
Content Curator
A polymer derived from the polymerisation of more than one species of monomers is called a copolymer. Such a polymerization reaction is called copolymerization. Copolymers are of different types and different processes of polymerization can be used to form copolymers. Examples include Butadiene styrene copolymer, nitrile rubber, styrene isoprene styrene etc. When two monomer species are involved the resultant copolymer is called a bipolymer.
| Table of Content |
Keyterms: Polymer, Monomer, Polymerisation, Bipolymer, Nitrile rubber, Styrene isoprene styrene, Butadiene styrene copolymer, Copolymerization
Read More: Polythene
What Is Copolymerisation?
[Click Here for Sample Questions]
There are basically two types of polymerisation reaction:
- Step-growth Polymerisation
- Chain-growth Polymerisation
Copolymerisation can be done by both methods. As we already know copolymerisation is a polymerisation reaction (step-growth or chain growth) where multiple species of monomers are polymerised together.
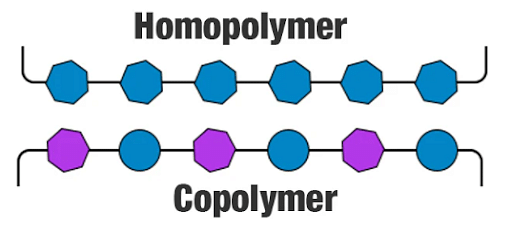
Copolymer
As it is clear from the picture above that a copolymer is a mixture of different species of monomers. In the above case, it is a bipolymer copolymer. Copolymerisation infuses better properties than what is present in a homopolymer. It is just like a hybrid version of a polymer where the best properties of different species of monomers get together to give a better product.
Consider the following copolymerisation reaction below.

Copolymerisation Reaction
The above copolymerisation of 1,3-Butadiene and styrene gives the Butadiene-Styrene copolymer which is quite tough and a good substitute for natural rubber and is used to manufacture auto tyres, floor tiles, cable insulations, footwear components etc. Similarly, Nylon 6-6 is a copolymer of hexamethylene diamine and adipic acid.

Nylon 6,6
Read More: Polypropylene
Types Of Copolymers
[Click Here for Previous Year Questions]
A copolymer has at least two types of monomers, so on the basis of how these monomers are arranged in the chain, the copolymers are classified into certain types. The basic two types are:
- Linear Copolymers
- Branched Copolymers
Again the linear copolymers contain a single main chain and are sub-classified as alternating, statistical and block copolymers. Branched copolymers are sub-classified into grafted and star-shaped copolymers.

Types of Copolymers
Alternating Copolymers
These copolymers have a regular, repetitive and alternating arrangement of two monomeric species. Example: Nylon 6,6. The general formula for the monomers A and B can be given as
-(-A-B-)n- or -A-B-A-B-A-B-A-B-A-B-

Alternating Copolymers
Statistical Copolymers
When the sequence of monomeric units follows a certain statistical rule, it is called a statistical copolymer. Moreover, if the probability of finding a given monomer at a particular point in a chain is equal to its mole fraction in the chain then it can be called a random polymer.

Statistical Copolymers
Example: Rubbers made from styrene-butadiene copolymers, resins from styrene-acrylic etc.
Block Copolymers
When two or more homopolymer chains are joined by covalent bonds the resultant polymeric chain is called a Block copolymer. The intermediate unit where they join is called a junction block. They can be diblock or triblock copolymers. Examples Acrylonitrile butadiene styrene (SBS rubber)

Block Polymers
Grafted Copolymer
Special branched copolymers in which side chains are structurally different from the main chain. Here, the main chain can be covalently bonded to one or more side chains.

Grafted Copolymer
Example: Polystyrene chains can be grafted into polybutadiene.
Star Copolymer
Several polymeric chains can be connected into the same central core unit.

Star Copolymer
Brush and comb copolymers are other kinds of branched copolymers.
Read More:
Applications Of Copolymers
[Click Here for Sample Questions]
- Copolymers of hygroscopic and hydrophilic polymers are frequently used in cosmetics, drug delivery and self-polishing paint applications.
- Acrylic copolymers are used for nail polish, eyeliners, lipsticks, sunscreens, skin lotions, etc.
- Graft polymers can be used as membranes to separate gas and liquids, they can also be used in emulsifiers and biomedical applications.
- Spandex used as synthetic fibre is a block copolymer, other block polymers like polyester and polyamide TPEs have applications in hose tubing, sports goods and automotive components.
- Nylon -6-6- is frequently used in textiles, carpeting, as an engineering material in bearings and gears due to its self-lubricating properties.
Read More: Polyamides
Things To Remember
- Copolymers have more than one monomeric unit/species joined together in either linear or branched fashion.
- Different classes of copolymers include block,graft,star,brush,comb,periodic,statistical and alternating copolymers.
- Copolymers have a vast range of applications in many fields.nylon -6-6- ,spandex,High strength polystyrene, SBS rubber,etc all have various uses .This wide range of applications is possible due to the highly modified chemical properties of copolymers which make them usable.
- Block copolymers have covalent bonds between two monomeric chains/blocks.
Previous Year Questions
- Which one of the following is amorphous… [VITEEE 2006]
- Nylon 66 is formed by… [UPSEE 2019]
- In Buna-S, the symbol ′Bu’ stands… [KCET 2013]
- The monomer used in Novolac, a polymer used in paints… [KCET 2017]
- The monomer of polystyrene is… [KEAM]
- Among the following, the branched-chain polymer is… [KCET 2018]
- Cis-1, 4-polyisoprene is called… [KCET 2019]
- The monomers of Buna… [KCET 2008]
- Poly-β-hydroxybutyrate-co-β hydroxyvalerate… [JEE 2019]
- The monomer used to produce orlon is…..[WBJEE 2009]
- Which of the following is a natural polymer?...[NEET 2020]
- Which of the following is not a condensation polymer ?
- Bakelite is obtained from phenol by reaction with….[AIEEE 2007]
- Which polymers occur naturally ?...[PMET 2009]
- Bakelite is obtained from phenol by reacting with..
- Which of the following is a polyamide ?...[AIEEE 2005]
Sample Questions
Ques.Differentiate between copolymers and homopolymers. (2 Marks)
Ans. Copolymers: Two or more monomeric species are involved. Example copolymerisation of butadiene styrene.
Homopolymers: Single monomeric species are present. Example homopolymerisation of polystyrene.
Ques.Write short notes on the following: (3 Marks)
(1) Copolymer
(2) Synthetic rubber
Ans.
- Copolymer: Copolymer is formed when more than one species of an unsaturated monomer combines to polymerize. The process of forming such polymers is called copolymerization. Example: rubber, spandex, PEVA, etc. They can be formed by either of the two methods: chain-growth polymerization and step-by-step growth polymerization.
- Synthetic rubber: Synthetic Rubber is a more flexible vulcanized rubber-like polymer that is better to use than natural rubber because of its ability to stretch far more than a natural one. Synthetic polymers can be formed by the intervention of copolymerization and can either be homopolymer or copolymer. Example: Neoprene, Buna-S, Thiokol, Buna-N, etc.
Ques.Which is a copolymer out of the following? (2 Marks)
(i) Polytetrafluoroethylene
(ii) Polyvinyl Chloride
(iii) Polyethylene
(iv) Natural Rubber
(v) Nylon 6,6
Ans. Option (v), Nylon 6,6 is a copolymer out of all. It is because as per the definition of copolymer, they have to be formed out of more than one species of monomers. But all the others are formed out of one species of monomer, except Nylon 6,6.
Ques. Explain the process of Addition Polymerisation. (2 Marks)
Ans. Addition polymerization, one of the ways to form copolymers, incorporates the formation of polymers without any intermediate product (kind of like SN2 reaction in haloalkanes and arenes). They take place generally in the presence of a catalyst. Also known as chain-growth polymerization reaction, there is the direct addition of the monomeric subunits.

Example: Using ethylene molecules to make polyethylene when the double bonds in ethylene break to give rise to single C-C bonds.
Ques. What is the Condensation reaction? Give an example. (2 marks)
Ans. Condensation polymerization reaction, also known as step-by-step growth polymerization, that is involved in either method of copolymerization, happens between two different monomeric species (either bi-functional or tri-functional). Substances like water, alcohol, and ammonia are eliminated in the final product due to repeated condensation reactions.
Example: Nylon 6,6 which is obtained from hexamethylene diamine is condensed repeatedly with adipic acid. The pressure and temperature required for the condition to succeed are quite high.
Ques. Write 3 applications of copolymers giving examples of copolymers. (2 marks)
Ans.
- Spandex used as synthetic fibre is a block copolymer, other block polymers like polyester and polyamide TPEs have applications in hose tubing, sports goods and automotive components.
- Nylon -6-6- is frequently used in textiles, carpeting, as an engineering material in bearings and gears due to its self-lubricating properties.
- Graft polymers can be used as membranes to separate gas and liquids, they can also be used in emulsifiers and biomedical applications.
Ques. What are the different types of linear copolymers? Explain them in brief. (5 Marks)
Ans. Periodic, Gradient, Block, Statistical and Alternating copolymers.
Alternating Copolymers: These copolymers have a regular, repetitive and alternating arrangement of two monomeric species, eg A and B.The general formula can be given as -(-A-B-)n- or -A-B-A-B-A-B-A-B-A-B-.
Examples: Nylon -6-6-
Statistical Copolymers: When the sequence of monomeric units follows a certain statistical rule it is called a statistical copolymer. Moreover, if the probability of finding a given monomer at a particular point in a chain is equal to its mole fraction in the chain then it can be called a random polymer.
Examples: Rubbers made from styrene-butadiene copolymers, resins from styrene acrylic etc.
Block Copolymers: When two or more homopolymer chains are joined by covalent bonds the resultant polymeric chain is called a Block copolymer.The intermediate unit where they join is called a junction block.They can be diblock or triblock copolymers.
Examples: Acrylonitrile butadiene styrene (SBS rubber).
Ques. How do you explain the functionality of a monomer? (2 marks)
Ans. The functionality of a monomer implies the number of bonding sites present in it. For example, monomers like propene, styrene, acrylonitrile have the functionality of one which means that they have one bonding site.
Monomers such as ethylene glycol, hexamethylenediamine, adipic acid have the functionality of two which means that they have two bonding sites.
Ques. What are natural and synthetic polymers? Give two examples of each. (3 Marks)
Ans.
- Natural polymers: The polymers which occur in nature mostly in plants and animals are called natural polymers. A few common examples are starch, cellulose, proteins, rubber nucleic acids, etc. Among them, starch and cellulose are the polymers of glucose molecules. Proteins are formed from amino acids which may be linked in different ways. These have been discussed in detail in unit 15 on biomolecules. Natural rubber is yet another useful polymer which is obtained from the latex of the rubber tree. The monomer units are of the unsaturated hydrocarbon 2-methyl-i, 3-butadiene, also called isoprene. Examples of natural polymers: Natural rubber, cellulose, nucleic acids, proteins etc.
- Synthetic polymers: The polymers which are prepared in the laboratory are called synthetic polymers. These are also called man-made polymers and have been developed in the present century to meet the ever-increasing demand of modern civilization. Examples of synthetic polymers: Dacron (or terylene), Bakelite, PVC, Nylon-66, Nylon-6 etc.
Ques. What are polymers? (2 marks)
Ans. Polymers are high molecular mass substances (103 — 107u) consisting of a very large number of simple repeating structural units joined together through covalent bonds in a linear fashion. They are also called macromolecules. Ex: polythene, nylon 6,6, bakelite, rubber, etc.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check Out:





Comments