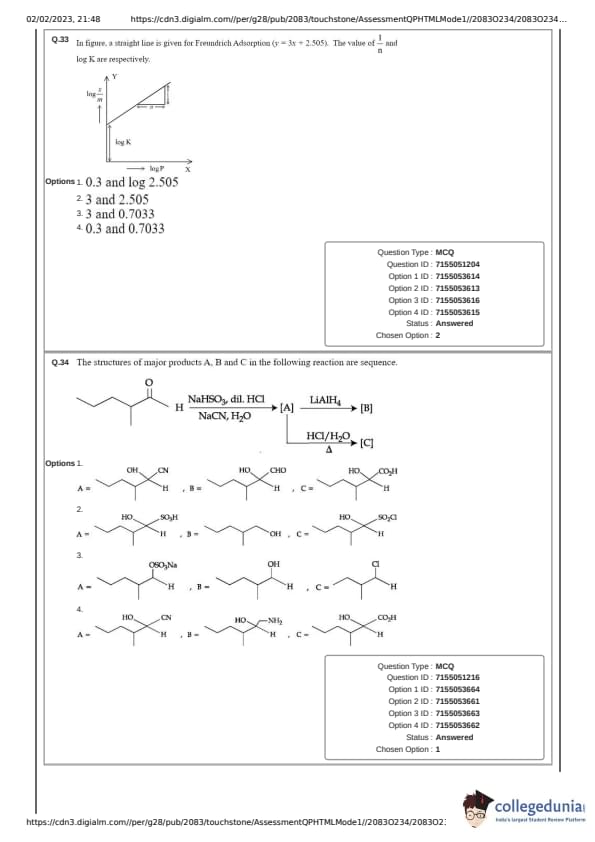
In figure, a straight line is given for Freundrich Adsorption (\(y = 3x + 2.505\)). The value of \(\frac{1}{n}\) and log K are respectively.
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : An aqueous solution of KOH when for volumetric analysis, its concentration should be checked before the use.
Reason (R) : On aging, KOH solution absorbs atmospheric CO\(_{2}\).
In the light of the above statements, choose the correct answer from the options given below.
(3) Both (A) and (R) are correct and (R) is the correct explanation of (A)
View Solution
Which one of the following sets of ions represents a collection of isoelectronic species?
(Given : Atomic Number : F :9, Cl : 17, Na = 11, Mg = 12, Al = 13, K = 19, Ca = 20, Sc = 21)
The effect of addition of helium gas to the following reaction in equilibrium state, is :
\[ PCl_5(g) \rightleftharpoons PCl_3(g) + Cl_2(g) \]
(1) the equilibrium will shift in the forward direction and more of Cl\(_{2}\) and PCl\(_{3}\) gases will be produced.
View Solution
For electron gain enthalpies of the elements denoted as \(\Delta_{eg}H\), the incorrect option is :
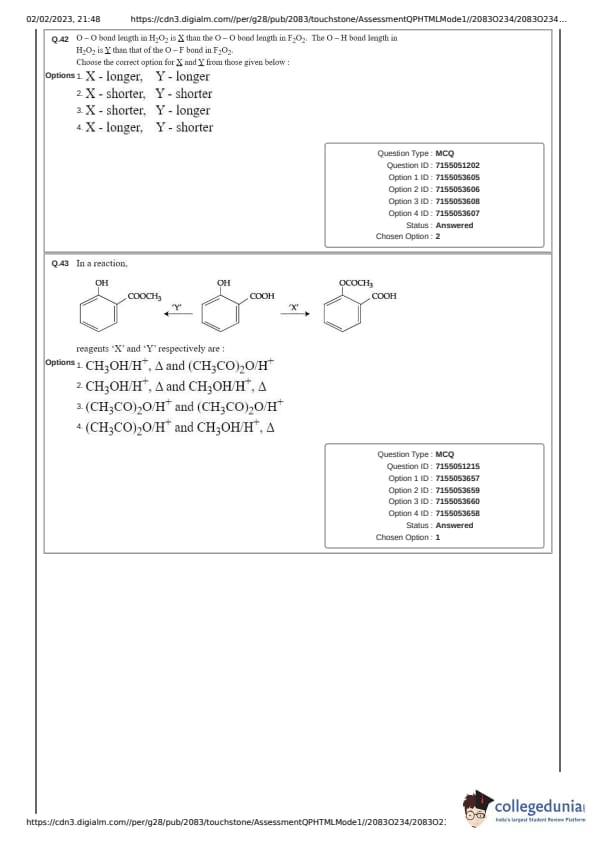
O-O bond length in H\(_{2}\)O\(_{2}\) is _X_ than the O-O bond length in F\(_{2}\)O\(_{2}\). The O -- H bond length in H\(_{2}\)O\(_{2}\) is ___Y__ than that of the O-F bond in F\(_{2}\)O\(_{2}\). Choose the correct option for _X_ and _Y_ from the given below.
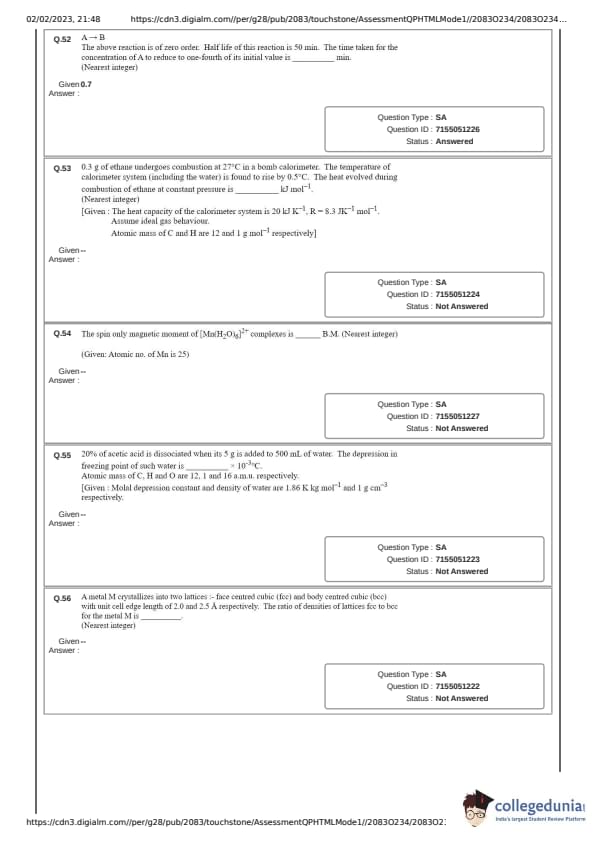
0.3 g of ethane undergoes combustion at 27\(^\circ\)C in a bomb calorimeter. The temperature of calorimeter system (including the water) is found to rise by 0.5\(^\circ\)C. The heat evolved during combustion of ethane at constant pressure is _____ kJ mol\(^{-1}\). (Nearest integer)
[Given : The heat capacity of the calorimeter system is 20 kJ K\(^{-1}\), R = 8.3 JK\(^{-1}\) mol\(^{-1}\). Assume ideal gas behaviour. Atomic mass of C and H are 12 and 1 g mol\(^{-1}\) respectively]
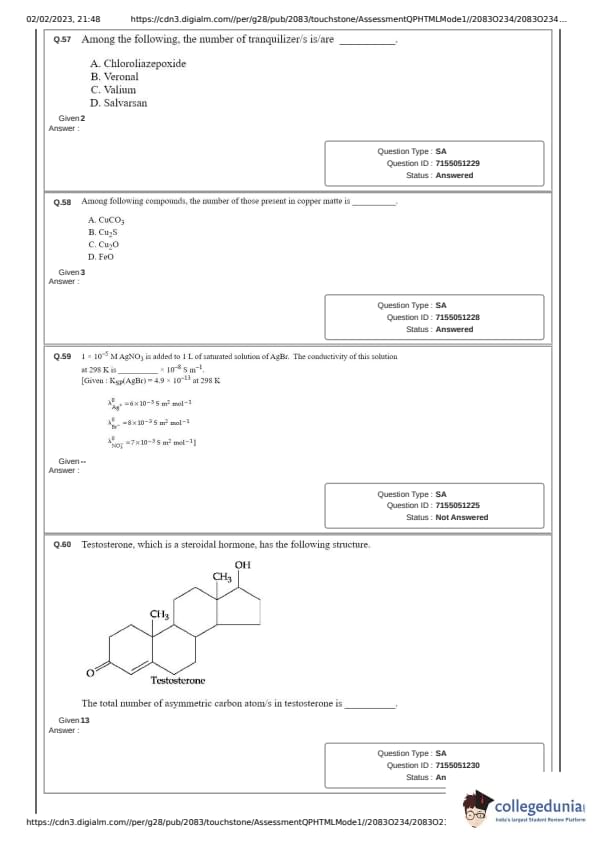
Among following compounds, the number of those present in copper matte is ____________.
A. CuCO\(_{3}\)
B. Cu\(_{2}\)S
C. Cu\(_{2}\)O
D. FeO
View Solution
Among the following, the number of tranquilizer/s is/are ____________.
A. Chlordiazepoxide
B. Veronal
C. Valium
D. Salvarsan
View Solution
A \(\rightarrow\) B
The above reaction is of zero order. Half life of this reaction is 50 min. The time taken for the concentration of A to reduce to one-fourth of its initial value is ____ min. (Nearest integer)
View Solution
20% of acetic acid is dissociated when its 5 g is added to 500 mL of water. The depression in freezing point of such water is ____ \(\times 10^{-3} \, ^\circ C\). Atomic mass of C, H and O are 12, 1 and 16 a.m.u. respectively.
[Given : Molal depression constant and density of water are 1.86 K kg mol\(^{-1}\) and 1 g cm\(^{-3}\) respectively.]
The molality of a 10% (v/v) solution of di-bromine solution in CCl\(_4\) (carbon tetrachloride) is 'x'. x = ____ \( \times 10^{-2} \) M. (Nearest integer)
Given:
Molar mass of Br\(_2\) = 160 g mol\(^{-1}\)
Atomic mass of C = 12 g mol\(^{-1}\)
Atomic mass of Cl = 35.5 g mol\(^{-1}\)
Density of dibromine = 3.2 g cm\(^{-3}\)
Density of CCl\(_4\) = 1.6 g cm\(^{-3}\)
1 \( \times 10^{-5} \) M AgNO\(_3\) is added to 1 L of saturated solution of AgBr. The conductivity of this solution at 298 K is ____ \( \times 10^{-8} \) S m\(^{-1}\).
Given:
K\(_{sp}\)(AgBr) = 4.9 \( \times 10^{-13} \) at 298K
\(\lambda^0_{Ag^+}\) = 6 \( \times 10^{-3} \) Sm\(^{2}\) mol\(^{-1}\)
\(\lambda^0_{Br^-}\) = 8 \( \times 10^{-3} \) Sm\(^{2}\) mol\(^{-1}\)
\(\lambda^0_{NO_3^-}\) = 7 \( \times 10^{-3} \) Sm\(^{2}\) mol\(^{-1}\)
Testosterone, which is a steroidal hormone, has the following structure.
The total number of asymmetric carbon atom/s in testosterone is ______
The spin only magnetic moment of [Mn(H\(_2\)O)\(_6\)]\(^{2+}\) complexes is _____ B.M. (Nearest integer)
Given: Atomic no. of Mn is 25
A metal M crystallizes into two lattices: face centred cubic (fcc) and body centred cubic (bcc) with unit cell edge length of 2.0 and 2.5 \(\AA\) respectively. The ratio of densities of lattices fcc to bcc for the metal M is ______. (Nearest integer)




Also Check:
JEE Main 2023 Chemistry Analysis Feb 1 Shift 2
JEE Main 2023 Paper Analysis for Chemistry paper scheduled on February 1 Shift 2 will be updated here after the conclusion of the exam. Candidates will be able to check subject-wise paper analysis for Chemistry paper scheduled on February 1 Shift 2 here along with the topics with the highest weightage.
| JEE Main 2023 Paper Analysis Feb 1 Shift 2 (After Exam) |
JEE Main 2023 Chemistry Question Paper Pattern
| Feature | Question Paper Pattern |
|---|---|
| Examination Mode | Computer-based Test |
| Exam Language | 13 languages (English, Hindi, Assamese, Bengali, Gujarati, Kannada, Malayalam, Marathi, Odia, Punjabi, Tamil, Telugu, and Urdu) |
| Exam Duration | 3 hours |
| Sectional Time Limit | None |
| Chemistry Marks | 100 marks |
| Total Number of Questions Asked | 20 MCQs + 10 Numerical Type Questions |
| Total Number of Questions to be Answered | 20 MCQs + 5 Numerical Type Questions |
| Marking Scheme | +4 for each correct answer |
| Negative Marking | -1 for each incorrect answer |
Also Check:
JEE Main 2022 Question Paper
JEE Main 2023 aspirants can practice and check their exam prep level by attempting the previous year question papers as well. The table below shows JEE Main 2022 Question Paper PDF for B.E./B.Tech to practice.





Comments