NCERT Solutions for class 11 Chemistry Chapter 12 Organic Chemistry – Some Basic Principles and Techniques are provided in this article. Some of the important topics in this chapter includes:
- Carbanions
- Carbocation Stability
- Classification Organic Compounds
- Difference Between Electrophile and Nucleophile
- Adsorption Chromatography
- Distillation
- Named Reactions
Expected number of questions: 5 to 7 questions of total 11 marks
Download PDF: NCERT Solutions for Class 11 Chemistry Chapter 12 pdf
NCERT Solutions for Class 11 Chemistry Chapter 12
NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry is given below.
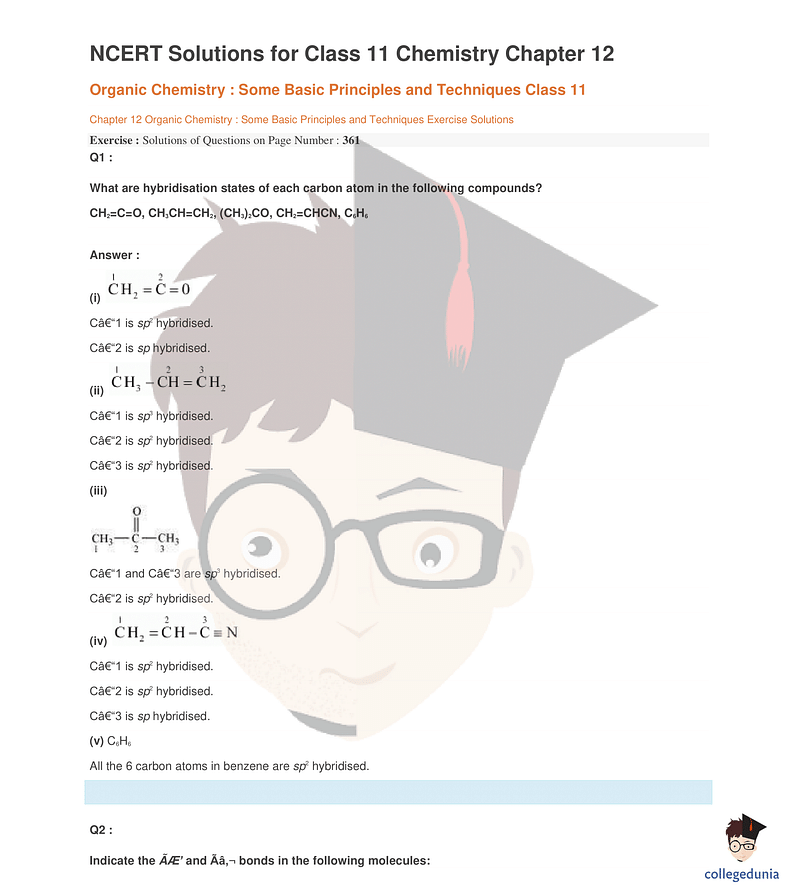




























Class 11 Chemistry Chapter 12 Organic Chemistry – Important Topics
Organic Chemistry is a branch of chemistry that deals with the study of hydrocarbons and their derivatives. The primary constituents of hydrocarbons include carbon and hydrogen.
The different types of saturated hydrocarbons include:
- Saturated Hydrocarbons
- Unsaturated Hydrocarbons
- Aliphatic Hydrocarbons
- Aromatic Hydrocarbons
| Properties of Hydrocarbons:
|
Reactions of Hydrocarbon:
- Substitution Reaction
- Addition Reaction
- Combustion Reaction
Related Articles:
| Nucleophile | Electrophile | Kjeldahl Method |
| Structural Formula | Radical Formula | Nomenclature of Functional Groups |
| Organic Rearrangement Reaction | Radicals | Woodward Reaction |
Chemistry Related Articles:






Comments