
Exams Prep Master
A polymer is a substance or material made up of a large number of repeating subunits that make up macromolecules. Because of their many properties, both manmade and natural polymers serve critical and pervasive roles in everyday life. Polymers range from ordinary synthetic plastics such as polystyrene to biopolymers such as DNA and proteins, which are required for biological structure and function. Natural and synthetic polymers are created by polymerizing a large number of small molecules known as monomers. In comparison to small molecule compounds, their huge molecular mass results in unusual physical features such as toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semi crystalline structures rather than crystals.
| Table of Content |
Key Terms: Polymers, Natural polymers, synthetic polymers, man-made polymers
What are polymers?
[Click Here for Sample Questions]
Polymers, by definition, are big molecules formed by chemically connecting a series of building pieces. Polymer is derived from the Greek words "many parts." Each of the components is referred to as a monomer. Consider a polymer to be a chain in which each link represents a monomer. Monomers can be basic — just one, two, or three atoms — or they can be complex ring-shaped formations with a dozen or more atoms.
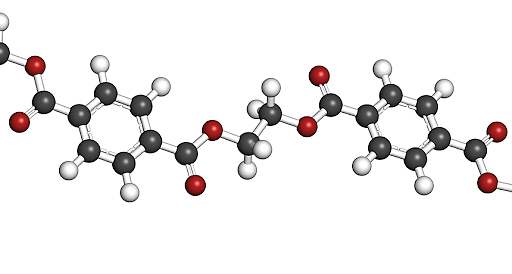
Each of the chain's links in an artificial polymer will often be similar to its neighbors. However, in proteins, DNA, and other natural polymers, chain links frequently differ from one another.
Also read: Polytetrafluoroethene (Teflon)
What are natural polymers?
[Click Here for Sample Questions]
Natural polymers are polymers that occur naturally in living systems and are made up of organic or inorganic subunits. These are found in nature and have a role in an organism's vital functions. Monosaccharides, amino acids, and nucleotides are the subunits of natural polymers. Polysaccharides, such as cellulose and starch, are sugar polymers; proteins, on the other hand, are a polymer of amino acids and other compounds. Natural polymers in the body perform a variety of roles, including providing structural integrity to cells, transferring genetic information across generations, functioning as a source of energy, and contributing to the biological system's different metabolic operations.
Other natural polymers have a high commercial value as well. In the industries, for example, plant exudate latex (rubber) is used. Rubber is an isoprene polymer. Carbon allotropes, such as graphite and diamond, are also natural inorganic polymers created via carbon catenation.
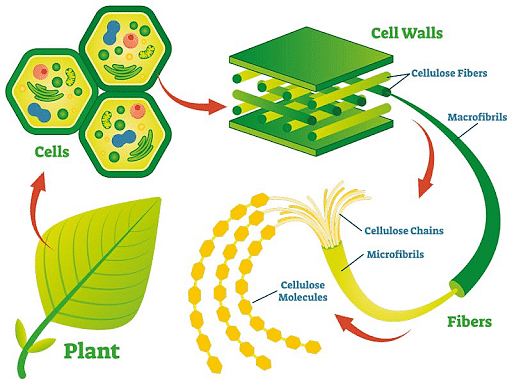
What are synthetic polymers?
[Click Here for Sample Questions]
Synthetic polymers are created as a by-product of certain chemical reactions in the laboratory. These macromolecules are not found in nature and must be manufactured. Long-chain polyethylene, which is made by adding ethylene subunits to a developing long chain, is an example of a synthetic polymer. Polyvinyl chloride is a thermolabile vinyl chloride polymer that is utilised on a daily basis.
Apart from these two types of polymers, there is a third type of polymer known as semi-synthetic polymers, which are made from naturally occurring cellulose. The phrase semi-synthetic polymer comes from the fact that the polymer's source is natural but it is eventually manufactured in a laboratory.

Also read: High-Density Polyethylene
Difference between Natural Polymers and Synthetic Polymers
[Click Here for Sample Questions]
| Characteristics | Natural Polymers | Synthetic Polymers |
|---|---|---|
| Definition | Natural polymers are macromolecules made up of small molecules called monomers that are bound together to produce larger macromolecules. | Chemical reactions are used to create synthetic polymers, which are molecules created artificially in laboratories and businesses. |
| Occurrence | Natural polymers occur naturally. | Synthetic polymers are created by chemical processes and do not occur naturally. |
| Processes | Natural polymers are produced from biological processes. | Synthetic polymers are produced via chemical processes. |
| Degradation | Natural polymers degrade easily by biological processes. | Synthetic polymers are rigid and do not degrade naturally by biological processes. |
| Handling | Natural polymers cannot easily be controlled as per need. | Under regulated conditions, synthetic polymers can be changed in laboratories. |
| Environmental impact | Natural polymers are environmentally friendly as these can be degraded via natural means. | Synthetic polymers are not environmentally friendly as they require long periods of time for degradation. |
| Examples | Some examples of natural polymers are proteins, nucleic acids, polysaccharides, etc. | Polystyrene, nylon, silicone, and other synthetic polymers are examples. |
Things to Remember
- A polymer is a substance or material made up of many repeating subunits that make up very big molecules, or macromolecules.
- Natural polymers are polymers that occur naturally in living systems and are made up of organic or inorganic subunits.
- Synthetic polymers are created as a by-product of certain chemical reactions in the laboratory. These macromolecules are not found in nature and must be manufactured.
- There is a third type of polymer known as semi-synthetic polymers, which are made from naturally occurring cellulose.
- Monosaccharides, amino acids, and nucleotides are the subunits of natural polymers.
- Monomers can be basic — just one, two, or three atoms — or they can be complex ring-shaped formations with a dozen or more atoms.
Sample questions
Ques. Is there a distinction between natural and synthetic resin? (2 marks)
Ans: They're made up of plant secretions and are soluble in a variety of organic liquids, but not water. Synthetic resins are a vast range of synthetic chemicals that resemble natural resins in appearance but differ chemically. Plastics and synthetic resins are difficult to tell apart.
Ques. Is it possible for natural polymers to degrade? (2 marks)
Ans: Biopolymers are natural biodegradable polymers. Polysaccharides, such as starch and cellulose, are the most well-known type of natural polymer. Proteins and other natural polymers can be used to create biodegradable products. These are the two most common biopolymer sources that are renewable.
Ques. What characteristics do synthetic polymers have? (2 marks)
Ans: High strength, or their property of being modulus to weight ratios (lightweight yet comparatively stiff and strong), toughness, resilience, corrosion resistance, lack of conductivity (heat and electrical), colour, transparency, processing, and low are the three main characteristics of synthetic polymers.
Ques. Why are synthetic polymers incapable of decomposition? (2 marks)
Ans: These polymers are made up of lengthy chai ns of carbon and hydrogen atoms. These polymers' interatomic bonding is extremely strong and adamant, making them resistant to bacteria attempting to break and digest them. In nature, all plastics and synthetic fibres are non-biodegradable.
Ques. What is the purpose of synthetic polymer? (2 marks)
Ans: Polyethylene is a polymer that is utilised in plastic bags and film wraps. Polyvinyl Chloride (PVC) is a plastic that is used for siding, pipelines, and floors. Polystyrene, a synthetic polymer, is used in cabinetry and packaging. Adhesives and latex paints contain polyvinyl acetate.
Ques. What are the benefits and drawbacks of utilising synthetic polymers? (2 marks)
Ans: They have always made life easier and more convenient in a variety of ways, but they do have a disadvantage. They do not come without drawbacks. The basic ingredients required to make them may become scarce, and getting rid of synthetic polymers is a difficult and time-consuming process.
Ques. What is the most durable polymer? (2 marks)
Ans: The most powerful polymer on the planet
The melting point of Zylon is above 1470 degrees Fahrenheit (780 degrees Celsius).
Zylon is 1.6 times stronger than Kevlar in terms of tensile strength.
Zylon is resistant to burning, needing circumstances of at least 68 percent oxygen.
Ques. Is rubber made of natural or synthetic materials? (1 mark)
Ans: Rubber is a natural commodity made by plants that may be found in many of the items we use on a daily basis.
Also Read:
| Related Links | ||
|---|---|---|
| Monomers | Saturated and Unsaturated Fats | Synthetic Polymers |
| Thermosetting Polymers | Copolymer | polymerisation |
Read more:





Comments