Arpita Srivastava Content Writer
Content Writer
Pyranose is used for mentioning saccharides that have a six-membered ring-type chemical structure of five carbon atoms and one oxygen atom, and sometimes with other carbons external to the ring. Oxygen heterocyclic pyran has inspired Pyranose’s name. There are no double bonds present in the pyranose rings. Pyranoside is a pyranose whose anomeric OH at C(l) is converted into an OR group. Tetrahydropyran is the ring system used in Pyranose Ring Structures.
| Table of Content |
Keyterms: Pyranose, Carbon, Atoms, Oxygen, Tetrahydropyran, Pyranose Ring, Glucose, Aldehyde group, Heterocyclic compound
Pyranose Formation
[Click Here for Sample Questions]
Glucose’s pyranose ring is formed when a hydroxyl group at the 5th carbon position (C-5) of a glucose molecule is reacted with an aldehyde group attached to the 1st carbon position (C-1) of the same glucose molecule. Hence, intramolecular hemiacetal molecules are formed. The name pyranose is derived from a heterocyclic compound called pyranose, which contains an oxygen atom with five carbon atoms that form a cyclic structure.

In this pyranose glucose ring structure, there are 5 carbon atoms and 1 oxygen atom, forming a 6-membered ring structure. There are a total of 38 pyranose ring formations, 2 in the shape of a chair, 6 in the shape of a boat, 6 in the shape of a sloping boat, 12 in the shape of a half chair, and 12 in the shape of an envelope.
If the reaction is between C-4 hydroxyl and aldehyde, furanose is formed. The pyranose type is thermodynamically more stable than the furanose type. This can be seen from these two cyclic distributions in solution.

Also Read:
| Related Articles | ||
|---|---|---|
| Monomers | Cellulose | Zwitterion |
| Maltose | Sucrose | Chemical Reaction |
Pyranose: Origins and History
[Click Here for Sample Questions]
The structure of D-aldohexose was first determined by Hermann Emil Fischer and he was granted the Nobel Prize in Chemistry in the year 1902. However, the linear free aldehyde structure proposed by Fisher represents a very small proportion of the form of hexose sugar in the solution.
Edmund Hirst and Clifford Purves of Walter Haworth's research group have determined that it is preferable for hexose sugars to form a 6-membered ring or a pyranose ring. The Haworth projection is when he drew the ring as a flat hexagon with groups below and above the ring plane.
The conformation of the pyranose ring was further refined when Sponsler and Dore (1926) realized that the mathematical treatment of the 6-membered ring of the saxophone could be applied to the X-ray structure of cellulose. The pyranose ring is puckered so that all carbon atoms in the ring could have a nearly ideal tetrahedral structure.
Conformations of Pyranose
[Click Here for Sample Questions]
The puckering of Pyranose leads to a total of 38 different basic pyranose foams with 2 chairs, 6 boats, 6 sloping boats, 12 half chairs and 12 envelopes.
These fits can interact with each other. There can be significant barriers to interconversion as each form has different relative energies. The energies of these conformations can be calculated from quantum mechanics. Examples of possible interconversions of glucopyranose are shown.

The conformation of the pyranose ring is similar to that of the cyclohexane ring. However, certain nomenclatures for pyranose include a reference to ring oxygen, and the presence of hydroxyl in the ring has a different effect on the conformational priority. There are also certain stereochemical and conformational effects of pyranose rings.
Pyranose: Nomenclature
[Click Here for Sample Questions]
The conformation is determined first in order to name the conformation of the b. The general conformation is similar to that found in cyclohexane and forms the basis of the name. The General conformations are boats (B), chairs (C), skews (S), envelopes (E) or half seats (H). The ring atoms are then numbered. Anomer carbon, or hemiacetal, is always 1. Oxygen atoms in a structure are commonly named after the cyclically bonded carbon atoms and are called O.
Now continue the steps as follows:
- Look at the top level and place the rings so that the atoms are numbered clockwise.
- For chair and skew formations, you need to select a reference level. The reference plane is chosen so that the smallest number of atoms (usually C-1) are out of the plane when it comes to the conformation of the chair. In a distorted conformation, the plane contains three adjacent atoms and another atom, including one with as few exoplanars as possible.
- Atoms above the level are written as superscripts before the conformer label
- Atoms below the plane are written as a subscript after the label of the conform.

Things To Remember
- Pyranose is used for mentioning saccharides that have a six-membered ring-type chemical structure of five carbon atoms and one oxygen atom, and sometimes with other carbons external to the ring. Oxygen heterocyclic pyran has inspired Pyranose’s name, however, there are no double bonds present in the pyranose rings.
- If the reaction is between C-4 hydroxyl and aldehyde, furanose is formed. The pyranose type is thermodynamically more stable than the furanose type. This can be seen from these two cyclic distributions in solution.
- The conformation is determined first in order to name the conformation of the pyranose. The general conformation is similar to that found in cyclohexane and forms the basis of the name. The General conformations are boats (B), chairs (C), skews (S), envelopes (E) or half seats (H). The ring atoms are then numbered. Anomer carbon, or hemiacetal, is always 1. Oxygen atoms in a structure are commonly named after the cyclically bonded carbon atoms and are called O.
Also Read:
| Related Articles | ||
|---|---|---|
| Oxidation and Reduction | Precipitation Reaction | Hydrogen |
| Enzymes | Hormones | Nucleic Acids |
Sample Questions
Ques: What is the pyranose structure of glucose? (2 marks)
Ans: The pyranose structure of glucose has a 6-membered ring and 5 carbon and oxygen atoms. There are no double bonds in this glucose structure.
Ques: How many Conformations of Pyranose are present? (3 marks)
Ans: There are a total of 38 different basic pyranose conformations with 2 chairs, 6 boats, 6 skew-boats, 12 half-chairs and 12 envelopes.
These fits can interact with each other. There can be significant barriers to interconversion as each form has different relative energies. The energies of these conformations can be calculated from quantum mechanics.
Ques: What are the Origins of Pyranose Structure? (4 marks)
Ans: The structure of D-aldohexose was first determined by Hermann Emil Fischer and he was granted the Nobel Prize in Chemistry in the year 1902. Edmund Hirst and Clifford Purves of Walter Haworth's research group have determined that it is preferable for hexose sugars to form a 6-membered ring or a pyranose ring. The conformation of the pyranose ring was further refined when Sponsler and Dore (1926) realized that the mathematical treatment of the 6-membered ring of the saxophone could be applied to the X-ray structure of cellulose.
Ques: Draw and illustrate the Pyranose structural diagram of Glucose. (4 marks)
Ans:
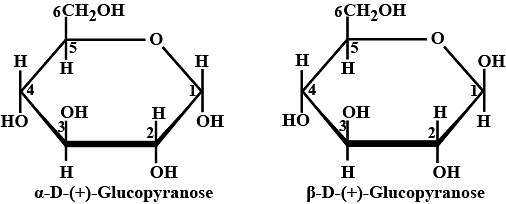
Pyranose structural diagram of Glucose
Ques: Explain the meaning of the pyranose structure of glucose. (2 marks)
Ans: The 6-membered ring structure of glucose is called the pyranose structure of glucose, similar to the heterocyclic compound pyranose.
Ques: What is the structural difference between pyranose and glucose? (3 marks)
Ans: The 5-membered carbohydrate cyclic hemiacetal is called the furanose type. The six members are called pyranose foam. The structural diagram of carbohydrates in the form of furanose or pyranose is called the Haworth formula after the British chemist Sir Walter Norman Howarth. Therefore, Howarth's formula is a way to explain the structure of pyranose or furanose in the form of glucose or other carbohydrates.
Ques: How does glucose have four asymmetric carbons? (3 marks)
Ans: If it has an "open-chain" polyhydroxy aldehyde structure, it has only four asymmetric carbons. The most abundant cyclic pyranose structure results in five asymmetric carbons, as the previous aldehyde carbons are converted to hemiacetals, each linked into four different groups.
Ques: What is the IUPAC name for glucose? (3 marks)
Ans: Glucose is preferably present in the form of a 6-membered pyranose ring. The preferred IUPAC name for this structure is D-glucopyranose, but the systematic name includes (3R, 4S, 5S, 6R) -6- (hydroxymethyl) oxano-2,3,4,5-tetrol. increase. This designation uses the IUPAC name "oxane" for the tetrahydropyran ring. Comparing the shape of the ring with the acyclic aldehyde (2R, 3S, 4R, 5R) -2,3,4,5,6-pentahydroxyhexanal, the position of the oxygen atom in the ring is 1, which causes confusion. increase. All chiral carbons are off by one number. The stereochemistry at the 2-position of the ring (anomeric carbon 1 of the acyclic structure) is R or S, depending on whether the beta anomer or the alpha anomer is referenced.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:



Comments