
Content Strategy Manager
Haber Process or the Haber-Bosch process is one of the most cost-effective and efficient industrial processes for producing ammonia. A German scientist, Fritz Haber and his assistant invented the Haber process catalyst and high-pressure equipment to perform this process in a laboratory throughout the twentieth century. Later, Carl Bosch took the concept and turned it into a machine for industrial production in the year 1910. This was a significant advancement in the realm of science.
| Table of Content |
Keyterms: Ammonia, Hydrogen, Air, Nitrogen, Iron, Reaction rate, Catalyst, Equilibrium, water, Metal, Temperature
Raw Materials used in the Haber Process
[Click Here for Sample Questions]
- The Haber Bosch process is an excellent example of how industrial chemists utilise their understanding of chemical equilibria impacting variables. This is used to determine the optimal conditions for producing a high yield of products at a reasonable cost.
- By reacting nitrogen gas with hydrogen gas, the Haber-Bosch process produces ammonia. A metal catalyst is used in this process, which is carried out at high temperatures and pressures.
The following is a list of the raw materials used in the process.
- The nitrogen is supplied via air.
- Hydrogen and the energy required to heat the reactants are supplied by natural gas and water.
- The catalyst, iron, is not consumed during the process.
Also Read:
Haber Process Diagram
[Click Here for Sample Questions]
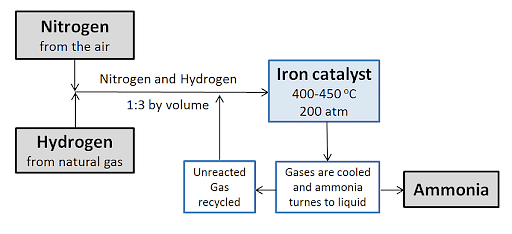
- In the Haber Bosch process, nitrogen gas is taken from the air and combined in a 1:3 volume ratio with hydrogen atoms acquired from natural gas.
- Over four catalyst beds, the gases are circulated with each pass cooling them. It is this process that maintains the equilibrium.
- Each pass has different conversion thresholds where unreacted gases are recycled.
- In most cases, an iron catalyst is used in the process. It is carried out at a temperature of 400–450 degrees Celsius and a pressure of 150–200 atmospheres.
- Carbon dioxide removal, shift conversion, methanation, and steam reforming are all part of this process.
- The ammonia gas is cooled to produce a liquid solution, which is collected and kept in storage containers at the end of the process.
Reaction Rate and Equilibrium of Haber Process
[Click Here for Sample Questions]
- The Haber process is based on the reaction of nitrogen and hydrogen gas to produce ammonia. The chemical reaction is shown below. Furthermore, the reaction is referred to as an exothermic reaction in this approach. It includes the release of energy.
N2(g) + 3H2(g) → 2NH3(g)
- During the reaction, nitrogen is obtained by liquefying it from the air, and hydrogen by reforming or steaming the natural gas.
CH4(g) + H2O → H2(g) + CO(g)
- The generation of ammonia is favoured by high pressure and low temperature, according to the Le Chatteleir principle. The Haber Process is usually carried out at a pressure of 200 to 400 atmospheres and a temperature of 500 degrees Celsius.
- NH3 is continually eliminated as ammonia is generated in commercial manufacturing. According to Le Chatelier's principle, removing the products causes additional nitrogen and hydrogen to mix.
- Changes in temperature, pressure, and catalyst, which are mostly utilised in the composition of the equilibrium mixture, the rate of the reaction, and the overall economics of the process.
Catalyst used in the Haber’s Process
[Click Here for Sample Questions]
- Although iron may be employed as a catalyst, the catalyst in this reaction is not pure iron. To increase its effectiveness, potassium hydroxide is added to it.
- This procedure is usually carried out at high temperatures and pressures.
- The reaction rate can be enhanced by utilising the catalyst, which consists of finely splitting the iron-containing molybdenum either as iron oxide or as a promoter due to the low working temperature.
- Instead of potassium hydroxide, we may also use CaO, K2O, Al2O3, and SiO2 as iron promoters.
- Osmium was employed as a catalyst in the first Haber process reaction chambers, but it was only accessible in very small amounts.
- Uranium was almost as easy and effective to obtain as osmium.
- A considerably less costly iron-based catalyst was found after extensive study.
Uses of Ammonia
[Click Here for Sample Questions]
- Agriculture: Ammonia production is crucial since it is one of the most essential ingredients in fertiliser production.
- Explosives: Ammonia may be used to make nitro-based explosives such as RDX, TNT, and others.
- Pharmaceuticals: It is utilised in the production of sulfonamides, antimalarials, and vitamins like thiamine and nicotinamide.
- Refrigeration: Ammonia is also used in large-scale refrigeration facilities, air-conditioning systems in buildings, and other applications.
- Consumer Products: Ammonia is an efficient cleaning ingredient that may be found in a variety of cleaning products.
Things to Remember
- The Haber Process is a technique for producing ammonia from hydrogen and nitrogen.
- The Haber Process is also known as the Harber-Bosh, Haber ammonia process and ammonia synthetic process.
- Carl Bosch used a catalyst and high-pressure techniques to transform the approach into a large-scale process.
- Pressures of 200 to 400 atmospheres and temperatures of 400° to 650° C (750° to 1200° F) are used in commercial production.
- The Haber-Bosch process is the most cost-effective for nitrogen fixation.
- It is still used as one of the basic chemical processes across the globe with minor changes.
Also Read:
Sample Questions
Ques: List the factors that influence the Haber process. (1 Marks)
Ans: Because the Haber cycle is a reversible process, changing the temperature or pressure of the reaction changes the ammonia production. The ammonia yield increases when the reaction pressure is increased.
Ques: How does Haber's process produce ammonia? (2 Marks)
Ans: The Haber process produces ammonia, which is extensively generated via the hydrogen (H2) and nitrogen (N2) Haber cycles. This Haber process produces ammonia gas by combining nitrogen gas from the environment with molecular hydrogen gas.
Ques: What are the advantages of using iron in the Haber Process? (2 Marks)
Ans: Advantages of using iron in Haber Process-
- The Haber cycle uses iron as a low-cost catalyst.
- It provides for a decent amount of time to get at a reasonable yield.
Ques: In what way does the Haber process get hydrogen? (2 Marks)
Ans: Natural gas methane is the primary source of hydrogen. Steam reforming is a technique that separates the carbon and hydrogen atoms in natural gas in a high-temperature and high-pressure pipe within a reformer using a nickel catalyst.
Ques: What is the significance of the Haber Process? (2 Marks)
Ans: The Haber process is still important today because it generates ammonia, which is needed for fertiliser and other reasons. The Haber cycle generates about 500 million tonnes of fertiliser each year (453 billion kg). This fertiliser aids in the feeding of around 40% of the world's population.
Ques: What are the other catalysts apart from iron that can be used in the Haber process? (2 Marks)
Ans: In haber process potassium hydroxide added to iron as a promoter to improve its effectiveness. Instead of potassium hydroxide, we may utilise CaO, K2O, Al2O3, and SiO2 as iron promoters. Osmium and Uranium were employed as a catalyst in the first Haber process reaction chambers.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:





Comments