Jasmine Grover Study Abroad Expert
Study Abroad Expert
Radiation refers to the particles of energy from a source, traveling through space or other mediums. There are various types of radiation such as particle radiation, gravitational radiation, acoustic, and electromagnetic radiation. In this article, we will understand what radiation is, its properties, diagrams, formulas, applications, and related sample questions.
| Table of Contents |
Key Terms: Radiation, heat transmission process, thermal radiation, nuclear process, law of radiation, Energy, particle radiation, gravitational radiation, acoustic radiation, electromagnetic radiation
What is Radiation?
[Click Here for Sample Questions]
Radiation refers to the release of energy in the form of electromagnetic waves or as moving subatomic particles, especially the high-energy particles that cause ionization. Radiation does not require a medium, and the speed of transition is equal to the speed of light in that medium. Heat may be transferred through a vacuum or transparent material through the process of radiation. It is the continuous emission of energy.
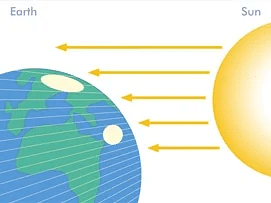
Radiation
For example, it is no secret that hot surfaces emit energy in the form of electromagnetic waves. This process is known as thermal radiation. For our earth’s existence, solar energy is important. It is really the location of our planet in our solar system that permits life on our planet to exist. But how is the sun's heat reaching us? How does the heat energy flow so long?
By the heat transmission process, radiation is released. The process of heat transmission by the emission of electromagnetic waves is called thermal radiation. These waves convey the body's thermal radiation-emitting energy and aid the heat of the sun in reaching us.
Also Read:
Types of Radiation
[Click Here for Previous Year Questions]
The main two types of radiation that exists in nature on the basis of the radiated particle’s energy are:
- Ionizing Radiation: These radiations carry the energy of more than 10 eV. This energy is enough to break the chemical bonds through the ionization of atoms and molecules. It consists of microwave radiation, radio waves, and infrared radiation.
- Non-Ionizing Radiation: These radiations consist of alpha, beta, and gamma particles.

Non-Ionizing Radiation
Other types of radiation are:
- Thermal Radiation: Thermal radiation is released by a body because of its temperature. In deciding how much radiant energy the item will release or absorb, the surface of an object plays a significant function.

Thermal Radiation
Here the sunlight is hitting the ground which makes it hot, which then makes the atmosphere warm with the help of thermal radiation.
- Nuclear Radiation: It is an energy that is released by the nuclear decay mechanism through elementary particles of the atomic nucleus.
Alpha Radiation: It is a short-range and heavy particle. It mainly is an ejected helium nucleus. Alpha radiation is also known as alpha decay, which is just radioactive decay. The formula for alpha particles is helium-4 (4He) nuclei.
Beta Radiation: This is classified under two categories.
- Beta Minus \(\beta \)-: This comprises an energetic electron. This in comparison to gamma radiation is way less penetrating.
- Beta Plus \(\beta \)+: This is the emission of positrons which are basically just antimatter forms of electrons.
Gamma Radiation: This comprises photons. The wavelength of photons is less than 3×10−11meters. The emission is a nuclear process that takes place to get rid of an unstable nucleus. These unstable nuclei are of unstable energy which happens after most nuclear reactions.
X-Ray Radiation: This is a type of electromagnetic radiation. It is no secret that X-rays are powerful forms of radiation. Most of the x-ray machines usually run on a wavelength range of 0.01 to 10 nanometers. These correspond to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 100 eV to 100 keV.

Nuclear Radiation
This type of radiation includes alpha, beta, gamma, and x-ray radiation (in the diagram neutron). All of these radiations are of different strengths and wavelengths.
- Gravitational Radiation: This radiation is like gravitational waves or ripples in the space-time curvature.’

- Black body Radiation: A body that absorbs heat radiation in its entirety from any wavelength is called a black body. It emits radiations of all wavelengths when such a body is heated. A perfect radiator is often referred to as a black body. The black body is not ideal, but the Ferry cavity is a flawlessly black body. The greatest example of a practical black body is a tiny hole in a box having a black interior. This is because virtually no radiation that enters this hole could escape back.

Properties Of Thermal Radiation
[Click Here for Sample Questions]
- Thermal Radiation does not need any material medium for heat transfer.
- This energy is called radiant energy. This radiation of energy is done from the surface of all bodies.
- This energy is a form of electromagnetic waves.
- The energy emitted largely depends on the nature and the temperature of the surface.
- Regardless of the state, all bodies radiate energy.
- The electromagnetic waves emitted by a body of increased temperature are called thermal radiation.
- Thermal radiation can either be absorbed or be reflected on the body depending on its color.
- Thermal radiation travels in a vacuum, in a straight line with the velocity of light.
- Thermal radiations can be reflected and refracted.
Related Terms to Radiation
[Click Here for Previous Year Questions]
- Total Emissive Power (e): At a certain temperature, the total emissive power of a body is defined as the total quantity of radiant energy radiated per second by the unit surface of the body. This is indicated by the symbol (e). The emissive power of a Black body is represented by the symbol E. The SI unit of Total Emissive Power is Jm-2s-1.
- Emissivity (?): The body's emissivity is a relationship between a body's total transmitting power and a black body's complete emissivity. This is shown by the symbol (?).
It means that
ε=e/E
or e=εE
For a black body,
ε=1
- Total Absorptive Power (a): The body's total absorptive power is defined as the ratio, in a particular time interval, of the total radiant energy absorbed by the body to the total energy that falls upon it at the same time interval. It is symbolically portrayed (a). The symbol that represents the complete absorption power of a black body is A. By default, A=1.
Radiation Formula
[Click Here for Sample Questions]
The radiation formula helps us determine the rate at which heat has been transferred by emitted radiation. This formula has been determined by Stefan-Boltzmann. The formula is:
Q/t = σeAT4
Here,
- σ is the Stefan-Boltzmann constant, 5.67×10−8 J/s.m2
- A is the surface area of the object
- T is the absolute temperature in Kelvin.
- e is the emissivity of the object
A jet-black (or black body) radiator has e = 1, whereas a perfect reflector has e = 0.
Applications of Radiation
[Click Here for Previous Year Questions]
- Radiation is widely used in many fields from medical, communication, to science.
- Radioactive chemicals are used for diagnosis, treatment, and research in the field of medicine.
- X-rays penetrate through the muscles and other soft tissues. X-rays, therefore, enable doctors to detect fractured bones and detect any malignancy in the body.
- Doctors utilize it for cancer therapy.
- It destroys or alters genes in order to inhibit cell development. Its other forms are known to be non-ionizing, such as radio waves, microwaves, and light waves.

Radiation in day-to-day life
Science
- It also has numerous uses in science like the utilization of radioactive atoms to determine the age of materials that previously belonged to a living being.
- They determine the age of such things through radiocarbon dating.
- Similarly, the age of rocks and other geological characteristics may be determined by various radioactive elements.
- Moreover, environmental scientists employ radioactive atoms, known as atoms for tracer, to detect the environmental routes of contaminants.

Radiation Therapy
Communications
- Electromagnetic radiation is used in all current communications systems. The fluctuations in the intensity of the sound, images, or other information delivered indicate the changes.
- For example, by making the wave fluctuate according to the fluctuation in the voice, we may broadcast a human voice as a radio or microwave.
- Likewise, musicians also explore sound and music via gamma rays.
Laws of Radiation
[Click Here for Sample Questions]
- Kirchhoff’s Law: The law of Kirchhoff says that the ratio of emission power to body absorption is a constant for all bodies and is equivalent to the emissive power of a fully black body at a certain temperature at a certain wavelength. An item radiates electromagnetic energy at a non-zero temperature, and it radiates energy according to a formula for black-body radiation if it is a perfect black body. The emissivity of a body (or surface) is equal to its absorption in the thermal equilibrium.
- Stefan’s Law: The entire quantity of radiant energy released in one second by a completely black body unit area is, according to Stefan's rule, exactly proportional to the fourth force of its absolute temperature. If E be the amount of radiant energy emitted by unit area in one second and T be the absolute temperature of the body, then
E/A = σT4
where σ is Stefan’s constant = 5.67×10–8Wm–2K4.
A body that isn’t black tends to absorb, which results in less radiation emission.
For such a body, E= eσAT4
e = emissivity (which is equal to absorptive power), which lies between 0 and 1.
With the surroundings of temperature T0 net energy radiated by an area A per unit time:
ΔE = E – E0
= eσA [T4–T04]
- Wien’s Displacement Law: Wien displacement law says that the wavelength (λm) is inversely related to its absolute temperature(T).
(λm) ∝ 1/T or
(λm)T= b
Where
b = 2.9×10–3mK is Wien’s constant.
Also Read:
Things To Remember
- Radiation is the transmission of heat from a hot body to a cold body
- Electromagnetic waves don’t need any material medium
- All bodies continuously radiate energy
- Thermal radiation is the electromagnetic radiation released by the body because of its temperature.
- Black body radiation completely absorbs thermal radiation.
- Kirchoff law says that the ratio of emission power to body absorption is a constant for all bodies and is equivalent to the emissive power of a fully black body at a certain temperature at a certain wavelength.
- Stefan’s law states that the entire quantity of radiant energy radiated in one second by the area of the unit of a completely black body is precisely proportional to its absolute fourth power, i.e. E/A = σT4.
- Wein’s Displacement law states that the wavelength of a black body is inversely related to its absolute temperature.
Previous Year Questions
- The de Broglie wavelength associated with the particle will be…..[NEET 2012]
- A radio transmitter operates at a frequency 880 kHz and a power of 10 kW. The number of photons emitted per second is….[NEET 1990]
- An example for the best source of monochromatic light is… [JKCET 2019]
- A photoelectric surface is illuminated successively by monochromatic light of wavelength ?...[NEET 2015]
- A photocell employs photoelectric effect to convert ….[NEET 2006]
- A particle of mass 1 mg has the same wavelength as an electron moving with a velocity…...[NEET 2008]
Sample Questions
Ques. What is radiation? What are the various types of radiation? (3 marks)
Ans. Radiation refers to the particles of energy from a source, traveling through space or other mediums. The various types of radiation are:
- Thermal
- Nuclear
- Gravitational
- Black Body
Ques. Ultrasound is which type of radiation? (2 marks)
Ans. Acoustic Radiation: The phenomena of the creation of acoustic radioactive power arises from an ultrasonic wave transferring momentum into an attenuating medium where both pressure and particle velocity are eliminated.
Ques. Radioactive atoms emit which type of radiation? (2 marks)
Ans. The three types of nuclear radiation emitted by radioactive atoms are Alpha, Beta, and Gamma radiation.
Ques. What does Gamma Radiation consist of? (2 marks)
Ans. Gamma Radiation consists of photons with a wavelength of less than 3 x 10-11 meters. The emission is a nuclear process that takes place to get rid of an unstable nucleus.
Ques. Which type of radiation is used in X-Ray? (2 marks)
Ans. Electromagnetic Radiation. They have sufficient energy to split molecules, damaging living cells. Some are absorbed and others go through when X-rays strike a substance.
Ques. What is the wavelength of X-ray? (2 marks)
Ans. Most of the x-ray machines usually run on a wavelength range of 0.01 to 10 nanometers. These correspond to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 100 eV to 100 keV.
Ques. State few applications of radiation. (5 marks)
Ans. Applications:
- Radiation is widely used in many fields from medical, communication, to science.
- X-rays penetrate through the muscles and other soft tissues, enabling doctors to detect fractured bones and detect any malignancy in the body.
- They are used for cancer therapy.
- It destroys or alters genes in order to inhibit cell development.
- Electromagnetic radiation is used in all current communications systems.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Also check:




Comments