Jasmine Grover Content Strategy Manager
Content Strategy Manager
Biomolecules are important organic molecules that fuse together to form complex organic compounds and govern the metabolism of living organisms. Proteins, carbohydrates, nucleic acids, enzymes and lipids form the living system of biomolecules.
- Carbohydrates are essential polyhydroxy aldehydes or ketones, forming the building blocks of energy in living organisms.
- They encompass monosaccharides, disaccharides, and polysaccharides, with glucose being a vital energy source derived from starch digestion.
- Proteins are composed of amino acids linked by peptide bonds.
- Denaturation of proteins, disrupting protein structure, occurs with changes in pH or temperature.
- Enzymes, primarily proteins, accelerate biochemical reactions with specificity.
- Vitamins are crucial for health and are classified into fat-soluble (A, D, E, K) and water-soluble (B, C) types.
- Nucleic acids, polymers of nucleotides, store genetic information as DNA and RNA.
- DNA, with deoxyribose sugar, encodes hereditary data, while RNA, containing ribose, aids in protein synthesis via mRNA, rRNA, and tRNA.
According to the CBSE Class 12 Chemistry Syllabus 2023-24, this chapter has been renumbered as Chapter 10. Biomolecules Class 12 Notes are important for revision as the chapter holds a weightage of 7 marks in the Class 12 Chemistry examination 2024.
Read More:
| Biomolecules Preparation Resources | |
|---|---|
| Biomolecules | NCERT Solutions For Class 12 Chemistry Chapter 14 Biomolecules |
| MCQs on Biomolecules | Biomolecules Important Questions |
Biomolecules Notes
Carbohydrates
- Carbohydrates are organic compounds primarily produced by plants.
- Examples include cane sugar, glucose, and starch.
- They are characterized by a general formula, Cx(H2O)y, but not all compounds fitting this formula are classified as carbohydrates.
- Carbohydrates are defined as optically active polyhydroxy aldehydes or ketones, or compounds that produce such units upon hydrolysis.
Classification of Carbohydrates
Carbohydrates are classified into three groups based on their behavior on hydrolysis.
Monosaccharides
Monosaccharides cannot be further hydrolyzed to simpler units. Examples include glucose, fructose, and ribose.
- Monosaccharides are classified into aldoses and ketoses based on their functional groups and the number of carbon atoms they contain.
Glucose
- Glucose is obtained from sucrose through hydrolysis with dilute acids or from starch via hydrolysis with dilute sulfuric acid under pressure and elevated temperature.
- Its structure comprises six carbon atoms arranged in a straight chain, featuring functional groups such as aldehyde and hydroxyl.
- Glucose, an aldohexose, is also known as dextrose and exists as the monomer of larger carbohydrates like starch and cellulose.
- It forms two cyclic hemiacetal structures (a and b forms) known as anomers, with the configuration determined by the hydroxyl group at C1.
- The cyclic structure is depicted using the Haworth projection, representing the pyranose structure.
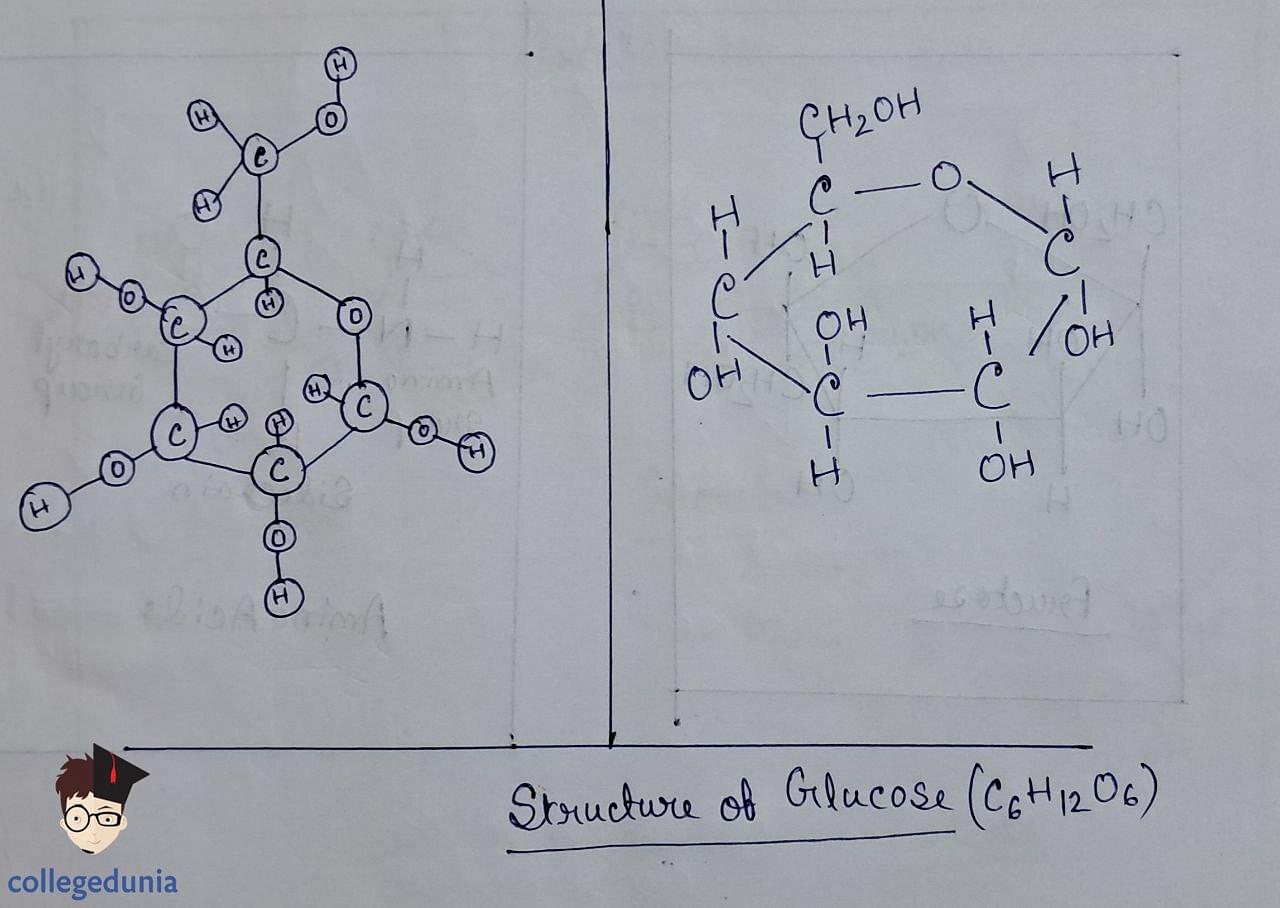
Glucose
Fructose
- Fructose, a ketohexose, is derived from sucrose hydrolysis and is naturally present in fruits, honey, and vegetables, serving as a natural sweetener.
- With the molecular formula C6H12O6, fructose contains a ketonic functional group at carbon number 2 and belongs to the D-series, exhibiting laevorotatory properties.
- Fructose exists in both open-chain and cyclic forms, with the cyclic form forming a five-membered ring called furanose upon the addition of a hydroxyl group at C5.
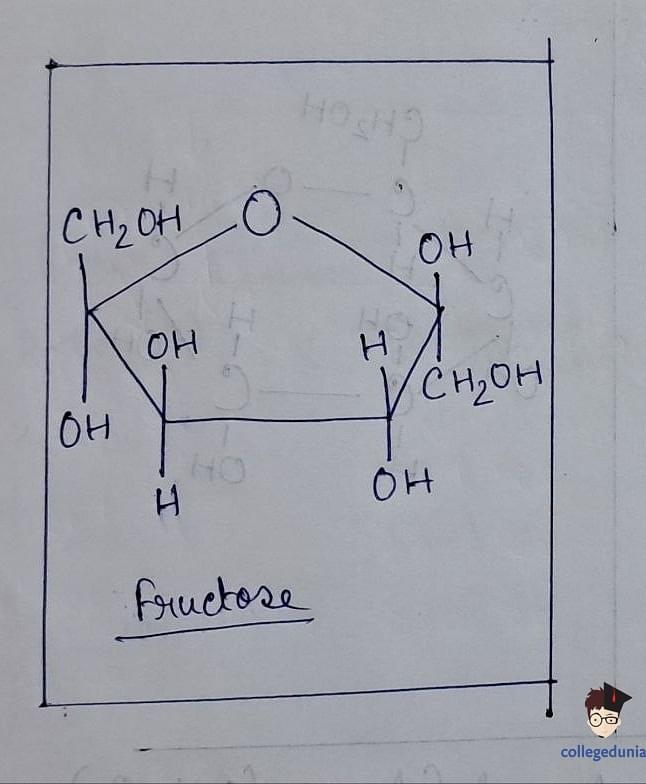
Fructose
Disaccharides
- Disaccharides form via glycosidic linkage between two monosaccharide units, resulting from the loss of water.
- Sucrose is a non-reducing sugar composed of glucose and fructose, while maltose and lactose are reducing sugars with free functional groups.
- Sucrose yields invert sugar upon hydrolysis and exhibits laevorotatory properties.
- Maltose comprises two glucose units linked by a glycosidic linkage between C1 and C4.
- Lactose, found in milk, consists of galactose and glucose units linked between C1 and C4, and is a reducing sugar.
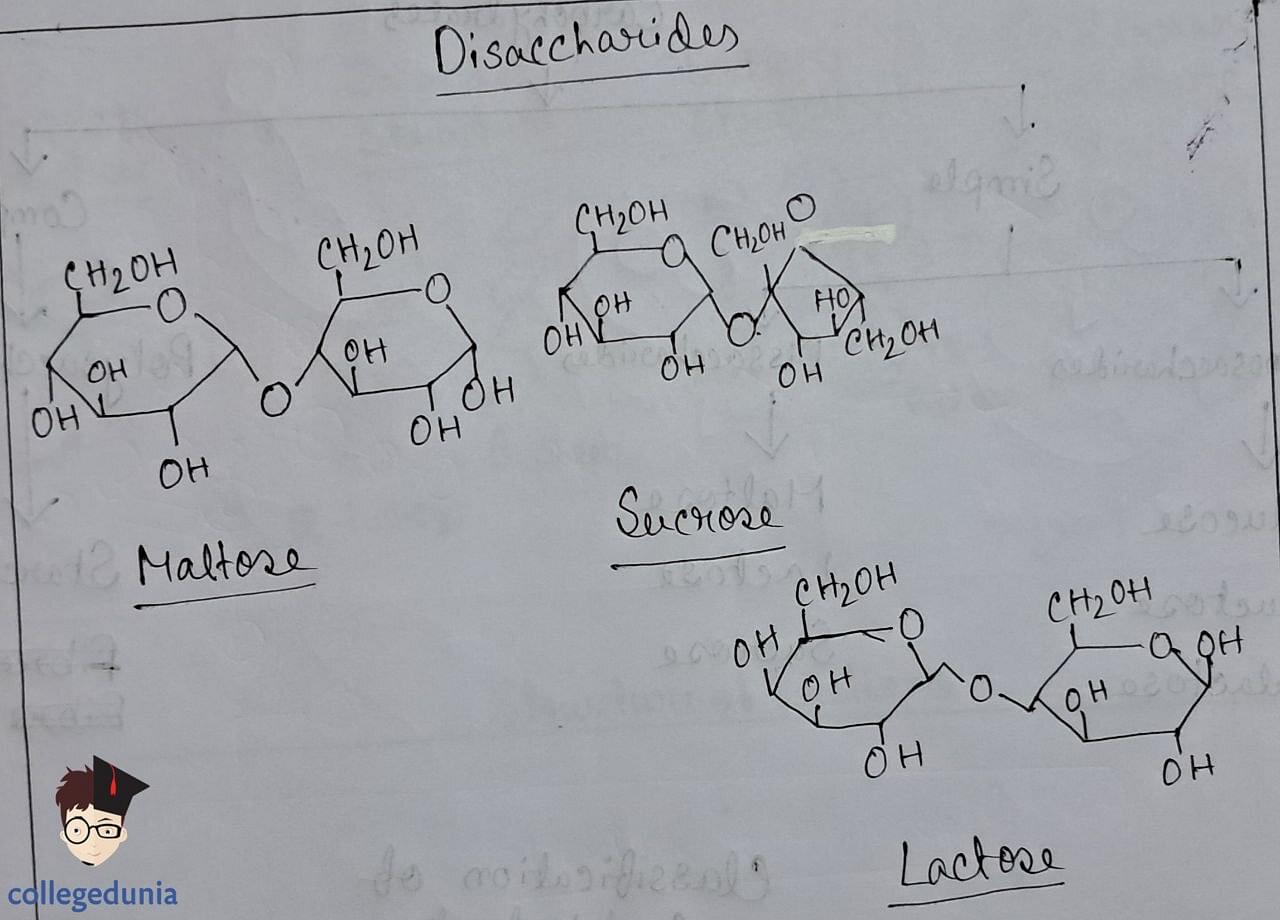
Disaccharides
Polysaccharides
- Polysaccharides, composed of multiple monosaccharide units, function as vital food storage and structural materials in living organisms.
- Starch, a primary plant storage polysaccharide, contains amylose and amylopectin.
- Amylose is water-soluble and unbranched, while amylopectin is insoluble and branched.
- Cellulose, predominant in plant cell walls, comprises straight chains of β-D-glucose units linked by glycosidic bonds between C1 and C4.
- Glycogen, akin to animal starch, is stored in the liver, muscles, and brain, characterized by high branching and serving as a glucose reserve.
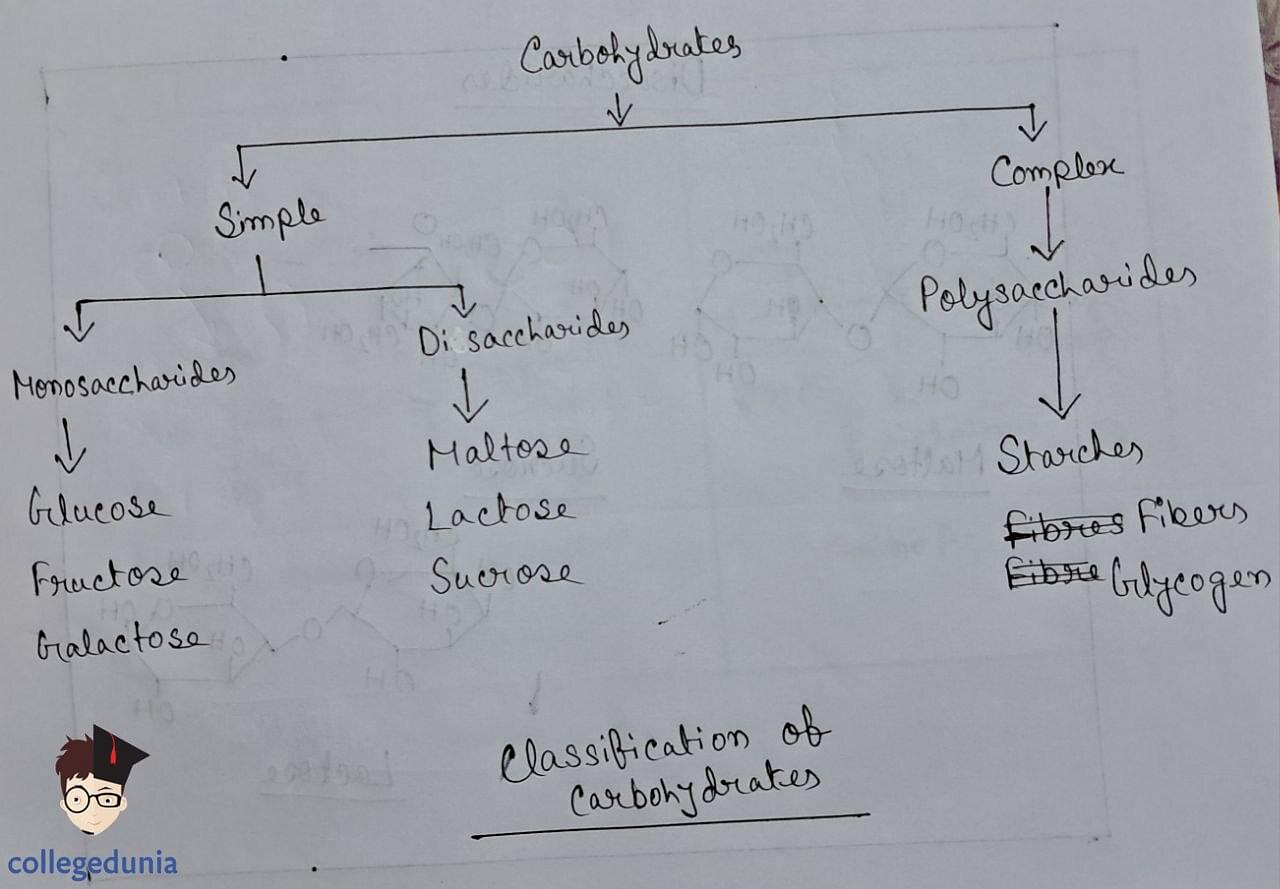
Classification of Carbohydrates
Importance of carbohydrates
- Carbohydrates provide a significant portion of the diet of plants and animals, serving as vital energy sources.
- Starch in plants and glycogen in animals function as storage forms of carbohydrates, offering easily accessible energy reserves.
- Cellulose constitutes the cell walls of bacteria and plants, with industrial applications including wood and cotton fiber products.
- Carbohydrates are crucial raw materials in industries like textiles, paper, lacquers, and breweries.
- Aldopentoses like D-ribose and 2-deoxy-D-ribose are key components of nucleic acids, underscoring their importance in biological systems.
Proteins
- Proteins are abundant biomolecules in living organisms, vital for biological processes.
- Classification of proteins is done based on the shape, constituents and nature of molecules.
- Composed of α-amino acids, proteins are sourced from foods like milk, cheese, pulses, peanuts, fish, and meat.
- They contribute to growth, maintenance, and bodily functions.
Amino Acids
- Amino acids contain amino (–NH2) and carboxyl (–COOH) functional groups.
- They are categorized as acidic, basic, or neutral depending on the relative number of amino and carboxyl groups in their molecule.
- Essential amino acids must be obtained through diet, while non-essential amino acids can be synthesized in the body.
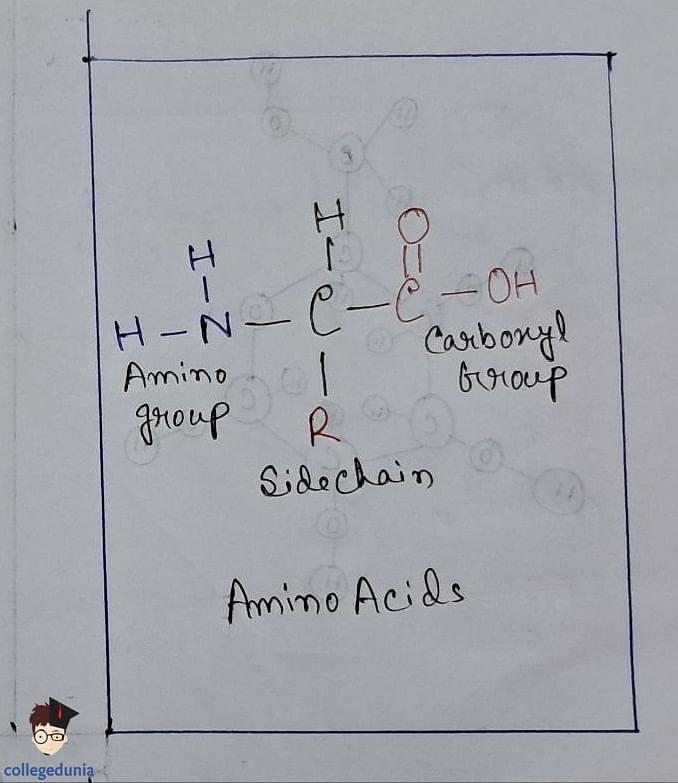
Amino Acids
Structure of proteins
Proteins are composed of amino acids linked by peptide bonds, formed by the condensation of amino and carboxyl groups.
- Dipeptides, tripeptides, and polypeptides result from the combination of amino acids, with proteins containing more than a hundred amino acid residues.
- Proteins are classified into fibrous and globular types based on their molecular shape.
- Fibrous proteins have parallel polypeptide chains held by hydrogen and disulfide bonds, while globular proteins have coiled polypeptide chains, giving them a spherical shape.
- Protein structure is analyzed at four levels -
- Primary (amino acid sequence)
- Secondary (a-helix, b-pleated sheet)
- Tertiary (overall folding)
- Quaternary (spatial arrangement of subunits).
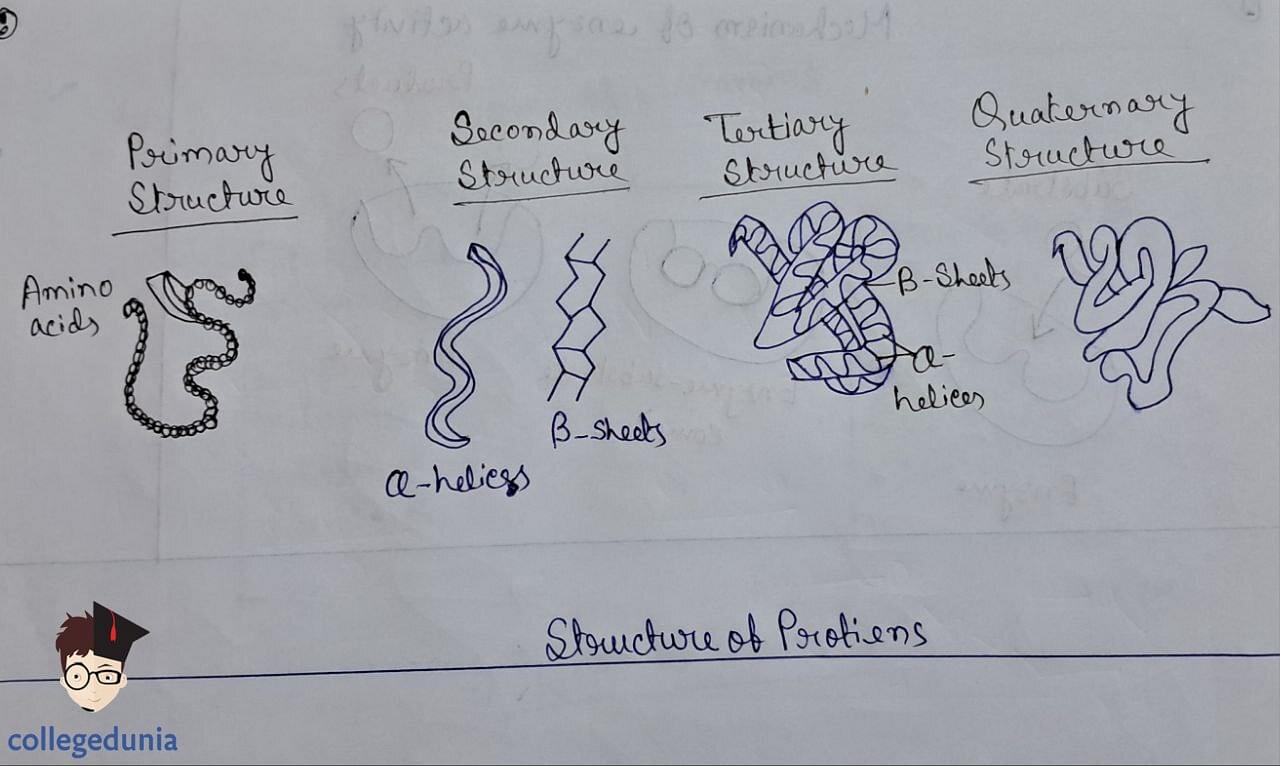
Structure of Proteins
Denaturation of Proteins
- Proteins in biological systems with unique three-dimensional structures and biological activity are termed native proteins.
- Denaturation occurs when native proteins undergo physical or chemical changes such as temperature fluctuations or pH alterations.
- It disrupts hydrogen bonds, causing globules to unfold and helices to uncoil, resulting in the loss of the protein's biological activity.
- During denaturation, secondary and tertiary protein structures are destroyed, while the primary structure remains intact.
- Common examples include the coagulation of egg white upon boiling and the curdling of milk due to lactic acid formation by bacteria.
Enzymes
- Enzymes facilitate chemical reactions vital for life, such as food digestion and energy production.
- It acts as biocatalysts and are mostly globular proteins, enabling reactions to occur under mild conditions in the body.
- The compounds are highly specific for particular reactions and substrates, often named after the compounds they act upon or the reactions they catalyze.
- It reduces the activation energy required for reactions, similar to chemical catalysts, allowing reactions to proceed efficiently.
- Enzymes typically end with "-ase" and may be named based on the reaction they catalyze or the substrate they act upon.
- For instance, maltase catalyzes the hydrolysis of maltose to glucose.

Enzymes
Vitamins
- Vitamins are essential organic compounds required in small amounts in the diet to maintain optimum growth and health.
- Most of these compounds cannot be synthesized in the human body but are abundant in various foods.
- Plants can synthesize most vitamins.
- They are classified into fat-soluble (A, D, E, K) and water-soluble (B complex, C) groups based on their solubility properties.
- While these compounds are essential, excess intake can be harmful.
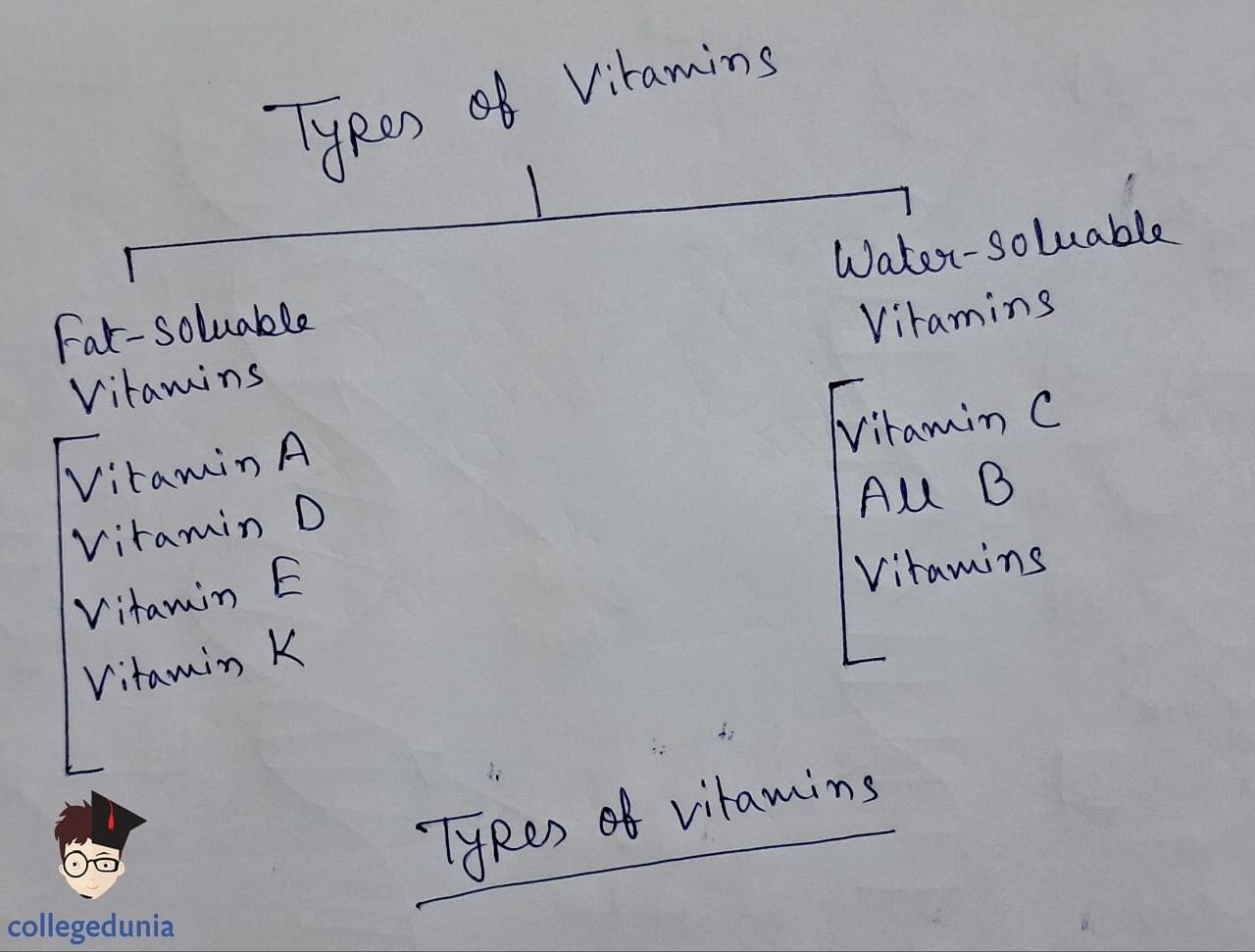
Types of vitamins
Nucleic acids
- Nucleic acids are polymers of nucleotides, comprising a-pentose sugar, phosphoric acid, and nitrogen-containing bases.
- In the nucleus of living cells, characteristics are transmitted via chromosomes, composed of proteins and nucleic acids.
- DNA and RNA are the two main types of nucleic acids.
- They are responsible for the transmission of characteristics from one generation to another.
- Nucleotides are joined by phosphodiester linkages between the 5' and 3' carbon atoms of the pentose sugar.
- DNA forms a double-stranded helix held together by hydrogen bonds between specific base pairs.
- RNA, categorized into messenger RNA (m-RNA), ribosomal RNA (r-RNA), and transfer RNA (t-RNA), plays distinct roles in protein synthesis.
- DNA serves as the repository of genetic information and is responsible for species identity maintenance.
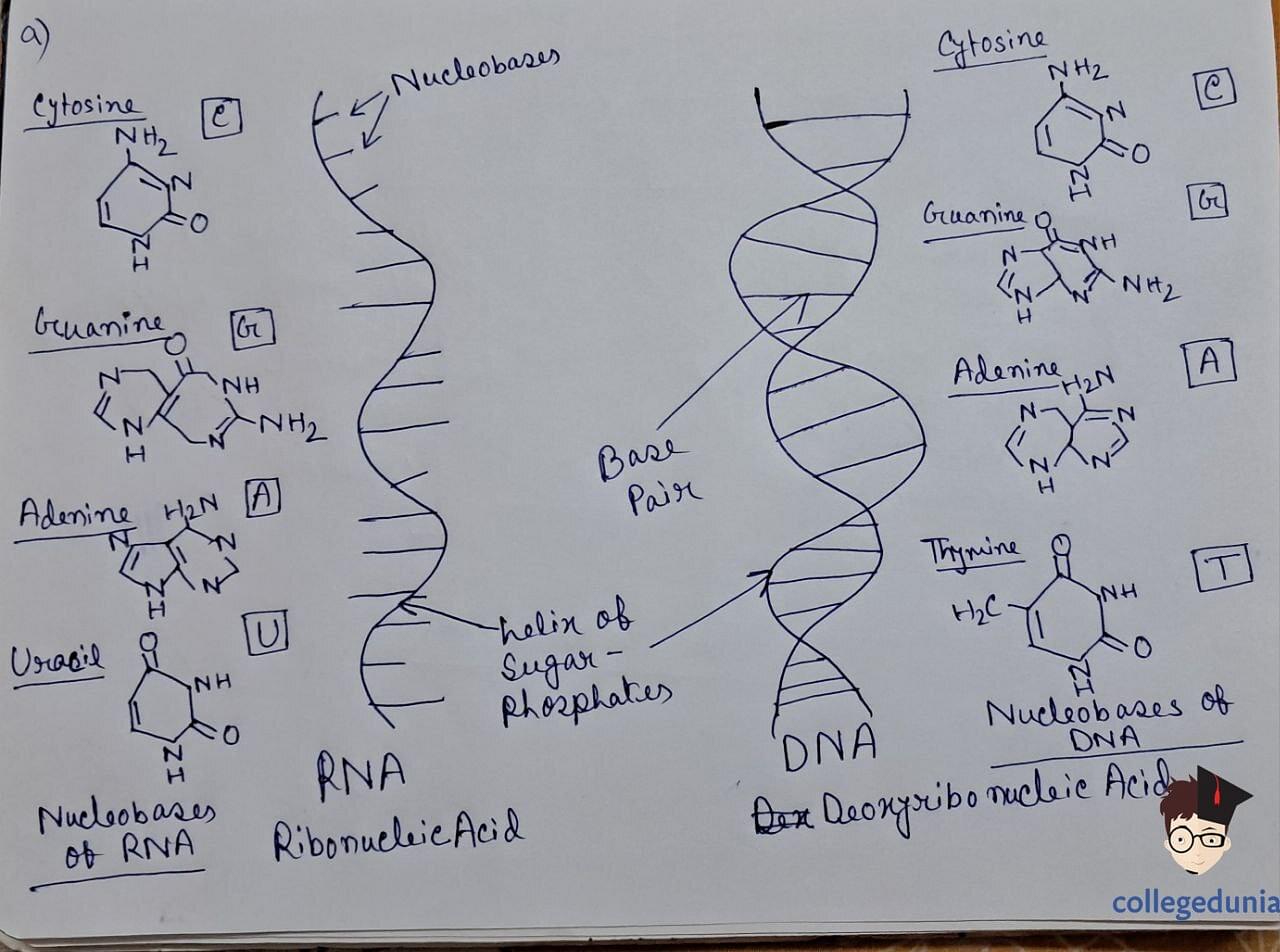
Nucleic Acid
Hormones
- Hormones, produced by endocrine glands, act as intracellular messengers in the body.
- They include steroids (e.g., estrogens, androgens), polypeptides (e.g., insulin, endorphins), and amino acid derivatives (e.g., epinephrine, norepinephrine).
- Peptide and steroid hormones are two types of hormones.
- They maintain biological balance, such as insulin regulating blood glucose levels, and glucagon increasing glucose levels.
- Thyroxine, produced by the thyroid gland, regulates metabolism, with abnormal levels leading to conditions like hypothyroidism or hyperthyroidism.
- Steroid hormones from adrenal cortex and gonads (testes, ovaries) regulate various body functions, such as carbohydrate metabolism and secondary sex characteristics.
- An imbalance in hormone levels can lead to disorders like Addison's disease (adrenal cortex dysfunction) or menstrual irregularities.
There are Some important List Of Top Chemistry Questions On Biomolecules Asked In CBSE CLASS XII



Comments