
Content Strategy Manager
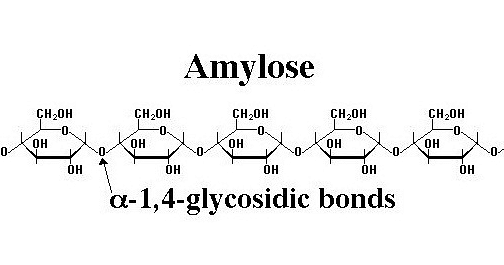
Amylose is a polysaccharide that is utilised as a functional biomaterial in a variety of industries. It is mostly a linear component made up of 100-10,000 glucose monomers connected together by 1,4 alpha bonds. Meyer discovered amylose in 1940, and his coworkers noticed that its characteristics differed from those of native maize starch. It can be found in algae and other lower plant types. It is a polymer composed of around 6000 glucose deposits with branches on 1 in every 24 glucose rings. Let’s have a closer look at the topic and discuss some important questions.
| Table of Content |
Key Terms: Amylose, Polysaccharide, Glucose, Monomers, Alpha bonds, Starch, Algae, Polymer, Glucose rings, Plastics, Paper pulp fibre bonding, Enzyme
Uses of Amylose
[Click Here for Sample Questions]
Amylose is used in permanent textile treatments, film production, plastics, and paper pulp fibre bonding.
- Higher amylose starches are used with food gum or an instant starch as a binder to provide a crisp coating while producing french fries, therefore reducing oil absorption.
- It's also used as a starch in food wrappers and sausage casings, as well as in pasta and bread crusts to provide equal cooking in the microwave.
Read More:
Physical Properties of Amylose
[Click Here for Sample Questions]
The physical properties of Amylose are tabulated below for your convenience,
| Category | Property |
|---|---|
| IUPAC name | (1→4)-α-D-Glucopyranan |
| Formula of amylose | (C6H10O5)n |
| Density | 1.25 g/mL |
| Molecular Weight or Molecular Mass | Variable |
| Boiling point | 627.7 ± 55.0 °C at 760 mmHg |
| Bond type | α glycosidic bonds |
| Chemical formula | Variable since it is a polymer |
| Odour | Unpleasant |
| Appearance | White powder |
| Surface tension | 74.4 ± 5.0 dyne/cm |
| Solubility | Insoluble in water |
Chemical Properties of Amylose
[Click Here for Sample Questions]
Amylose, in conjunction with iodine, forms a unique blue colour complex. This analysis is given via chromatography's high-performance size-exclusion technique, as well as a variety of other techniques.
Amylose molecules create numerous hydrogen bonds, making them less vulnerable to enzyme destruction.
General Properties of Amylose
[Click Here for Sample Questions]
The general properties of Amylose are tabulated below,
| Category | Property |
|---|---|
| (C6H10O5)n | Amylose |
| Density | 1.25 g/mL |
| Molecular Weight/ Molar Mass | Variable |
| Boiling Point | 627.7±55.0 °C at 760 mmHg |
| Bond Type | α glycosidic bonds |
| Chemical Formula | Variable because it’s a polymer |
Functions of Amylose
[Click Here for Sample Questions]
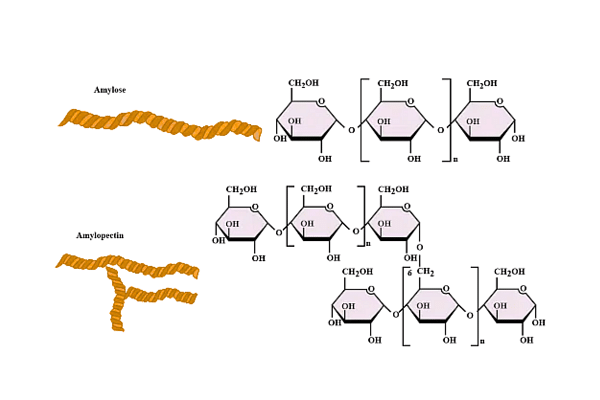
Amylose is essential for plant energy storage. However, it is less easily absorbed than amylopectin. This is due to its helical shape, which allows it to take up less space than amylopectin. As a result, it is the starch of choice for plant storage. It accounts for around 30% of total stored starch in plants, however the percentage varies by species and variation.
The digestive enzyme -amylase degrades the starch molecule into maltose and maltotriose, which are used as energy sources.
Amylose is also used as a thickening, emulsion stabilizers, water binder, and gelling agent in both industrial and culinary applications.
Amylose may be found in a variety of foods that we consume on a daily basis. These are listed below.
- Potatoes with rice
- Beans and legumes
- Fruits and vegetables that are starchy
- Grain (whole)
Functions of Amylose
Things to Remember
- Amylose is a polysaccharide that is utilised as a functional biomaterial in a variety of industries. It is mostly a linear component made up of 100-10,000 glucose monomers connected together by 1,4 alpha bonds.
- Amylose is used in permanent textile treatments, film production, plastics, and paper pulp fibre bonding.
- Amylose molecules create numerous hydrogen bonds, making them less vulnerable to enzyme destruction.
- Amylose is essential for plant energy storage. However, it is less easily absorbed than amylopectin. This is due to its helical shape, which allows it to take up less space than amylopectin. As a result, it is the starch of choice for plant storage.
Also Read:
Sample Questions
Ques: What Is the Distinction Between Amylose and Amylopectin? (4 marks)
Ans: The distinction between amylose and amylopectin is as follows.
- Amylose is a polysaccharide composed of many D-glucose molecules. The 1,4-glycosidic linkages connect them. Because of the presence of amylose in the starch, when iodine is added to it, the colour tends to shift to a deeper blue or black. Amylose is water-soluble and may be readily digested into glucose units using the enzymes -amylase and -amylase.
- Amylopectin is a polymer composed of numerous D-glucose molecules. Amylopectin accounts for 80% of the amylopectin found in starch. The amylopectin molecules are held together by -1,4-glycosidic and -1,6-glycosidic linkages. Because of the presence of amylopectin, when iodine is added to starch, it takes on a reddish-brown colour. It also dissolves easily in hot water. When chilled, it solidifies as a starch gel or paste.
Ques: What exactly is Amylase? (3 marks)
Ans: An amylase is an enzyme that catalyses the breakdown of starch into sugars. Amylase is a component of human and many other mammals' saliva, where it helps to initiate the chemical process of digestion. Foods with a high starch content but a low sugar content, such as rice and potatoes, may have a somewhat sweet taste when chewed because amylase degrades part of the starch into sugar. Our pancreas and salivary glands produce alpha-amylase, which hydrolyzes dietary starch and converts it to disaccharides and trisaccharides. Several additional enzymes subsequently turn them into glucose, which our bodies use for energy.
Amylase is also produced by plants and certain microorganisms.
Ques: What foods are high in amylose? (2 marks)
Ans: Amylose may be found in the following foods:
- legumes and beans
- Grain (whole)
- Fruits and vegetables that are starchy
- Potatoes with rice
Ques: What roles do amylose and amylopectin play? (3 marks)
Ans: Amylose, Amylopectin, Cellulose, and Glycogen's primary functions include energy storage and food reserve. Starch is an excellent example of this since it includes 10-20% amylose and 80-90% amylopectin. Starch is the most important carbohydrate eaten by humans and the primary energy source for green plants.
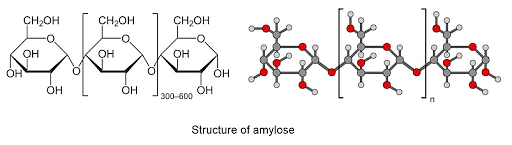
Ques: What is the structure of amylose? (3 marks)
Ans:
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:





Comments