
Content Writer
Cellulose is an organic compound. It goes by the chemical formula (C6H10O5)n. It is a complex carbohydrate that consists of oxygen, carbon, and hydrogen. Cellulose is tasteless and has no odor. It is the main polysaccharide used for structural function in plants.
| Table of Content |
Key Terms: Cellulose, crystalline, beta-acetal connection, Cellulolysis, cellodextrins, organic compounds, Plant cell, glucose polymers, glucose, molecule
What is Cellulose?
[Click Here for Sample Questions]
Cellulose is among the most prevalent organic compounds. It is a polymer made up of unbranched glucose residues linked by beta-1,4 connections, allowing the molecule to form long, straight chains. This compound is an insoluble dietary fiber made up of glucose polymers that are found in all plant cell walls. Leafy green vegetables like kale, Brussels sprouts, and green peas are examples of cellulose-rich foods.
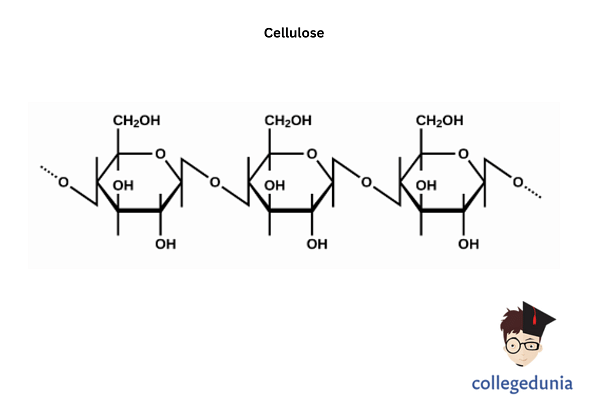
Cellulose can be broken up into glucose at high temperatures by treating concentrated mineral acids. Compared with starch, it is more crystalline. Starch, however, goes from crystalline to amorphous 60-70 degrees transition, but on the other side, cellulose takes 320 degrees and 25 megapascal pressure.
Also Read:
Properties of Cellulose- (C6H10O5)n
[Click Here for Sample Questions]
The degree of polymerization or chain length and the number of glucose molecules that make up the polymer molecule rely on several cellulose properties. Cellulose is odorless and in most organic solvents is insoluble. It is biodegradable and chiral.
Here are the physical and chemical properties of Cellulose:
| Physical Properties | Chemical Properties |
|---|---|
| Cellulose Chemical Formula | (C6H10O5)n |
| Molecular Weight/ Molar Mass | 162.1406 g/mol |
| Density | 1.5 g/cm3 |
| Appears | White powder |
| Melting Point | 260–270 °C |
Structure of Cellulose- (C6H10O5)n
[Click Here for Sample Questions]

Structure of Cellulose
The cellulose structure comprises long glucose unit polymer chains linked by beta-acetal connection. A very small part of the cellulose chain appears in the graph on the left. The monomer units are beta-d-glucose and are connected with CH1 of one glucose by all the beta-acetal linkages to CH4 of the next glucose. Carbon number 1 is known as anomeric carbon and is the center of the functional acetal group. An acetal is a carbon linked to two ether oxygen. The beta is set to the ether oxygen on the same ringside as the CH6. This leads to a horizontal or upwards protrusion in the chair construction.
Applications of Cellulose- (C6H10O5)n
[Click Here for Sample Questions]

- Used as a fiber supplement in diet
- Used as an additive in several food items
- Used in rayon production
- Used as a preservative in cheese as it works as an anti-clumping agent
- Used in making explosives
- Used to manufacture nitrocellulose
Breakdown of Cellulolysis
[Click Here for Sample Questions]
Cellulolysis is defined as the process of breaking down the Cellulose molecules. The molecules are broken into smaller polysaccharides, called cellodextrins. They can also be broken down completely into glucose units. This is called a hydrolysis reaction. Due to the strong binding of cellulose molecules, the cellulolysis process is comparatively difficult for other polysaccharides.

The glycoside hydrolases, which include exo-acting glucosidases and endo-acting cellulases, are enzymes that break the glycosidic linkage in Cellulose. These enzymes are usually released as part of multienzyme complexes that may also contain carbohydrate-binding doctrines and modules.
Structural Difference Between Starch and Cellulose
[Click Here for Sample Questions]
Cellulose is a linear chain of glucose molecules bound by beta 1,4 glycosidic bonds. Whereas, starch is present in both linear and branched chains.

Further comparison between Starch and Cellulose has been explained in the table below:
| Parameter | Starch | Cellulose |
|---|---|---|
| Glucose Range | Uses about 200-1000 glucose molecules to form one starch molecule | Takes up 500 glucose molecules to form one starch molecule |
| Bonding | Hydrogen bonding | None |
| Role | To store energy in the form of carbohydrates | To form a specific structure of plants |
| Type of chain | Coiled and unbranched (amylose) or long, branched (amylopectin) | Long, straight, unbranched chains forming H- bonds with the adjacent chains |
| Solubility in water | Amylose is soluble in water, and amylopectin is insoluble in water | Insoluble |
| Forms | Grain form | Fibres form |
| Found in | It is found in plants | It is found only in plants’ cell wall |
| Glucose unit linkages | Contains glucose residues as 1-6, otherwise1-4bonds | Constitutes their residues of glucose as glycosidic bonds with 1-4 |
| Molar mass | The molar starch mass varies | 162.1406 g/mol |
Things to Remember
- Cellulose is an important structural polysaccharide found in plants.
- Cellulose is composed of unbranched chains of glucose molecules linked via beta 1-4 glycosidic bonds.
- In cellulose chains, each alternate glucose molecule is inverted. The chains are placed parallel to each other to form microfibrils.
- It's made in the plasma membrane of plant cells by special rosette transmembrane complexes.
- The cellulose microfibrils are cross-linked via hemicellulose molecules.
- Polysaccharide matrix with acidic polysaccharides can also be found along with cellulose microfibrils in the plant cell walls.
- Cellulose is found in the cell walls of plants, algae, and bacteria, as well as tunicates' shells.
- Cellulose is digested only in herbivores.
- In plants, cellulose is degraded by pathogenic enzymes. It also goes through degradation at 350 degree temperature.
- Plant and bacterial cells, as well as algae, get their strength and rigidity from cellulose.
- Cellulose has widespread industrial applications such as paper and paper products production, insulation paper production, as a biofuel, a stationary phase in chromatography, etc.
Read More:
| Chapter Related Concepts | ||
|---|---|---|
| Carbohydrate Metabolism | RNA and DNA | |
| Lipoproteins | Fats and Oils | Sucrose |
Sample Questions
Ques. Write a short note on cellulose. (3 Marks)
Ans. Cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae, and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40−50%, and that of dried hemp is approximately 57%.
Ques. Can we digest cellulose? (3 Marks)
Ans. Humans lack the enzyme necessary to digest cellulose. Hay and grasses are particularly abundant in cellulose, and both are indigestible by humans (although humans can digest starch). Animals such as termites and herbivores such as cows, koalas, and horses all digest cellulose, but even these animals do not themselves have an enzyme that digests this material. Instead, these animals harbor microbes that can digest cellulose.
The termite, for instance, contains protists (single-celled organisms) called mastigophorans in their guts that carry out cellulose digestion. The species of mastigophorans that performs this service for termites is called Trichonympha, which, interestingly, can cause a serious parasitic infection in humans.
Ques. What are the main sources of starch? Explain its structure in brief. (4 Marks)
Ans. Sources: Cellulose occurs exclusively in plants and is the most abundant organic substance in the plant kingdom. It is a predominant constituent of the cell wall of the plant cell. It is present in wood, cotton clothes, jute, cotton, etc. in wood, it is present 50%, in dry grasses, it is 40−45%, injure 60−65%, in cotton 90−95 %, in cotton clothes 90% cellulose and rest in fats and waxes.
Structure: Cellulose is composed of β-D-glucose units linked by (1→4) glycosidic bonds. The X-ray analysis has shown that there are large linear chains of β - D(+) glucose molecules lying side by side in the form of bundles held together by H - bonding in the neighboring hydroxyl groups. In each linear chain, the D(+) glucose units are attached by C1 to C4 bonds through β – glycosidic linkages.
Ques. Structurally, cellulose is a linear polymer of: (4 Marks)
(a) β -D-glucose unit
(b) Sucrose molecules
(c) α -d glucose units
(d) Glucose and fructose unit
Ans. Glucose is an organic compound that has many polymers. These polymers exist to form various chemical compounds. It is a monosaccharide. Cellulose is a polymer of glucose but it is a polysaccharide. It is formed with long linear chains of β -D-glucose units.
We can draw the structure of β−D−glucose as

This structure forms a long chain by replacing one hydrogen atom from the alcohol group and bonding with the adjacent oxygen atom of the other group. Thus, to form a new compound called cellulose.

Cellulose
So here, we have the answer that cellulose is obtained by a long chain of β−D−glucose.
Ques. What is the final product obtained by hydrolysis of the Cellulose? (5 Marks)
Ans. As the main component of lignocelluloses, cellulose is a biopolymer consisting of many glucose units connected through β-1,4-glycosidic bonds. Breakage of the β-1,4-glycosidic bonds by acids leads to the hydrolysis of cellulose polymers, resulting in the sugar molecule glucose or oligosaccharides.
Mineral acids, such as HCl and H2SO4, have been used in the hydrolysis of cellulose. However, they suffer from problems of product separation, reactor corrosion, poor catalyst recyclability, and the need for the treatment of waste effluent.
The use of heterogeneous solid acids can solve some of these problems through the ease of product separation and good catalyst recyclability. The acid strength, acid site density, adsorption of the substance and micropores of the solid material are all key factors for effective hydrolysis processes.
Upon hydrolysis at high pressure in the presence of dilute acid cellulose gives β - D glucose.
\((C_6H_{10}O_5)_n+nH_2O \xrightarrow[{(\triangle ) }]{\text{Dil. Acid}}nC_6H_{12}O_6\\Cellulose \; \:\, \; \;\; \;\beta-D\;Glucose\)
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:





Comments