Jasmine Grover Study Abroad Expert
Study Abroad Expert
Reduction potential can be referred to as the tendency of the chemical species to gain an electron and get reduced as a result of it. It can be explained with the help of the hydrogen’s electrochemical reference which can be globally said to have a zero reduction potential.
| Table of Content |
Keyterms: Electron, Potential, Half Cells, Gibbs free Energy charge, Electrochemistry, Oxidation Potention, zero reduction potential, Electrolysis Products
Reduction Potential Definition
[Click Here for Sample Questions]
The electrode potential is oxidation potential and the reduction potential is named oxidation potential. This condition, if oxidation takes place at the electrode, Reduction comprises an increase of electrons. Then the tendency of an electrode to produce electrons is called its reduction potential.

Reduction Potential
The equilibrium potential changes between the solution surrounding and metal electrode; it is named the electrode potential. It is also cleared as the tendency of an electrode to increase and decrease electrons.
Also Read:
| Related Articles | ||
|---|---|---|
| Electrochemistry Important Questions | Variations Molar Conductivity | Electrochemistry Ncert Solutions |
| Electrochemical Cells | Nernst Equation | Isobaric Process |
Standard Reduction Potential
[Click Here for Sample Questions]
Standard electrode potential is an element (E°) in electrochemistry as computing the person’s revocable electrode potential at the standard state with ions at a changed concentration of 1 mol dm-3 atmosphere pressure. And then, the normal electrode potential is then commonly written as the standard potential for reduction.
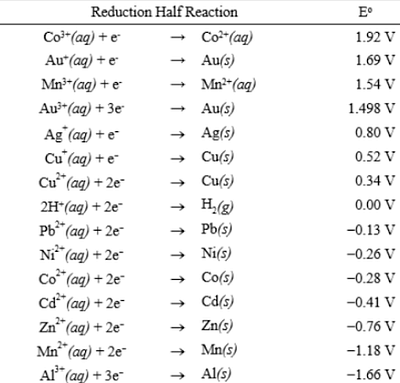
Standard Reduction Potential at 25ºC
By dedicating the standard reduction potential for the reaction going on at the anode from the standard reduction potential for the cathode reaction, the standard reduction potential can be determined. To get the minimum simple cell potential, put the cell potential together.
Reduction Potential Explanation
[Click Here for Sample Questions]
At the point when a metal piece is Solutions in its very own answer particles, a potential distinction is framed at the metal interface and the solution. The potential distinction size is a proportion of the cathode propensity to go through one or the other decrease of oxidation or the inclination to either lose or acquire the electrons.
The particle and metal signify half-cell, and the response is the half-reaction. The inundated metal is called an electrode, and the potential happened due to the reaction at the electrode interface. The solution is known as the electrode potential. Along these lines, the terminal potential is depicted as the propensity of a cathode either to lose or acquire electrons. On the off chance that the decrease happens at the terminal, it is characterized as the decreased potential.
Then the oxidation takes place at the electrode center, so, it is called the oxidation potential
M → M2+ + 2e–
As metal ions open depositing on the metal upper portion this grows a positive charge on the metal rod. Then, oxidation is just a reverse of reduction but reduction potential is obtained from the oxidation potential by normal charging the sign.
In normal for an electrode
Oxidation potential = – Reduction potential
Eo (Zn/Zn2+) = 0.76V
And standard reduction potential as equation
Eo (Zn2+/Zn) = -0.76V
All the more reduction potential has been adopted by the IUPAC (International Union of Pure and Applied Chemistry) for the assignment of electrode potential.
At the point when the half-cell reaction is completed at a temperature of 298K and the electrode is suspended in an answer of one molar focus, the electrode potential is named as the standard terminal potential and is represented by Eo. The Standard electrode potential Eo empowers one to evaluate the thermodynamics action of different synthetic substances. However, there are no techniques accessible by which we can quantify its total worth. The terminal capability of an anode is estimated regarding standard hydrogen electrodes.
Gibbs Free Energy Charge
[Click Here for Sample Questions]
The connection between the Gibbs free charge change and the standard reaction potential is:
ΔG = −nFE0ΔG = − nFE0
In this equation:
- ΔGΔG is the change in free energy
- n is the number of moles
- E0 is the standard potential
- F is the Faraday constant
Half Cells
[Click Here for Sample Questions]
As a cell, a battery has two half-cells isolated with an electrolyte. The anodes are needed to interface the half cells to the outer circuit. Each electrode can go about as a feature of a redox couple, however, none of these must be.
The standard conditions for the hydrogen half-cell are the grouping of hydrogen H+(AQ)H+(AQ), the pressing factor of hydrogen gas is given as 105Pa with a temperature of 298K.

The standard hydrogen half-cell is used as a position half-cell and all different half-cells are dignified against it. A list of electrode potential has been produced relative to the standard hydrogen half-cell. Then the reaction in this half-cell is:
2H+(aq) + 2e– ⇔ H2(g)
Electrode potential changes with temperature thus a standard temperature is characterized. This is 298K. Changing the grouping of any particles showing up in the half-reactions likewise influences the voltages, so a standard convergence of 1.00 mol dm-3 is picked. The standard pressing factor is 105Pa.
The potential of a standard hydrogen half-cell is characterized as 0.0V a worth picked for convenience.
The standard electrode potential of a half-cell Eo is characterized as the expected distinction between the half cell and a standard hydrogen half-cell.
Eo Values have a significant contingent upon whether the half-cell is at a sequential positive potential than the standard hydrogen half-cell. Estimations are made at 298 K with the metal plunging into a 1.00 mol dm-3 arrangement of a salt of the metal.
Also Read:
Things to Remember
- Reduction potential is a part of CBSE class 12, second term.
- It comes under unit 1 electrochemistry carrying a total of 7 periods and 4 to 6 marks.
- The reduction potential helps in the measurement of the tendency of molecules to gain new electrons and as a result gets reduced.
- The standard reduction potential can help in understanding a reaction’s directionality.
- Higher the positive value of a reduction potential the more it will get reduced.
Sample Questions
Ques 1. What are the changes between reduction potential and oxidation potential? (1 mark)
Ans: The important changes between the potential for oxidation and decrease are that the potential for oxidation shows a chemical element’s propensity to be oxidized. Alternately, the potential for decrease recommends the probability of a synthetic component is reduced.
Ques 2. Is reduction potential negative or positive? (1 mark)
Ans: When a solution has more positive reduction potential in comparison to the new species then it will gain electrons from the new species (i.e., to be reduced by oxidizing the new species) and a solution with a lower (more negative) reduction potential will, in general, lose electrons to the new species.
Ques 3. What influences reduction potential? (1 mark)
Ans: As a general rule, extremely late change metal particles those at the right finish of the progress metal chain, including copper, silver, and gold have a high reduction potential. Assuming the lithium's ordinary decrease potential is extremely negative, the lithium particle's oxidation potential is very positive.
Ques 4. How would you compute reduction potential? (1 mark)
Ans: The standard reduction potential can be dictated by taking away the standard reduction potential for the anode-incited response from the standard reduction potential for the cathode-actuated reaction. The less sign is significant the converse of the oxidation-reduction.
Ques 5. Which component has the biggest reduction potential? (1 mark)
Ans: Fluorine has the most elevated potential for decline. High oxidizing specialists like to oxidize different components and are decreased without help from anyone else. Thus, comparative with chlorine, bromine, and iodine, fluorine has the best potential for decrease. The high reduction potential is because of the low enthalpy of bonds and high Fluorine electronegativity.
Ques 6. Complete and balance the following half–equations, and indicate whether oxidation or reduction is involved.
(a) ClO2(g) → ClO3–(aq) (acidic solution)
(b) MnO4–(aq) → MnO2(s) (acidic solution)
(c) SbH3(g) → Sb(s) (basic solution)
(3 marks)
Ans. (a) ClO2(g) + H2O(l) → ClO3–(aq) + 2 H+(aq) + e– Oxidation
(b) MnO4–(aq) + 4 H+(aq) + 3 e– → MnO2(s) + 2 H2O(l) Reduction
(c) SbH3(g) + 3 OH–(aq) → Sb(s) + 3 H2O(l) + 3 e– Oxidation
Ques 7. What is the standard potential of a cell in which copper is reduced by iron? (1 mark)
(a) 0.96V
(b) 0.60V
(c) 0.08V
(d) 1.48V
Ans. a) 0.96V
Ques 8. The standard reduction potentials for some metals are listed below:
Au³+ + 3e-Au Eº = 1.50
Ag²+ + 2e-Ag Eº = 0.80
Cu+ + e-Cu Eº = 0.52
Ni²+ + 2e-Ni Eº = 0.23
Fe²+ + 2e- Fe Eº = -0.44
Select the metal having the strongest reducing agent and why? (2 marks)
(a) Ag
(b) Fe
(c) Cu
(d) Au
Ans. b) Fe.
A metal that will be oxidised through this reaction is the reducing agent. Since all the half reactions shown are reduction potentials, one needs to change the half reactions so as to regulate the result to the greatest cell potential when the metal is oxidised. The cell potential of iron will be 0.44 volts after oxidation which makes Iron the strongest reducing agent present on the list.
Ques 9. Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇔ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction? (3 marks)
(a) -1.08V
(b) 0.46V
(c) -1.76V
(d) -0.40V
(e) 1.08V
Ans. -1.08V
One need not multiply the coefficients that are needed to balance to the Eo cell, one just has to see that copper oxidation is taking place. Hence there is a change in the sign (0.34-0.34) and there is no change in the sign as the Cr decreases. Finally by adding the Eo cells one gets: -0.74 + (-0.34) = -1.08
Ques 10. State two differences between oxidation half reaction and reduction half reaction? (2 marks)
Ans. Two differences are:
| Oxidation half reaction | Reduction half reaction |
|---|---|
| In case of oxidation reaction an electron is removed from a substance. | In case of reduction reaction an electron is gained by the substance. |
| In oxidation there is a decrease in the total number of electrons. | In reduction there is an increase in the total number of electrons. |
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:



Comments