Jasmine Grover Study Abroad Expert
Study Abroad Expert
An electrolytic cell is an electrochemical cell that converts electrical energy into chemical energy. This process drives a non-spontaneous redox action. Electrolytic cells decompose chemical compounds in electrolysis. It is a process that breaks down ionic substances. Two half-cells create one electrolytic cell: reduction half-cell and oxidation half-cell.
| Table of Content |
Keyterms: Electrolytic Cell, Galvanic Cells, Electrolysis, Electron, Faraday’s Law, Salt Bridge, Cathode, Anode, Electrochemistry
Comparison Between Galvanic Cells and Electrolytic Cells
[Click Here for Sample Questions]
Electrolytic cells and Galvanic cells work on a similar mechanism. The similarities between electrolytic cells and galvanic cells exist because of three key factors:
- Both cells need a salt bridge;
- Both have cathode and anode components;
- Flow of electrons from the anode to the cathode is consistent in both these cells.
3Nonetheless, the different flow of electrons distinct the two from each other. Read on to know the differences between the two cells:

| Galvanic Cell | Electrolytic Cell |
|---|---|
| Converts chemical energy into electrical energy. | Uses electrical energy to drive redox reactions. |
| Contains negatively charged anodes and positively charged cathodes. | Contains negatively charged cathodes and positively charged anodes. |
| Presents spontaneous cell reactions. | Features non-spontaneous cell reactions. |
| Oxidation half-reaction: Y→Y++e- | Oxidation half-reaction: Z-→Z+e- |
| Reduction half-reaction: Z++e-→Z | Reduction half-reaction: Y++e-→Y |
| Overall cell reaction: Y+Z→Y++Z-(G<0) | Overall cell reaction: Y++Z-→Y+Z(G>0) |
Read More:
| Related Articles | ||
|---|---|---|
| Difference Between Cell and Battery | Reduction Potential | Concentration Cell |
| Nonelectrolyte | Standard Hydrogen Electrode | Cadmium Sulfate Formula |
What are the components of an electrolytic cell?
[Click Here for Sample Questions]
An electrolytic cell has three primary components:
- Cathode
- Anode
- Electrolyte
The cathode is negatively charged and the anode is positively charged for these cells. The electrolyte is a medium of exchange of electrons between the cathode and anode. Water (containing dissolved ions) and molten sodium chloride are most commonly-used electrolytes.
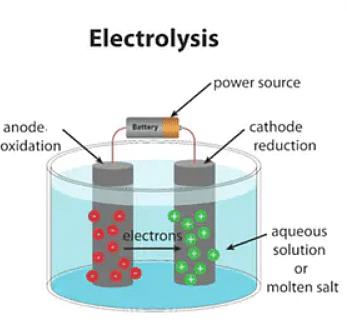
How does an Electrolytic Cell work?
[Click Here for Sample Questions]
Electrolysis in electrolytic cells is an essential process which decides whether or not the Electrolytic Cell Molten sodium chloride (NaCl) would work.

As seen in the above diagram, two inert electrodes are dipped into molten sodium chloride. This contains dissociated Na+ cations and Cl– anions. An electric current is passed into this circuit and the electrons gather around the cathode. This creates a negative charge. As a result, the positively charged sodium cations move towards the negatively charged cathode. This leads to the formation of metallic sodium at the cathode.
Alongside this process, the chlorine atoms pass onto the positively charged cathode. It results in the formation of chlorine gas (Cl2) at the anode. This releases 2 electrons, completing the circuit.
Here are the relevant chemical equations and the overall cell reaction:
- Reaction at Cathode: [Na+ + e– → Na] x 2
- Reaction at Anode: 2Cl– → Cl2 + 2e–
- Cell Reaction: 2NaCl → 2Na + Cl2
Thus, molten sodium chloride has to undergo electrolysis in an electrolytic cell to produce metallic sodium and chlorine gas.
Faraday’s Law of Electrolysis
[Click Here for Sample Questions]
Faraday's laws are critical to understanding when learning about electrolytic cells. The laws are used to express magnitudes of electrolytic effects.
In 1832, Michael Faraday introduced two laws of electrolysis:
| “The weights of substances forming at an electrode during electrolysis is directly proportional to the quantity of electricity that passes through the electrolyte.” |
| “The weights of different substances formed by the passage of the same quantity of electricity are proportional to the equivalent weight of each substance.” |
Applications of Electrolytic Cells
[Click Here for Sample Questions]
- An electrolytic cell can be used to produce oxygen gas and hydrogen gas from water.
- Electrolytic cells can be used to extract aluminium from bauxite.
- An electrolytic cell can also be used in electroplating. This process helps to form a thin protective layer of metal on another metal's surface.
- Electrolytic cells are also used in the electrorefining of various non-ferrous metals.
- Electrolytic cells can be used in electrowinning processes.
- Electrolytic cells are widely used in high-purity industrial productions such as copper, zinc, and aluminium.
Things to Remember
- An electrolytic cell converts electrical energy to chemical energy through a non-spontaneous redox action.
- Electrolytic cells are composed of two half-cells: reduction half-cell and oxidation half-cell.
- Three primary components of an electrolytic cell: Cathode, Anode, and Electrolyte.
- Electrolytic cells and Galvanic cells are very similar yet are very different from each other. For example, the electron flow is different in both cells.
- Faraday's laws are a critical aspect in understanding electrolysis. They define the magnitudes of electrolytic effects.
Read More:
Sample Questions
Ques: Aqueous solution of copper sulphate and silver nitrate are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be the same or different? Explain your answer. (2 Marks)
Ans: It will be different. According to Faraday's second law, the amounts of different substances liberated by the same quantity of electricity passing through the electrolytic solution are proportional to their chemical equivalent weights Atomic mass of metal/No. of electrons electrons required to reduce the cation.
Here, for the electrode reactions:
Cu²++2e-→Cu(s)
Ag-+e-→Ag(s)+
Hence, one mole of Cu²+ and Ag³+ require 2 mol of electron (2F) and 1 mol of electrons (F1) respectively.
Ques: State Faraday’s laws of electrolysis. How much charge in terms of Faraday is required for reduction of 1 mol of Cr2O7²- to Cr³+ (3 Marks)
Ans: First law-the chemical deposition due to flow of current through an electrolyte is directly proportional to the quantity of electricity (coulombs) passed through it.
Faraday's second law of electrolysis states that, when the same quantity of electricity is passed through several electrolytes, the mass of the substances deposited are proportional to their respective chemical equivalent or equivalent weight.
Ques. Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging. (4 Marks)
Ans: A lead storage battery consists of anode of lead, cathode of a grid of lead packed with lead dioxide (PbO2) and 38% H2SO4 solution as electrolyte. When the battery is in use, the reaction taking place are:
Anode: Pb(s)+SO4²-(aq)→PbSO4(s)+2e-
Cathode: PbO2(s)+SO42-(aq)+4H+(aq)+2e- →PbSO4(s)+2H2O(l)
Overall reaction: Pb(s)+PbO2(s)+2H2SO4(aq)→2PbSO4(s)+2H2O(l)
On charging the battery, the reverse reaction takes place, i.e., PbSO4 deposited on electrodes is converted back to Pb and PbO2 and H2SO4 is regenerated.
Ques. Three electrolytic cells A, B, C containing solutions of ZnS04, AgNO3 and CuS04, respectively are connected in series. A steady current of 1.5 amperes passed through them until 45 g of silver deposited at the cathode of call B. How long did the current flow? What mass of copper and zinc were deposited? (4 Marks)
Ans. Given: I=1.5A, W=1.45g of Ag, t=?, E=108, n=1
Using Faraday's 1st law of electrolysis W = ZIt
or, WE/nFIt1.4596500/1.5108=863.73 seconds.
Now for Cu, W1=1.45g of Ag, E1=108, W2= ?, E2 =31.75
From Faraday's 2nd law of electrolysis W1W2= E1E2
1.45W2=10831.75∴ W2=1.4531.75108=0.426g of Cu.
Similarly, for Zn, W1,=1.45g of Ag, E1,=108, W=?, E=32.65
Using formula, W1/W2= E1/E2
1.45/W2= 10832.65∴ W2=1.45*32.65/108=0.438 of Zn.
Ques. Suggest a list of metals which can be extracted electrolytically. (3 Marks)
Ans: The highly reactive metals having large -ve E° values, which can themselves act as powerful reducing agents can be extracted electrolytically. The process is known as electrolytic reduction.
Metals which are higher in the electrochemical series such as sodium, potassium, magnesium , calcium and aluminium are extracted by electrolysis. These metals have a strong affinity for oxygen and their oxides are highly stable. It is not possible to reduce their oxides by common reducing agents such as carbon, carbon monoxide and hydrogen. Electro metallurgy provides a convenient method to extract these metals.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:



Comments