
Content Curator
Chromatography is a technique used for separating a mixture of chemical substances into their individual components.
- It is used for the separation, purification, and testing of compounds.
- The term ‘Chromatography’ was coined by Russian botanist Mikhail Tswett in 1906.
- There are two principles of Chromatography namely Adsorption Chromatography and Partition Chromatography.
- In Chromatography, The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a stationary phase.
- In the stationary phase, different components of the mixture travel at different speeds, causing them to separate.
- These phases determine which substances travel more quickly or slowly, and this is how they are separated.
- The different travel times are referred to as retention time.
There are many types of chromatography such as Liquid Chromatography, Gas Chromatography, and Ion-exchange Chromatography, however, all of these types employ the same basic principles.
Key Terms: Chromatography, Adsorption Chromatography, Partition Chromatography, Paper Chromatography, Thin Layer Chromatography, Mobile Phase
Chromatography Definition
[Click Here for Sample Questions]
Chromatography is a laboratory technique used to separate components or solutes of a mixture. The components are separated on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase and a contiguous stationary phase.
- The mobile phase in Chromatography can be either a liquid or a gas.
- The stationary phase is either a solid or a liquid.
- The solute molecules are continuously exchanged by the Kinetic molecular motion between the two phases.
- Different types of Chromatography can be categorized depending on the type of mobile phase used.
- If the mobile phase is liquid, the technique would fall under the category of liquid chromatography.
- If the mobile phase is gas, the technique will fall under the category of gas chromatography.
Read More:
Principles of Chromatography
[Click Here for Previous Years' Questions]
Chromatography is based on the principle where molecules are transferred between the mobile phase and the stationary phase due to the absorbance or partition of molecules present in the mixture which we want to separate.
In order to understand the principle of chromatography, one must be thoroughly clear with the following terms:
| Term | Definition |
|---|---|
| Analyte | Mixture whose individual components have to be separated and analyzed. |
| Mobile Phase | A solvent that moves through the column. |
| Stationary Phase | A substance that stays fixed inside the column. |
| Eluent | Fluid entering the column. |
| Eluate | Fluid exiting the column which collected in flasks. |
| Elution | Process of washing out a compound through a column using a suitable solvent. |
Chromatography is a separation technique where the analyte is combined within a mobile phase which is pumped through a stationary phase.
- Generally, one phase is hydrophilic and the other phase is lipophilic.
- The components of the analyte interact differently with both of these two phases.
- Depending on their polarity, they interact more or less with the stationary phase, retarding to a greater or lesser extent.
- Sample components separate as they travel through the stationary phase at different speeds.
- Each sample component elutes from the stationary phase at a specific time which is called retention time.
- As the components of the sample pass through the detector, their signal is recorded and plotted in the form of a chromatogram.
Types of Chromatography
[Click Here for Sample Questions]
There are four main types of chromatography. These are
Adsorption Chromatography
Based on the absorptivity property of the component, different components are adsorbed on the adsorbent to different extents.
- The most common adsorbents are silica gel and alumina.
- When the mobile phase is allowed to pass through the stationary phase (adsorbent phase) the compounds of the mixture are moved to different distances over the stationary phase.
Thin Layer Chromatography (TLC)
This is another type of chromatography which is used to separate the mixture over a thin layer of adsorbent coated on a plate made of glass. The adsorbent used is silica gel or alumina.
- An adsorbent layer is spread across a glass plate.
- The mixture to be separated is dissolved in the suitable solvent.
- With the help of a fine capillary at a distance of 2 cm, the mixture solution is placed on the glass plate.
- A glass jar is filled with a suitable solvent and then a glass plate is placed in the jar at a vertical position.
- It is important to note that the mixture placed on the adsorbent-coated plate is not dipped in the solvent.
- With the help of capillary action, the solvent will automatically increase as the compartment is closed this process is called elution.
- As the elution moves up, depending on the degree of adsorbent and separation the mixture is separated.
- The separated spots can be coloured and will be visible to the naked eye and the transparent spots can be viewed with the help of iodine or UV light.
- The relative adsorbent of each component of the mixture is expressed in terms of retardation factor (Rf value).
- Rf is the distance moved by the substance from the baseline Distance moved by the solvent from the baseline
- The Rf value of the substance depends on the nature of the substance, solvent, adsorbent and temperature.
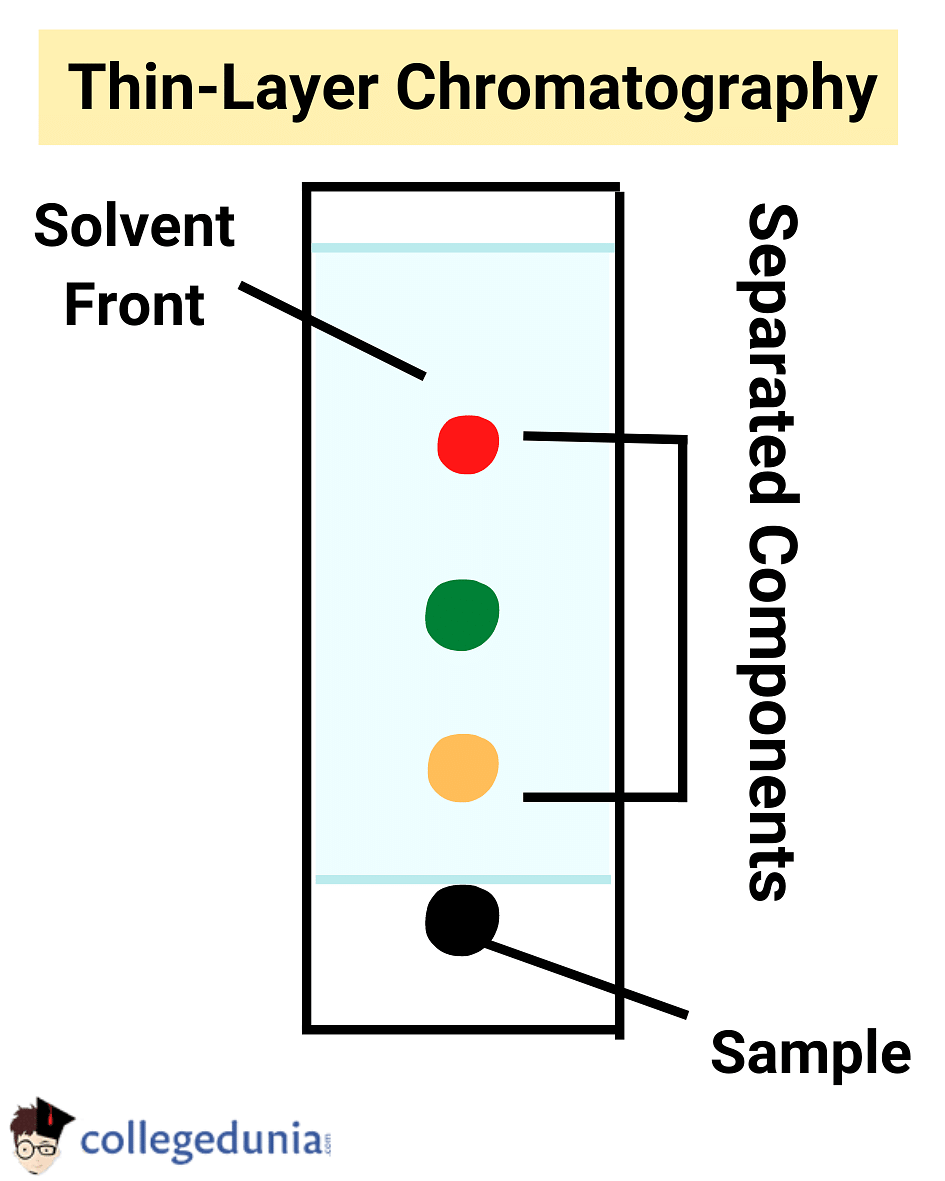
Thin Layer Chromatography
Column Chromatography
Separating a mixture over a column of absorbent (Stationary Phase) is called Column Chromatography.
- The stationary phase (adsorbent) is filled in the glassed tube.
- The adsorbents like Alumina, silica gel, activated charcoal, manganese, etc can be used.
- The mixture which is to be separated is mixed with the suitable solvent and is allowed to run through an adsorbent.
- When the mixture is run through the adsorbent it slowly gets separated and forms a layer at different heights.
- Due to the difference in properties, the mixture is separated when run through the stationary phase.
- The component with higher absorptivity remains at the top and the remaining components flow down.
- The components are separated and removed by dissolving into suitable solvents and this process is called elution.
- Elution acts as a moving phase. As it is passed from the adsorbent phase the component is slightly dissolved in elution depending on the component's nature.
- Thus the components get separated.
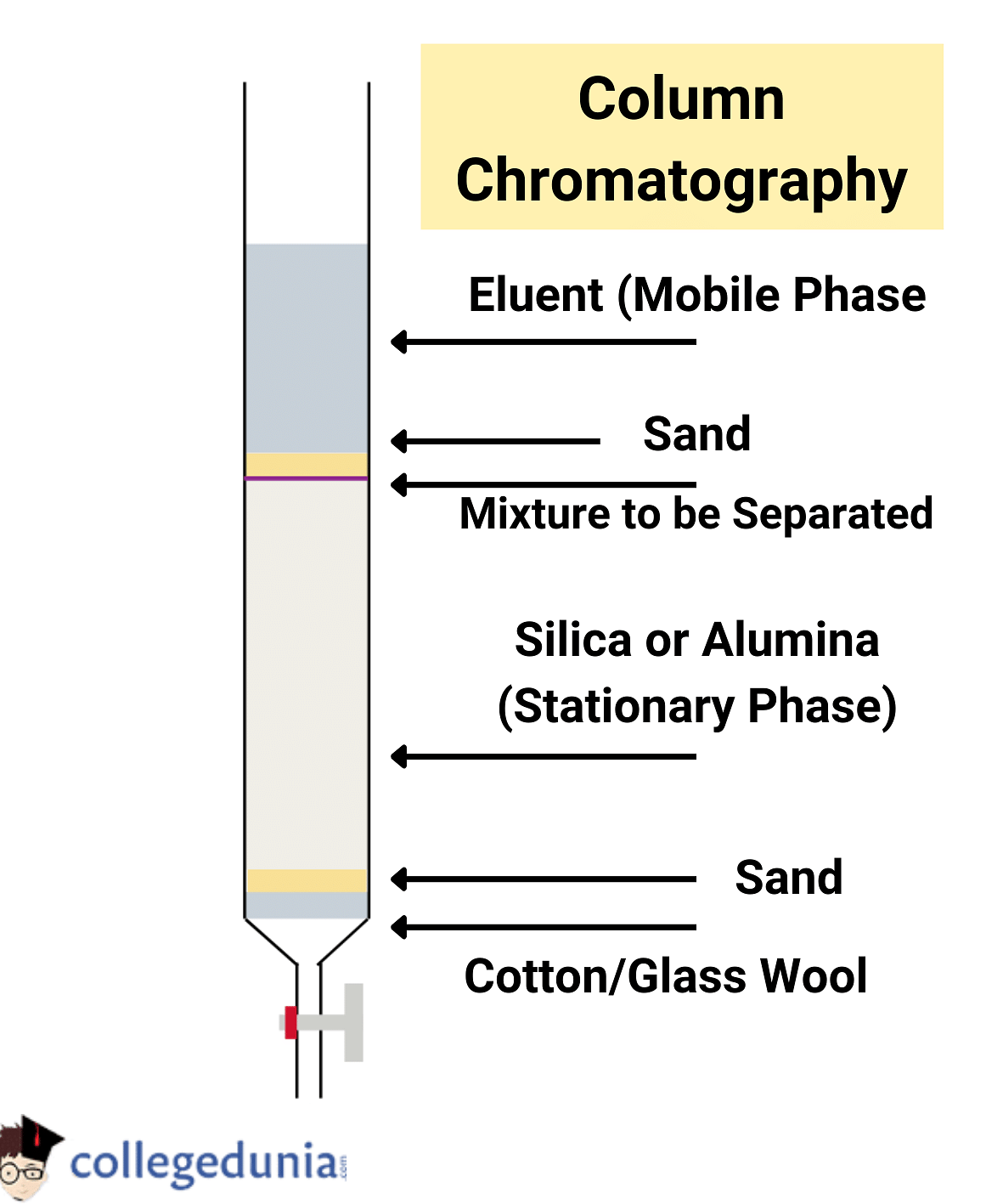
Column Chromatography
Partition Chromatography
Chromatography that is based on the continuous differential partitioning of components of a mixture is known as Partition Chromatography. The most common type of partition chromatography is Paper Chromatography.
Paper Chromatography
The below diagram shows the process of Paper Chromatography:
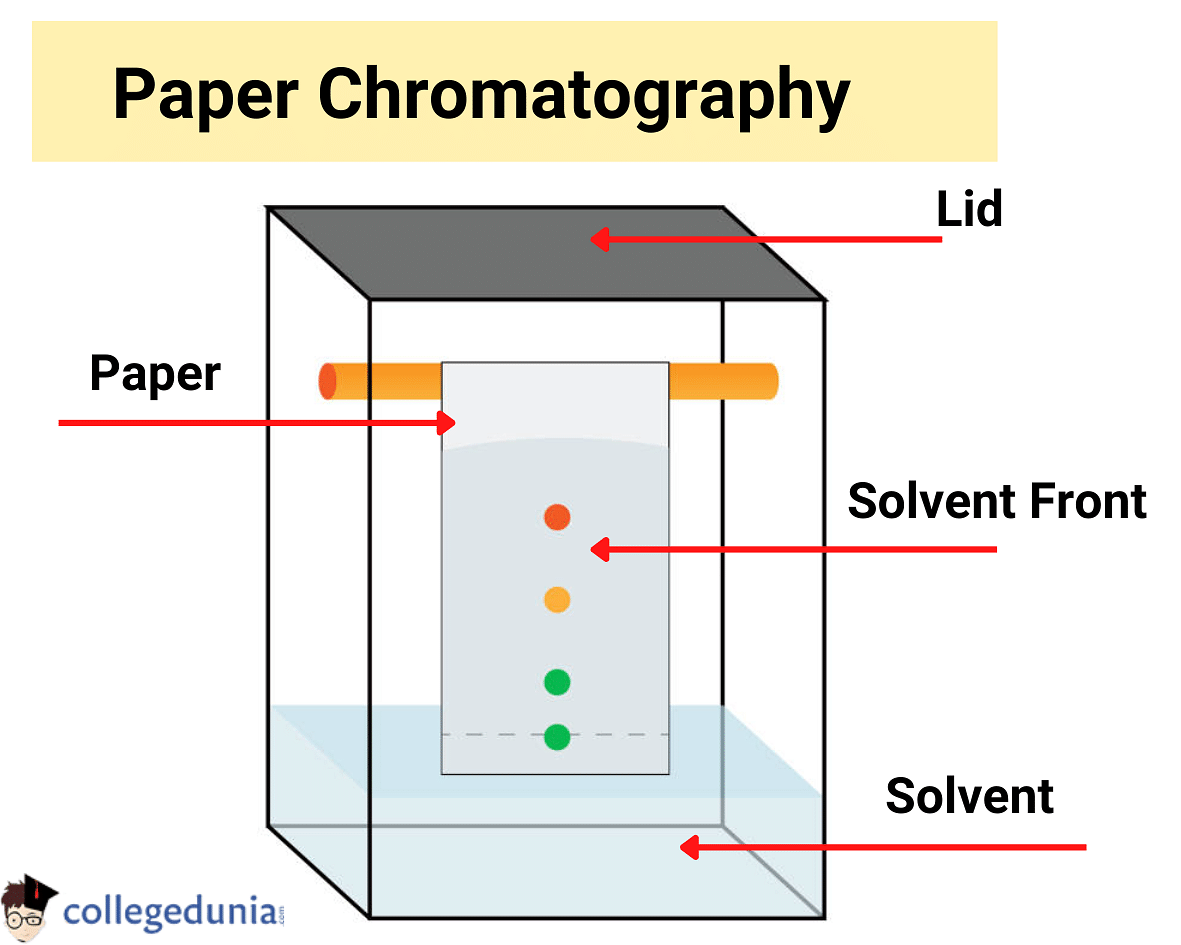
Paper Chromatography
In Paper Chromatography, a special type of paper is used which is called Chromatography paper. The paper contains water stored in it, which acts as a Stationary Phase.
- The mobile phase in this process is called the irrigant or developing solvent.
- The solvent travels through chromatography paper by the capillary action.
- The drop of mixed solution is dropped on chromatography paper and one end of the paper is dipped into the solvent.
- The mixture undergoes partly partition on the water molecules present in the paper and partly adsorption on the paper.
- A mixture is separated and displaced on the chromatography paper based on partition and adsorption rates.
- Components may be visible to the naked eye because they are of different colours, or they can be viewed under ultraviolet light or by spraying the reagents.
What is Differential Extraction?
[Click Here for Sample Questions]
Differential extraction refers to the method of separation of any organic component present in a given aqueous solution.
- An organic solvent is used for which the solubility of the desired compound is more than compared to that of water.
- Using a separating funnel, organic solvents are chosen so that they are immiscible with the aqueous solution and can form layers easily.
- It is later recovered by the process of distillation or evaporation.
- The process of continuous extraction is brought into use when the solubility of the compound is less in the organic solvent.
Applications of Chromatography
[Click Here for Sample Questions]
There are various uses of Chromatography. The most common uses are mentioned below-
- Chromatography is used for the separation, isolation and purification of proteins from complex sample matrices.
- It is an essential part of almost any protein purification strategy.
- Organic compounds like amino acids, alcohol, acids, antibiotics, etc are used for characterising and isolating with the help of Chromatography.
- It is used to determine chemicals in pharmaceutical products.
- Chromatography is used to determine the alcohol level present in blood samples at hospitals.
- Clinical tests like urine analysis and antibiotic analysis can be done with the help of chromatography.
- It is used to determine components present in samples collected from the crime scene or from the suspects.
- The nutritive value of food additives in food industries can be determined by using chromatography.
- It can be used to determine the level of pollution present in the water.
Also Read:
Chromatography Handwritten Notes
[Click Here for Previous Years' Questions]
Here are the handwritten notes on Chromatography and related topics:












































Things to Remember
- Chromatography is the technique used to separate the components of a mixture.
- Chromatography is used for the separation, purification, and testing of compounds in laboratories.
- The three major Chromatography techniques are Column Chromatography, Thin layer Chromatography and Paper chromatography.
- The principle of Chromatography states that the molecules are transferred between the mobile phase and the stationary phase due to the absorbance or partition of molecules present in the mixture.
- Chromatography has a wide range of applications in different sectors and industries such as pharmaceuticals, chemical, and food industries.
Previous Years’ Questions
- Paper chromatography is a type of partition chromatography….
- Those substances can be separated by steam distillation which are…
- The phenomenon in which adsorption and absorption takes place…
- If dichloromethane (DCM) and water (H2O) are used for differential extraction… [JEE Main 2019]
- Which of the following is not correct… [UP CPMT 2007]
- The Lassaigne's extract is boiled with concentrated…. [NEET 2011]
- For which of the following compounds Kjeldahl method… [JEE Main 2013]
- In Dumas method one gram of carbon compound gives… [AP EAMCET 2019]
- When metal M is treated with NaOH, a white gelatinous precipitate… [JEE Main 2018]
- Liquation process is used for the purification of… [COMEDK UGET 2005]
Sample Questions
Ques. What is Chromatography? (2 Marks)
Ans. Chromatography is a technique used for the separation, identification and purification of constituents of a mixture for qualitative and quantitative analysis. Adsorption, partition, ion exchange, exclusion, affinity, and electrophoresis are the main types of chromatography techniques that are widely used in our daily life or laboratory. Chromatography is classified into two categories:
- Adsorption Chromatography
- Partition Chromatography
Ques. What is absorption chromatography? (2 Marks)
Ans. Adsorption Chromatography is the process based on different adsorption properties of the component and its mixture. This technique makes use of the mobile phase which is either in liquid or gaseous form. The mobile phase is adsorbed onto the surface of a stationary solid phase. There are two main methods based on adsorption chromatography:
- Column Chromatography
- Thin Layer Chromatography
Ques. What is the principle of chromatography? (3 Marks)
Ans. The principle of chromatography is as follows:
- Chromatography is based on the principle in which the molecules in the mixture are applied onto the surface or into the solid, and the fluid stationary phase (stable phase) separates from each other while moving with the help of the mobile phase.
- The factors that affect this separation process include molecular characteristics related to adsorption (liquid-solid), partition (liquid-solid), and affinity or differences among their molecular weights.
- Owing to these differences, some components of the mixture stay for a longer time in the stationary phase, and they move slowly in the chromatography system, while others move on rapidly into the mobile phase, and leave the system faster.
Ques. What is Rf value? And How is the Rf value useful? (2 Marks)
Ans. Rf value stands for retardation factor in paper chromatography. It is the distance travelled by the fluid component. Rf value is used to equate unknown samples with known components. As Rf Value for particular solvents and a component are common.
Rf value is the distance moved by the substance from the baseline divided by the distance moved by the solvent from the baseline. It is the component characteristics used to clarify components for the given systems at known temperatures.
Ques. State the applications for Chromatography in the Pharmaceutical sector. (3 Marks)
Ans. There are various applications of chromatography in the Pharmaceutical sector such as:
- It is used to identify and analyze samples for the presence of trace elements or chemicals.
- It is used for the separation of compounds based on their molecular weight and element composition.
- It helps to detect unknown compounds and the purity of the mixture.
- It also helps in drug development.
Ques. What is a Mobile phase in Chromatography? (3 Marks)
Ans. The mobile phase moves through the stationary phase when different components are separated due to different rates of travelling.
- If the mobile phase is liquid, it is referred to as liquid chromatography (LC).
- If the mobile phase is gas then it is referred to as gas chromatography (GC).
The movement of the molecules depends on their affinity to the stationary and mobile phases. As a result, the components which are attached less by the stationary phase will move faster in mobile phases.
Ques. Explain Column Chromatography. (5 Marks)
Ans. Separating a mixture over a column of absorbent (Stationary Phase) is called Column Chromatography.

Column Chromatography
- A glass tube is filled with an adsorbent like Alumina, silica gel or activated manganese.
- A mixture to be separated is mixed with a suitable solvent and then run through an adsorbent.
- The mixture gets separated at different heights when passed through the adsorbent.
- Because of differences in properties, the mixture gets separated when passed through the stationary phase.
- Higher absorptivity components remain at the top and the rest flows down.
- The components are separated and removed by dissolving into suitable solvents and this process is called elution.
- Depending on the components' nature, some of the components are slightly dissolved in elution when used as the moving phase.
Thus, the components get separated.
Ques. What is the Stationary Phase in Chromatography? (2 Marks)
Ans. The stationary phase in chromatography may be a column, plate, or paper. It refers to a solid phase or a liquid phase coated on the surface of a solid phase. Depending upon the nature of the stationary phase, there are three chromatographic techniques:
- Liquid-solid absorption or column chromatography (LSC)
- Paper chromatography (PC)
- Thin-layer chromatography (TLC)
Ques. What is Paper Chromatography? (5 Marks)
Ans. Paper Chromatography is one of the types of chromatography. The below diagram shows the process of Paper Chromatography:

Paper Chromatography
- Chromatography paper is a special type of paper used in paper chromatography. Water is stored in the paper and acts as a stationary phase
- The solvent is called an irrigant in this process and it is in the mobile phase.
- One end of the paper is dipped in the solvent. The drop of the mixture is above the solvent layer. Due to the capillary action solvent travels upwards.
- The mixture undergoes partly partition on the water molecules present in the paper and partly adsorption on the paper.
- Due to partition and adsorption, the mixture is separated and displaced at different positions on the chromatography paper.
- Separated components are visible to the naked eye as they have different colours and can also be viewed under ultraviolet light or by spraying appropriate reagents.
Ques. What are the different types of chromatography? (3 Marks)
Ans. There are four types of Chromatography
- Adsorption Chromatography: Based on the absorptivity property of the component, different components are adsorbed on the adsorbent to different extents.
- Thin Layer Chromatography: This process separates mixtures by spotting them onto a glass plate coated with a thin layer of absorbent like silica gel or alumina.
- Column Chromatography: It is the technique used to separate the components of a mixture using a column of suitable adsorbent packed in a glass tube
- Partition Chromatography: Partition chromatography is the continuous differential partitioning of components of a mixture.
Check-Out:





Comments