CBSE Class 12 Chemistry Question Paper 2024 PDF (Set 3 - 56/1/3) is available for download here. CBSE conducted the Chemistry exam on February 27, 2024, from 10:30 AM to 1:30 PM. The total marks for the theory paper are 70. The question paper contains 20% MCQ-based questions, 40% competency-based questions, and 40% short and long answer type questions.
CBSE Class 12 Chemistry Question Paper 2024 (Set 3 - 56/1/3) with Answer Key
| CBSE Class 12 2024 Chemistry Question Paper with Answer Key | Check Solution |
CBSE Class 12 Chemistry Question Paper 2024 with Solution

Section A
The equilibrium \( Cr_2O_7^{2-} \rightleftharpoons 2CrO_4^{2-} \):
View Solution
Which of the following is the strongest acid?
View Solution
The acidity of benzoic acid derivatives depends on the electron-withdrawing or electron-donating nature of the substituent at the para position:
- Electron-withdrawing groups (like \( NO_2 \)) increase acidity by stabilizing the conjugate base through delocalization.
- Electron-donating groups (like \( OH \) or \( CH_3 \)) decrease acidity by increasing electron density on the carboxyl group.
Since \( p\)-NO\(_2\) is a strong electron-withdrawing group, it stabilizes the conjugate base, making benzoic acid more acidic. Quick Tip: The greater the electron-withdrawing effect, the stronger the acid due to better conjugate base stabilization.
Maltose is made up of:
View Solution
Maltose is a disaccharide composed of two glucose molecules linked by an \(\alpha\)-1,4-glycosidic bond.
It is formed from starch breakdown by the enzyme amylase and is further hydrolyzed by maltase to form glucose. Quick Tip: Maltose is a reducing sugar because it has a free anomeric carbon that can participate in oxidation reactions.
The system that forms maximum boiling azeotrope is:
View Solution
A maximum boiling azeotrope occurs when a mixture exhibits strong intermolecular interactions, leading to a lower vapor pressure and a higher boiling point.
The chloroform-acetone mixture forms strong hydrogen bonds, preventing easy separation and increasing boiling point. Quick Tip: Azeotropes can be minimum or maximum boiling. Maximum boiling azeotropes exhibit strong intermolecular attractions.
A zero-order reaction is one whose rate is independent of:
View Solution
For a zero-order reaction, the rate is independent of reactant concentration and follows: \[ Rate = k \]
This occurs when a catalyst or surface reaction dictates the rate rather than reactant concentration. Quick Tip: Common zero-order reactions include decomposition of ammonia on platinum and enzyme-catalyzed reactions at saturation.
In the Arrhenius equation, when \( \log k \) is plotted against \( 1/T \), a straight line is obtained whose:
View Solution
Arrhenius equation: \[ \log k = \log A - \frac{E_a}{2.303RT} \]
where slope \( = -\frac{E_a}{2.303 R} \) and intercept \( = \log A \). Quick Tip: A higher activation energy (\( E_a \)) means a stronger temperature dependence of the reaction rate.
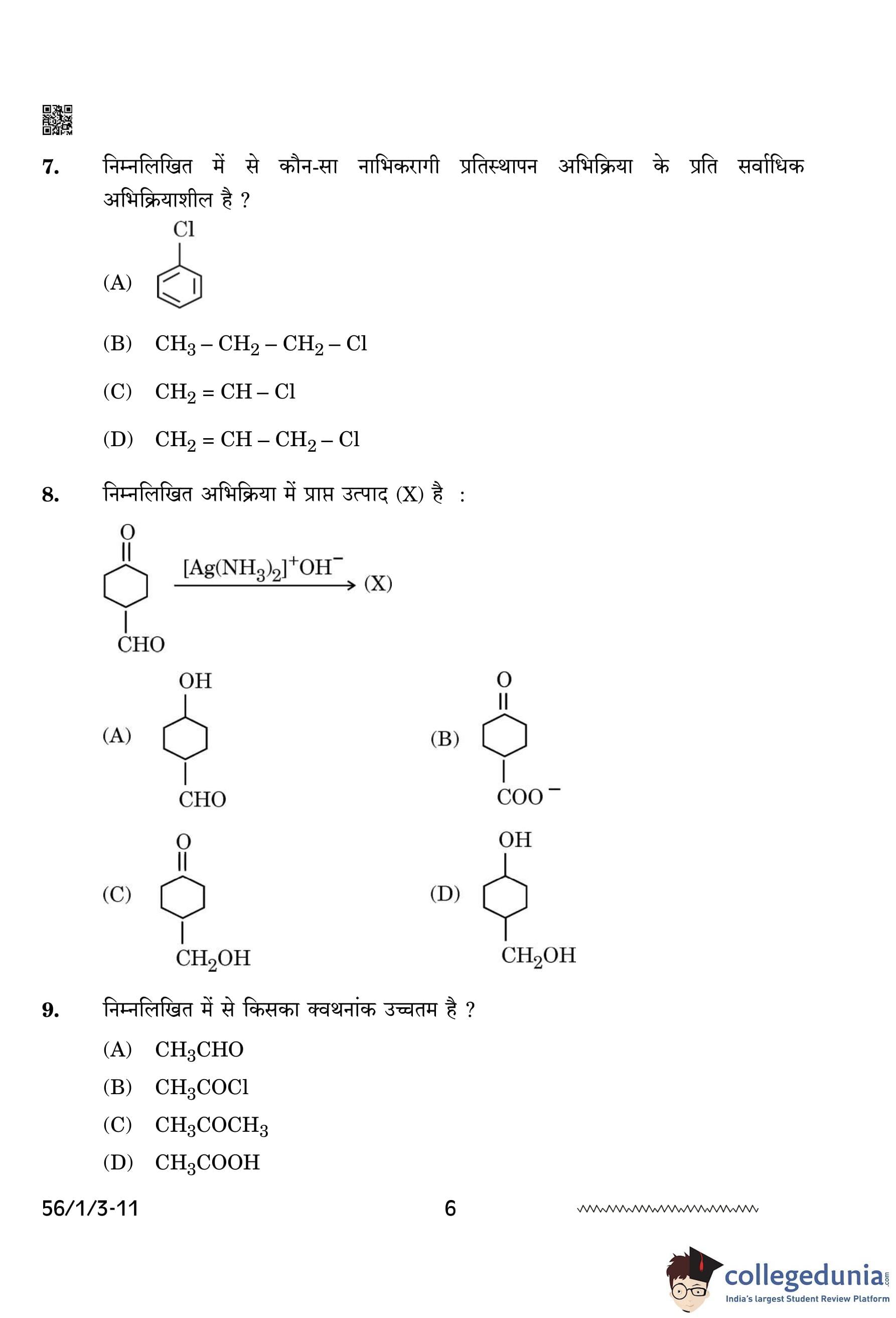
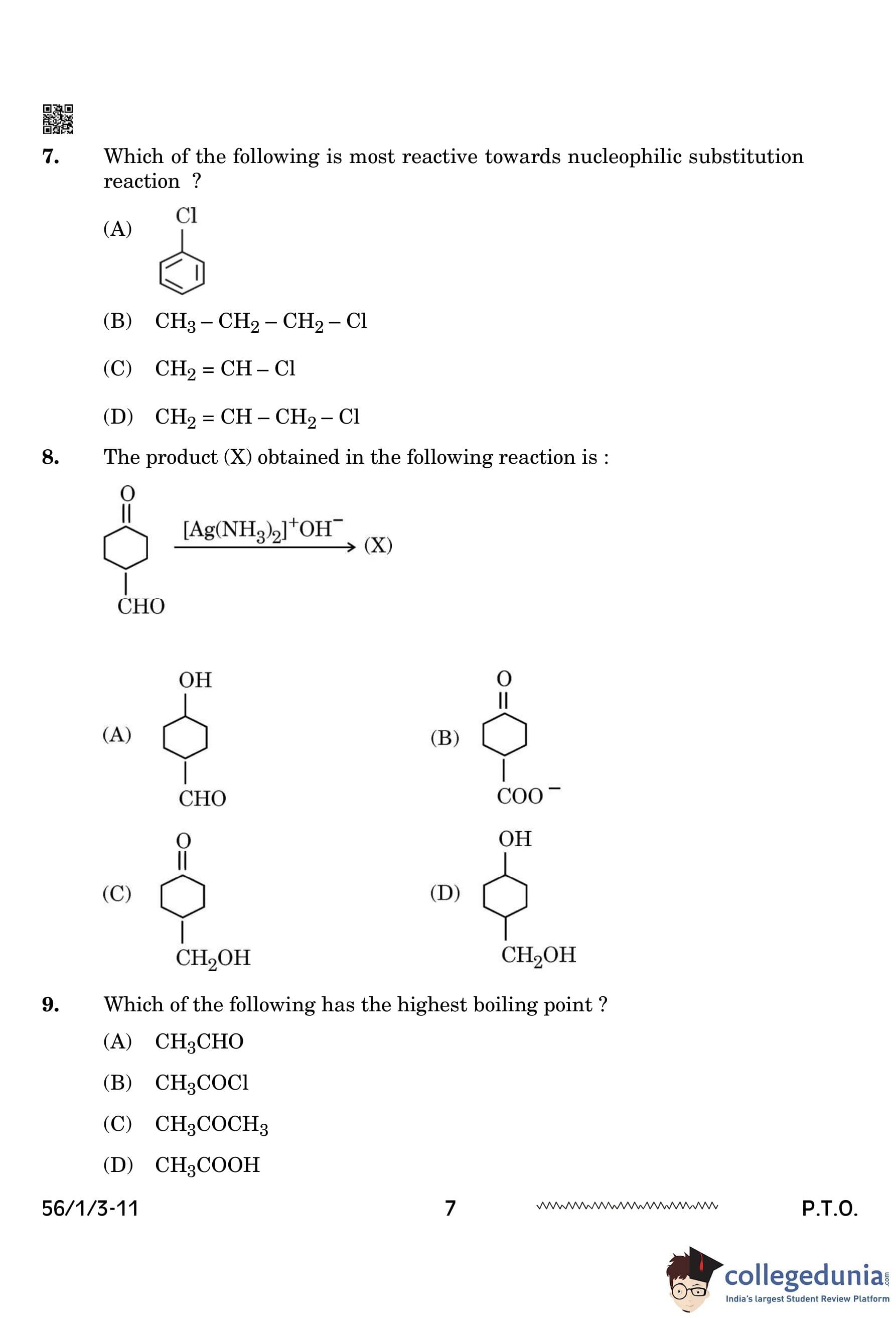
Which of the following is most reactive towards nucleophilic substitution reaction?
View Solution
Allyl chloride (\( CH_2=CH-CH_2-Cl \)) is most reactive towards nucleophilic substitution because the allylic carbocation formed during the reaction is stabilized by resonance.
Other compounds lack such stabilization, making them less reactive. Quick Tip: Carbocations that are resonance-stabilized undergo nucleophilic substitution reactions more readily.
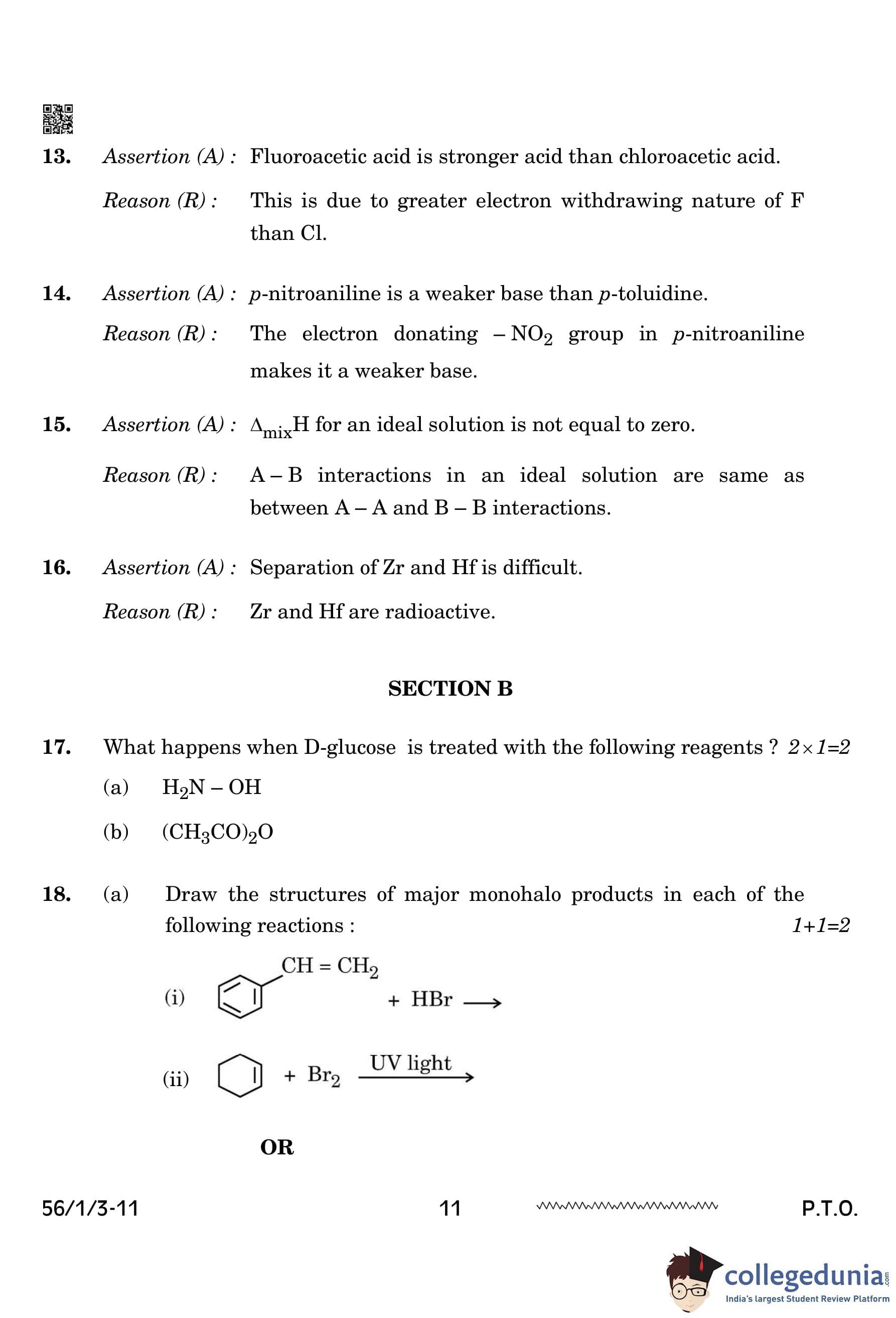
The product (X) obtained in the following reaction is:
View Solution
The given reaction is Tollens' test, which oxidizes aldehydes to carboxylates.
Since benzaldehyde lacks an alpha-hydrogen, it does not form a carboxylic acid but instead forms the corresponding benzoate ion. Quick Tip: Tollens' test is a specific test for aldehydes, giving a silver mirror as a positive result.
Which of the following has the highest boiling point?
View Solution
Carboxylic acids (\( CH_3COOH \)) have the highest boiling point due to intermolecular hydrogen bonding, forming dimers.
Other compounds exhibit weaker dipole-dipole interactions or van der Waals forces, leading to lower boiling points. Quick Tip: Compounds with hydrogen bonding generally have higher boiling points compared to those with dipole-dipole or van der Waals interactions.
Phenol does not undergo nucleophilic substitution reaction easily due to:
View Solution
The C-O bond in phenol has partial double bond character due to resonance, making it stronger and less reactive toward nucleophiles.
Thus, phenol resists nucleophilic substitution under normal conditions. Quick Tip: Due to resonance stabilization, phenol does not undergo SN1 or SN2 reactions easily.
The suitable Grignard reagent used for the synthesis of
with methanal is:
View Solution
A Grignard reagent reacts with methanal (\( HCHO \)) to give a primary alcohol.
Since the desired product is isopropanol, the correct Grignard reagent is isopropyl magnesium bromide (\( (CH_3)_2CHMgBr \)). Quick Tip: Grignard reagents react with formaldehyde to form primary alcohols and with ketones to form tertiary alcohols.
All proteins on hydrolysis give:
View Solution
Proteins are polymers of amino acids linked by peptide bonds.
Upon hydrolysis, they break down into \( \alpha \)-amino acids, which are the building blocks of proteins. Quick Tip: Proteins hydrolyze into amino acids, not sugars, because they are made from peptide bonds, not glycosidic bonds.
Assertion (A): Fluoroacetic acid is a stronger acid than chloroacetic acid.
Reason (R): This is due to greater electron-withdrawing nature of \( F \) than \( Cl \).
View Solution
Fluoroacetic acid is stronger than chloroacetic acid because fluorine has a higher electronegativity than chlorine. This increases the inductive (-I) effect, making the carboxylate ion more stable and enhancing acidity. Quick Tip: A stronger electron-withdrawing group increases acidity by stabilizing the conjugate base.
Assertion (A): \( p \)-nitroaniline is a weaker base than \( p \)-toluidine.
Reason (R): The electron-donating \( -NO_2 \) group in \( p \)-nitroaniline makes it a weaker base.
View Solution
\( p \)-Nitroaniline is weaker than \( p \)-toluidine because the \( -NO_2 \) group is electron-withdrawing (-M effect), which decreases the electron density on the nitrogen, making it less available for protonation. However, the given reason incorrectly states that \( -NO_2 \) is electron-donating, which is false. Quick Tip: Electron-withdrawing groups decrease basicity by reducing electron density on nitrogen.
Assertion (A): \( \Delta_{mix} H \) for an ideal solution is not equal to zero.
Reason (R): A–B interactions in an ideal solution are the same as between A–A and B–B interactions.
View Solution
In an ideal solution, the enthalpy of mixing (\( \Delta_{mix} H \)) is zero because intermolecular forces between A–B are equal to those between A–A and B–B. The assertion incorrectly states it is not equal to zero, making it false. The reason correctly describes an ideal solution. Quick Tip: For an ideal solution, \( \Delta_{mix} H = 0 \) and obeys Raoult’s Law.
Assertion (A): Separation of Zr and Hf is difficult.
Reason (R): Zr and Hf are radioactive.
View Solution
Zirconium (Zr) and Hafnium (Hf) have very similar atomic radii and chemical properties due to lanthanide contraction, making their separation difficult. However, they are not radioactive, making the given reason incorrect. Quick Tip: Lanthanide contraction results in similar properties of Zr and Hf, leading to difficult separation.
What happens when D-glucose is treated with the following reagents?
(a) \( H_2N-OH \)
(b) \( (CH_3CO)_2O \)
View Solution
(a) Reaction with Hydroxylamine (\( H_2N-OH \)):
When D-glucose is treated with hydroxylamine (\( H_2N-OH \)), it forms a glucose oxime.
The aldehyde group (\( -CHO \)) at C-1 reacts with hydroxylamine to form an oxime (\( -CH=N-OH \)):
(b) Reaction with Acetic Anhydride (\( (CH_3CO)_2O \)):
When D-glucose is treated with acetic anhydride, all the hydroxyl (\( -OH \)) groups of glucose undergo acetylation, forming penta-acetyl glucose.
This reaction protects all hydroxyl groups as ester groups (\( -OCOCH_3 \)), making the molecule less reactive in further chemical reactions. Quick Tip: - Oxime formation is a common test for aldehydes and ketones to confirm the presence of the carbonyl group. - Acetylation with acetic anhydride is used to protect hydroxyl groups in complex organic synthesis.
(a) Draw the structures of major monohalo products in each of the following reactions:
View Solution
(i) Reaction with HBr:
The addition of HBr to styrene (C\(_6\)H\(_5\)CH=CH\(_2\)) follows Markovnikov’s rule, leading to the formation of 1-bromoethylbenzene as the major product.
(ii) Bromination in UV light:
In the presence of UV light, free radical substitution occurs, resulting in the formation of bromocyclohexane as the major product.
Quick Tip: Markovnikov's rule states that in the electrophilic addition of HX to an alkene, the hydrogen atom adds to the carbon with more hydrogen atoms.
Give reasons for the following:
(i) Grignard reagent should be prepared under anhydrous conditions.
View Solution
Grignard reagents (RMgX) are highly reactive towards moisture. If water is present, it reacts with the Grignard reagent, forming an alkane instead of undergoing the desired reaction. This is why anhydrous conditions are necessary during preparation and storage. Quick Tip: Grignard reagents act as strong nucleophiles and bases, reacting rapidly with even small amounts of water, making anhydrous conditions essential.
For a chemical reaction \( R \to P \), the variation in the concentration \([R]\) vs time \( t \) plot is given as:
(a) Predict the order of the reaction and write the unit of rate constant (\( k \)) for this order of reaction.
(b) What is the slope of the curve?
View Solution
(a) Order of the Reaction and Unit of Rate Constant:
Since the plot of concentration \([R]\) versus time \( t \) is a straight line with a negative slope, the reaction follows zero-order kinetics.
For a zero-order reaction: \[ [R] = [R]_0 - kt \]
where \( k \) is the rate constant.
The unit of the rate constant for a zero-order reaction is: \[ mol L^{-1} s^{-1} \quad or \quad mol L^{-1} t^{-1} \]
(b) Slope of the Curve:
From the equation \( [R] = [R]_0 - kt \), the plot of \( [R] \) vs. \( t \) has a slope of \( -k \).
Thus, the slope of the curve is \( -k \). Quick Tip: For a zero-order reaction, the rate is independent of reactant concentration, and the half-life is given by: \[ t_{1/2} = \frac{[R]_0}{2k} \]
Write the chemical equations when:
(a) Ethanal is treated with 2,4-dinitrophenylhydrazine?
(b) Propanone is treated with Zn(Hg) and conc. HCl?
View Solution
(a) Reaction of Ethanal with 2,4-Dinitrophenylhydrazine (DNPH):
Ethanal (\( CH_3CHO \)) reacts with 2,4-dinitrophenylhydrazine (\( H_2N-NH\)-Ar-\( NO_2 \)) to form a hydrazone derivative.
This reaction is used to detect the presence of carbonyl groups (aldehydes and ketones).
(b) Reduction of Propanone using Zn(Hg) and Conc. HCl (Clemmensen Reduction):
Propanone (\( CH_3COCH_3 \)) undergoes Clemmensen reduction in the presence of zinc amalgam (Zn-Hg) and conc. HCl, reducing the carbonyl group to an alkane.
This method is commonly used to convert ketones into alkanes. Quick Tip: - The 2,4-DNPH test is a qualitative test for detecting aldehydes and ketones. - Clemmensen reduction is useful in removing carbonyl functional groups while retaining other sensitive groups.
Calculate the potential of an Iron electrode in which the concentration of Fe\( ^{2+} \) ion is 0.01 M.
\[ E^\circ_{Fe^{2+}/Fe} = -0.45 V \quad at \quad 298 K \]
\[ [Given: \log 10 = 1 ] \]
View Solution
The Nernst equation for the Fe\( ^{2+} \)/Fe electrode is:
\[ E_{Fe^{2+}/Fe} = E^\circ_{Fe^{2+}/Fe} - \frac{0.059}{2} \log \frac{1}{[Fe^{2+}]} \]
Substituting the given values:
\[ E_{Fe^{2+}/Fe} = -0.45 V - \frac{0.059}{2} \log \frac{1}{0.01} \]
\[ = -0.45 V - 0.059 \times 1 \]
\[ = -0.509 V \] Quick Tip: The Nernst equation is used to calculate electrode potential under non-standard conditions.
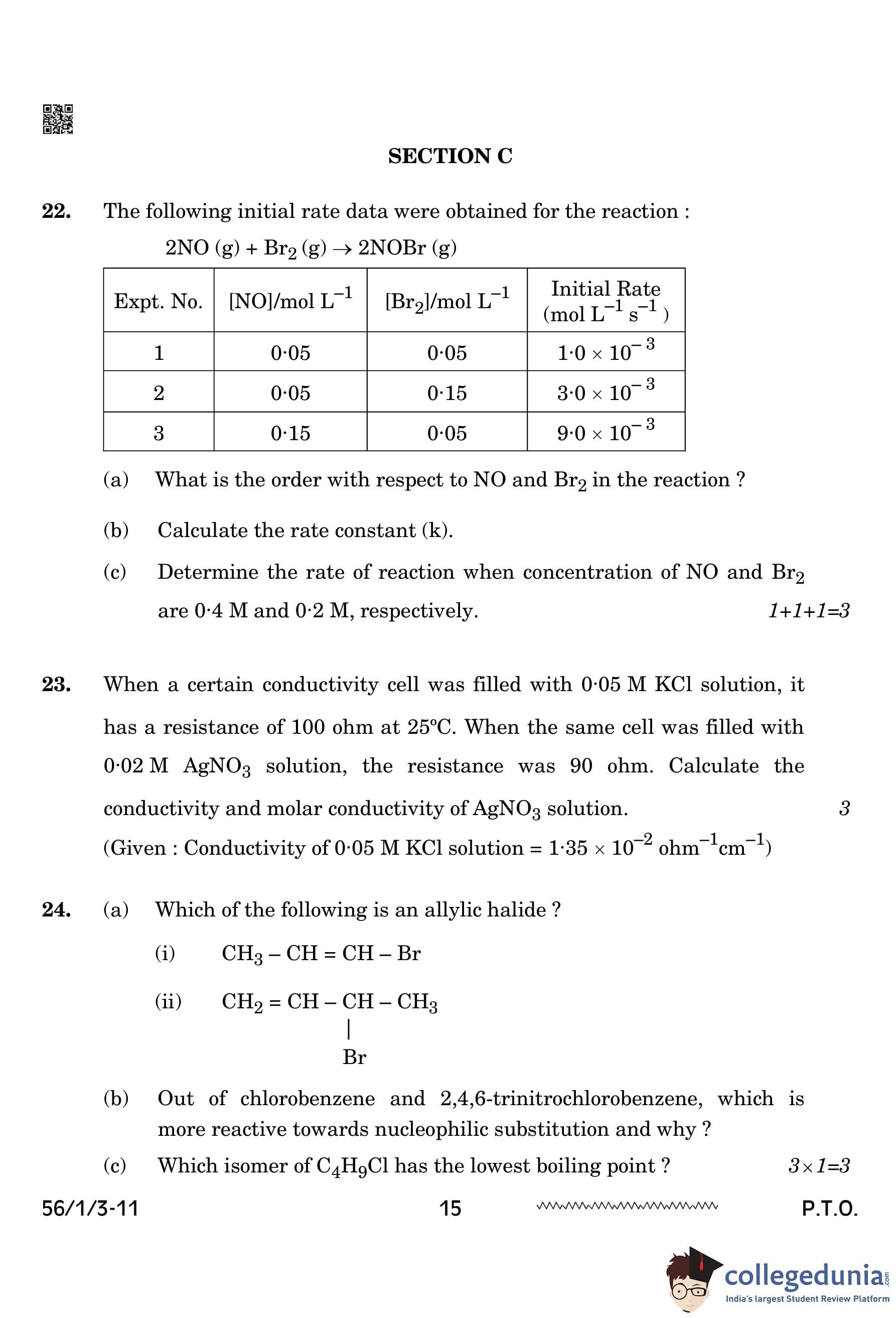
The following initial rate data were obtained for the reaction: \[ 2NO (g) + Br_2 (g) \rightarrow 2 NOBr (g) \]
(a) What is the order with respect to NO and \(Br_{2}\) in the reaction?
(b) Calculate the rate constant (k).
(c) Determine the rate of reaction when the concentrations of NO and \(Br_{2}\) are 0.4 M and 0.2 M, respectively.
View Solution
Rate law for the reaction can be expressed as: \[ Rate = k[NO]^p[Br_2]^q \]
Where \( p \) is the order with respect to NO and \( q \) is the order with respect to \(Br_{2}\).
(a) Determining the order with respect to NO (\( p \))
Compare experiments 1 and 2 to eliminate the effect of \(Br_{2}\) concentration and find \( p \).
From experiments 1 and 2: \[ \frac{1 \times 10^{-3}}{3 \times 10^{-3}} = \frac{k[0.05]^p[0.05]^q}{k[0.05]^p[0.15]^q} \] \[ \frac{1}{3} = \left( \frac{1}{3} \right)^q \quad \Rightarrow \quad q = 1 \]
Determining the order with respect to \(Br_{2}\) (\( q \))
Compare experiments 1 and 3 to eliminate the effect of NO concentration and find \( p \).
From experiments 1 and 3: \[ \frac{9 \times 10^{-3}}{1 \times 10^{-3}} = \frac{k[0.15]^p[0.05]^q}{k[0.05]^p[0.05]^q} \] \[ \frac{9}{1} = \left( \frac{3}{1} \right)^p \quad \Rightarrow \quad p = 2 \]
Thus, the order with respect to NO is 2 and the order with respect to \(Br_{2}\)s is 1.
(b) Calculating the rate constant (k)
Use the rate law and the data from any experiment to solve for \( k \).
Using experiment 1: \[ 1 \times 10^{-3} = k[0.05]^2[0.05] \] \[ k = \frac{1 \times 10^{-3}}{(0.05)^3} = 8 \, L^2 \, mol^{-2} \, s^{-1} \]
(c) Determining the rate when the concentrations of NO and \(Br_{2}\) are 0.4 M and 0.2 M
Now that we have the value of \( k \), we can use it to calculate the rate when [NO] = 0.4 M and [\(Br_{2}\)] = 0.2 M. \[ Rate = k[NO]^2[Br_2] \] \[ Rate = 8 \, L^2 \, mol^{-2} \, s^{-1} \times (0.4)^2 \times 0.2 \] \[ Rate = 8 \times 0.16 \times 0.2 = 2.56 \times 10^{-1} \, mol L^{-1} \, s^{-1} \] Quick Tip: When determining the order of a reaction with respect to reactants, use the method of comparing experiments with different concentrations of one reactant while keeping others constant. This will help isolate the effect of each reactant on the rate.
When a certain conductivity cell was filled with 0.05 M KCl solution, it has a resistance of 100 ohms at 25°C. When the same cell was filled with 0.02 M \(AgNO_{3}\) solution, the resistance was 90 ohms. Calculate the conductivity and molar conductivity of \(AgNO_{3}\) solution.
% Given:
Conductivity of 0.05 M KCl solution = \( 1.35 \times 10^{-2 \, \Omega^{-1} \, cm^{-1} \)
View Solution
To calculate the conductivity of \(AgNO_{3}\) solution, we will use the relationship between the conductivity, resistance, and the cell constant.
The cell constant (G*) is calculated using the following equation: \[ Cell constant (G*) = Conductivity \times Resistance \]
For KCl solution: \[ G^* = 1.35 \times 10^{-2} \, \Omega^{-1} \, cm^{-1} \times 100 \, \Omega = 1.35 \, cm^{-1} \]
Now, for the \(AgNO_{3}\) solution, we can use the same cell constant: \[ G^* = 1.35 \, cm^{-1} = Conductivity \times 90 \, \Omega \] \[ Conductivity = \frac{1.35}{90} = 0.015 \, S/cm \]
Next, we calculate the molar conductivity (\(\Lambda_m\)) using the formula: \[ \Lambda_m = Conductivity \times \frac{1000}{Concentration} \]
For \(AgNO_{3}\) solution with concentration \( 0.02 \, M \): \[ \Lambda_m = 0.015 \, S/cm \times \frac{1000}{0.02} = 750 \, Scm^2/mol \]
Thus, the molar conductivity of the \(AgNO_{3}\) solution is \( 750 \, Scm^2/mol \). Quick Tip: To calculate molar conductivity, the formula \( \Lambda_m = Conductivity \times \frac{1000}{C} \) is used, where \( C \) is the concentration of the solution in mol/L.
(a) Which of the following is an allylic halide?
(i)
(ii)
View Solution
An allylic halide is a compound in which the halogen is attached to a carbon that is adjacent to a double bond.
From the given options:
(i): is not an allylic halide because the halogen (Br) is not adjacent to the double bond.
(ii): is an allylic halide, as the halogen (Br) is attached to the carbon that is adjacent to the double bond.
Thus, the correct allylic halide is option (ii). Quick Tip: An allylic halide has the halogen attached to a carbon atom that is adjacent to a carbon-carbon double bond. This makes it more reactive in certain reactions, such as allylic substitution.
Out of chlorobenzene and 2,4,6-trinitrochlorobenzene, which is more reactive towards nucleophilic substitution and why?
View Solution
2,4,6-Trinitrochlorobenzene is more reactive towards nucleophilic substitution because the nitro (\( NO_2 \)) groups present on the benzene ring are electron-withdrawing in nature. These groups pull electron density away from the ring, making the carbon-chlorine bond weaker and easier to break, facilitating nucleophilic attack. In contrast, chlorobenzene does not have such electron-withdrawing groups, making it less reactive towards nucleophilic substitution. Quick Tip: Electron-withdrawing groups such as nitro (\( NO_2 \)) increase the reactivity of aromatic compounds in nucleophilic substitution reactions by destabilizing the carbon-halogen bond.
Which isomer of C\textsubscript{4}H\textsubscript{9}Cl has the lowest boiling point?
View Solution
The isomer of C\textsubscript{4H\textsubscript{9Cl with the lowest boiling point is tert-butyl chloride (CH\textsubscript{3)\textsubscript{3C-Cl. This is because branching reduces the surface area of the molecule, leading to weaker intermolecular forces (van der Waals forces) and consequently a lower boiling point compared to the straight-chain isomer. Quick Tip: Branched isomers generally have lower boiling points compared to their straight-chain counterparts due to weaker van der Waals forces arising from their reduced surface area.
(a) Write the formula for the following coordination compound:
Potassium tetrahydroxidozincate (II)
View Solution
N/A
Arrange the following complexes in the increasing order of conductivity of their solution: \[ [Cr(NH_3)_5Cl] Cl_2, \, [Cr(NH_3)_3Cl_3], \, [Cr(NH_3)_6Cl_3] \]
View Solution
N/A
Identify the type of isomerism exhibited by the following complexes:
View Solution
(i) \( [Co(NH_3)_5NO_2]^{2+} \) exhibits linkage isomerism, as the \({NO}_2\) ligand can bind through either the nitrogen or the oxygen atom.
(ii) \( [Co(en)_3]Cl_3 \) exhibits optical isomerism, as the complex is chiral and does not have a plane of symmetry. Quick Tip: Linkage isomerism occurs when a ligand can coordinate to the metal through two different atoms, while optical isomerism occurs when a molecule lacks any symmetry elements, such as a plane of symmetry, making it non-superimposable on its mirror image.
Compound (A) \( \left( C_6H_{12}O_2 \right) \) on reduction with LiAlH\textsubscript{4 gives two compounds (B) and (C). The compound (B) on oxidation with PCC gives compound (D) which upon treatment with dilute NaOH and subsequent heating gives compound (E). Compound (E) on catalytic hydrogenation gives compound (C). The compound (D) is oxidized further to give compound (F) which is found to be a monobasic acid (Molecular weight = 60). Identify the compounds (A), (B), (C), (D), (E) and (F).
View Solution
Identifying Compound (A)
The molecular formula of compound (A) is \( C_6H_{12}O_2 \), which could be either a carboxylic acid or an ester. Upon reduction with LiAlH\textsubscript{4, it gives two compounds, indicating the presence of both a carboxyl group and an ester group in compound (A). The most likely structure of compound (A) is ethyl acetate (CH\textsubscript{3COOCH\textsubscript{2CH\textsubscript{3).
\[ \, CH_3CH_2COOCH_3 \, or \, CH_3COOCH_2CH_3 \]
Identifying Compound (B)
Upon reduction with LiAlH\textsubscript{4, compound (A) gives compound (B), which is likely ethanol (CH\textsubscript{3CH\textsubscript{2OH).
\[ \, CH_3CH_2OH \]
Identifying Compound (C)
Upon reduction with LiAlH\textsubscript{4, compound (B) (acetaldehyde) gets reduced to propyl alcohol (CH\textsubscript{3CH\textsubscript{2CH\textsubscript{2OH). LiAlH\textsubscript{4 is a strong reducing agent, typically used to reduce aldehydes to primary alcohols.
\[ \, CH_3CH_2CH_2OH \]
Identifying Compound (D)
Compound (B) is ethanol (CH\textsubscript{3CH\textsubscript{2OH). When ethanol is oxidized using PCC (Pyridinium chlorochromate), it is converted to acetaldehyde (CH\textsubscript{3CHO). This reaction is typical for primary alcohols, where mild oxidants like PCC prevent further oxidation to carboxylic acids.
\[ D: \, CH_3CHO \]
Identifying Compound (E)
Upon catalytic hydrogenation of acetaldehyde (CH\textsubscript{3CHO), acrolein (CH\textsubscript{3CH=CHCHO) is formed. This reaction involves the reduction of the carbonyl group in acetaldehyde into an alkene group while keeping the aldehyde functional group intact.
\[ \, CH_3CH = CH - CHO \]
Identifying Compound (F)
Upon further oxidation of acrolein (CH\textsubscript{3CH=CHCHO), acetic acid (CH\textsubscript{3COOH) is produced. This is a common reaction where aldehydes undergo oxidation to form carboxylic acids.
\[ \, CH_3COOH \] Quick Tip: - Reduction with LiAlH\textsubscript{4} reduces ester and carboxyl groups to alcohols. - Oxidation with PCC selectively oxidizes alcohols to aldehydes. - Hydrogenation reduces aldehydes to alcohols.
(a) Write the mechanism of the following reaction: \[ CH_3CH_2OH \xrightarrow{H^+ \, 443\, K} CH_2 = CH_2 \]
View Solution
N/A
Write the main product in each of the following reactions:
View Solution
For the given reactions:
- (i) The oxidation of the hydroboration product with hydrogen peroxide (\(H_2O_2\)) and hydroxide (\(OH^-\)) leads to the formation of a diol.
The main product will be (CH\textsubscript{3–CH\textsubscript{2–CH\textsubscript{2–CH\textsubscript{2OH), 1-propanol.
- (ii) Phenol (C\textsubscript{6H\textsubscript{5OH) reacts with aqueous NaOH to form phenoxide ion (C\textsubscript{6H\textsubscript{5O\textsuperscript{−).
When phenol reacts with CO\textsubscript{2 in the presence of H\textsuperscript{+, it undergoes carboxylation to form salicylic acid (2-hydroxybenzoic acid).
\[ Main product: \, Salicylic acid (C\textsubscript{6H\textsubscript{4}(OH)COOH)} \] Quick Tip: In reactions of alcohols and phenols, the product formation can be understood by considering the reactivity of the hydroxyl group (-OH) with reagents such as borane, hydrogen peroxide, or carboxylic acids.
Answer the following: (any three)
(a) What is peptide linkage?
View Solution
N/A
What type of bonds hold a DNA double helix together?
View Solution
N/A
Which one of the following is a polysaccharide?
Sucrose, Glucose, Starch, Fructose
View Solution
N/A
Give one example each for water-soluble vitamins and fat-soluble vitamins.
View Solution
- Water-soluble vitamins: Vitamin B and Vitamin C.
- Fat-soluble vitamins: Vitamin A, D, E, K.
These vitamins are classified based on their solubility, with water-soluble vitamins dissolving in water and fat-soluble vitamins dissolving in fats and oils. Quick Tip: - Peptide bonds form the backbone of proteins. - Hydrogen bonds are weak but critical for stabilizing the structure of DNA. - Starch is a major energy storage polysaccharide in plants. - Water-soluble vitamins are essential for metabolic processes, while fat-soluble vitamins are stored in fat tissues.
What is crystal field splitting energy?
View Solution
The energy used in the splitting of degenerate d-orbitals due to the presence of ligands in a definite geometry is called Crystal Field Splitting Energy.
Step 1: Understanding Crystal Field Splitting Energy
When ligands approach a transition metal ion, the degeneracy of the d-orbitals is lifted due to electrostatic interactions, causing them to split into two sets of orbitals: \[ t_{2g} \quad (lower energy) \quad and \quad e_g \quad (higher energy) \]
The energy difference between these orbitals is called Crystal Field Splitting Energy (\(\Delta\)). Quick Tip: Crystal Field Splitting Energy (\(\Delta\)) depends on: - The nature of the ligand (strong field or weak field). - The oxidation state of the metal ion. - The geometry of the complex (octahedral, tetrahedral, square planar).
Give reason for the violet colour of the complex \( [Ti(H_2O)_6]^{3+} \) on the basis of crystal field theory.
View Solution
The violet colour arises due to the d-d electronic transition within the split d-orbitals.
Step 1: Electronic Configuration of \( [Ti(H_2O)_6]^{3+} \)
For \( Ti^{3+} \), the electronic configuration is: \[ Ti^{3+} = 3d^1 \]
Step 2: Crystal Field Splitting and d-d Transition
In an octahedral field, the d-orbitals split into: \[ t_{2g} \quad (lower energy) \quad and \quad e_g \quad (higher energy) \]
Since Ti\(^{3+}\) has one electron, it occupies the \( t_{2g} \) orbital as: \[ t_{2g}^1 e_g^0 \]
Step 3: Cause of Violet Colour
When visible light is absorbed, the electron gets excited from the \( t_{2g} \) to the \( e_g \) orbital, causing a d-d transition. The observed colour (violet) is due to the complementary colour of the absorbed wavelength. Quick Tip: The colour of transition metal complexes is due to d-d transitions. The nature of ligands and the splitting energy \(\Delta\) determine the observed colour.
\( [Cr(NH_3)_6]^{3+} \) is paramagnetic while \( [Ni(CN)_4]^{2-} \) is diamagnetic. Explain why.
View Solution
\( [Cr(NH_3)_6]^{3+} \) is paramagnetic due to unpaired electrons, while \( [Ni(CN)_4]^{2-} \) is diamagnetic due to electron pairing.
Step 1: Electronic Configuration of Cr\(^{3+}\) and Ni\(^{2+}\) \[ Cr^{3+} = 3d^3, \quad Ni^{2+} = 3d^8 \]
Step 2: Effect of Ligands on Magnetic Properties
- \( NH_3 \) is a weak field ligand, so it does not cause electron pairing in \( [Cr(NH_3)_6]^{3+} \), leaving unpaired electrons in the \( t_{2g} \) orbitals.
- \( CN^- \) is a strong field ligand, so it pairs electrons in \( [Ni(CN)_4]^{2-} \), making it diamagnetic. Quick Tip: - Strong field ligands like \( CN^- \) cause pairing of electrons, leading to diamagnetic behaviour. - Weak field ligands like \( NH_3 \) do not cause pairing, leading to paramagnetism.
Explain why \( [Fe(CN)_6]^{3-} \) is an inner orbital complex, whereas \( [Fe(H_2O)_6]^{3+} \) is an outer orbital complex.
View Solution
\( [Fe(CN)_6]^{3-} \) is an inner orbital complex due to strong ligand-induced pairing, whereas \( [Fe(H_2O)_6]^{3+} \) is an outer orbital complex due to weak ligand-induced hybridization.
Electronic Configuration of Fe\(^{3+}\) \[ Fe^{3+} = 3d^5 \]
Effect of Ligands on Hybridization
- \( CN^- \) is a strong field ligand, causing electron pairing and leading to \( d^2sp^3 \) hybridization (inner orbital complex).
- \( H_2O \) is a weak field ligand, preventing electron pairing and leading to \( sp^3d^2 \) hybridization (outer orbital complex). Quick Tip: - Inner orbital complexes involve \( d^2sp^3 \) hybridization with strong ligands. - Outer orbital complexes involve \( sp^3d^2 \) hybridization with weak ligands.
N,N-diethylbenzenesulphonamide is insoluble in alkali. Give reason.
View Solution
N,N-diethylbenzenesulphonamide does not contain any hydrogen atom attached to nitrogen, making it non-acidic and thus insoluble in alkali.
Step 1: Understanding the Nature of N,N-Diethylbenzenesulphonamide
- In order for a compound to dissolve in alkali, it must have an acidic hydrogen atom that can react with OH\(^-\).
Step 2: Why It Is Insoluble
- Sulphonamides that contain a hydrogen attached to nitrogen are acidic and dissolve in alkali.
- However, in N,N-diethylbenzenesulphonamide, both hydrogen atoms on nitrogen are replaced by ethyl groups, making it non-acidic and hence, insoluble in alkali. Quick Tip: - Only acidic compounds dissolve in alkali. - The absence of an acidic hydrogen makes a compound insoluble in bases.
Aniline does not undergo Friedel-Crafts reaction. Why?
View Solution
Due to salt formation with AlCl\(_3\), aniline becomes a Lewis base, deactivating the benzene ring.
Step 1: Role of Lewis Acid Catalyst in Friedel-Crafts Reaction
- Friedel-Crafts alkylation and acylation require a Lewis acid catalyst like AlCl\(_3\) to generate the electrophile.
Step 2: Interaction Between Aniline and AlCl\(_3\)
- Aniline (\( C_6H_5NH_2 \)) has a lone pair on nitrogen, which interacts with AlCl\(_3\), forming a salt. \[ C_6H_5NH_2 + AlCl_3 \rightarrow [C_6H_5NH_2]^+ [AlCl_3]^- \]
- This deactivates the benzene ring, making it less reactive toward electrophilic substitution.
Step 3: Conclusion
Due to this salt formation, aniline does not undergo Friedel-Crafts reaction. Quick Tip: - AlCl\(_3\) forms a salt with aniline, deactivating the benzene ring. - Electrophilic substitution is hindered, preventing Friedel-Crafts reaction.
Write a simple chemical test to distinguish between methylamine and aniline.
View Solution
Diazotization Test: Aniline forms a diazonium salt, which gives an orange dye with phenol, whereas methylamine does not.
Diazotization of Aniline
- Aniline reacts with nitrous acid (\( HNO_2 \)) at low temperature (0–5°C) to form benzene diazonium chloride. \[ C_6H_5NH_2 + HNO_2 \rightarrow C_6H_5N_2^+Cl^- \]
Coupling Reaction with Phenol
- The diazonium salt reacts with phenol to form an orange dye.
- Methylamine (\( CH_3NH_2 \)) does not undergo diazotization and does not form the orange dye. Quick Tip: - Aniline undergoes diazotization and forms a coloured dye. - Methylamine does not form a diazonium salt.
Write the chemical reaction involved in Gabriel phthalimide synthesis.
View Solution
Gabriel phthalimide synthesis is used for the preparation of primary amines.
Step 1: Formation of N-Alkyl Phthalimide \[ Phthalimide + KOH \rightarrow Potassium Phthalimide \] \[ Potassium Phthalimide + R-X \rightarrow N-Alkyl Phthalimide \]
Step 2: Hydrolysis to Form Primary Amine \[ N-Alkyl Phthalimide + NaOH \rightarrow Primary Amine + Phthalic Acid \] Quick Tip: - Gabriel Phthalimide Synthesis is specific for primary amines. - Secondary and tertiary amines are not formed in this reaction.
How will you convert aniline to p-bromoaniline?
View Solution
Aniline is acetylated, then brominated, and finally hydrolyzed to obtain p-bromoaniline.
Step 1: Acetylation of Aniline \[ Aniline + (CH_3CO)_2O \rightarrow Acetanilide \]
Step 2: Bromination of Acetanilide \[ Acetanilide + Br_2 + CH_3COOH \rightarrow p-Bromoacetanilide \]
Step 3: Hydrolysis to p-Bromoaniline \[ p-Bromoacetanilide + OH^- \rightarrow p-Bromoaniline \] Quick Tip: - Direct bromination of aniline gives both ortho and para isomers. - Acetylation controls the reaction to favor p-bromoaniline.
Complete the following reaction:
\[ C_6H_5N_2^+Cl^- \xrightarrow{(i) HBF_4} \xrightarrow{(ii) NaNO_2/Cu, \Delta} \quad ? \]
View Solution
The given reaction follows the Sandmeyer and Balz-Schiemann reactions, leading to the formation of fluorobenzene and nitrobenzene.
Step 1: Balz-Schiemann Reaction (Fluorination)
The first step involves treating benzene diazonium chloride with HBF\(_4\), forming benzene diazonium tetrafluoroborate. \[ C_6H_5N_2^+Cl^- + HBF_4 \rightarrow C_6H_5N_2^+BF_4^- \]
On heating, it decomposes to form fluorobenzene: \[ C_6H_5N_2^+BF_4^- \xrightarrow{\Delta} C_6H_5F + N_2 + BF_3 \]
Step 2: Sandmeyer Reaction (Nitration)
The second step involves treating benzene diazonium chloride with NaNO\(_2\)/Cu, which replaces diazonium with a nitro (-NO\(_2\)) group, forming nitrobenzene. \[ C_6H_5N_2^+Cl^- + NaNO_2/Cu, \Delta \rightarrow C_6H_5NO_2 + N_2 \] Quick Tip: - The Balz-Schiemann reaction is used to introduce fluorine into the benzene ring. - The Sandmeyer reaction is used to introduce nitro (-NO\(_2\)) and other functional groups.
Write the structures of A and B in the following reaction:
\[ C_6H_5COOH \xrightarrow{NH_3, \Delta} A \xrightarrow{Br_2/NaOH} B \]
View Solution
- \( A \) = Benzamide (\( C_6H_5CONH_2 \))
- \( B \) = Aniline (\( C_6H_5NH_2 \))
Step 1: Conversion of Benzoic Acid to Benzamide
When benzoic acid (\( C_6H_5COOH \)) is heated with ammonia, it undergoes amide formation, forming benzamide (\( C_6H_5CONH_2 \)). \[ C_6H_5COOH + NH_3 \xrightarrow{\Delta} C_6H_5CONH_2 \]
Step 2: Hoffmann Bromamide Degradation
When benzamide (\( C_6H_5CONH_2 \)) reacts with Br\(_2\)/NaOH, the carbonyl (-CO) group is removed, leading to the formation of aniline (\( C_6H_5NH_2 \)). \[ C_6H_5CONH_2 + Br_2 + NaOH \rightarrow C_6H_5NH_2 + Na_2CO_3 \] Quick Tip: - The Hoffmann Bromamide Reaction is used for amide to amine conversion by removing the carbonyl group. - This reaction reduces carbon count by 1, making it useful for amine synthesis.
(1) The melting and boiling points of Zn, Cd, and Hg are low.
View Solution
The melting and boiling points of Zn, Cd, and Hg are low due to the absence of unpaired electrons in their d-orbitals, leading to weak metallic bonding between atoms.
Electronic Configuration and Bonding
- Zn, Cd, and Hg belong to Group 12 and have a completely filled d\(^ {10}\) configuration.
- The lack of unpaired d-electrons reduces the extent of metallic bonding, making these metals soft with low melting and boiling points.
Weak Interatomic Forces
- In transition metals, strong metallic bonding arises due to overlapping of d-orbitals.
- However, in Zn, Cd, and Hg, fully filled d-orbitals do not contribute to bonding, resulting in weak interatomic forces and low melting/boiling points. Quick Tip: - Fully filled d-orbitals weaken metallic bonding. - Strong metallic bonding requires unpaired d-electrons.
Of the \( d^4 \) species, Cr\(^{2+}\) is strongly reducing while Mn\(^{3+}\) is strongly oxidizing.
View Solution
Cr\(^{2+}\) is strongly reducing because it tends to oxidize to the stable \( d^3 \) configuration in Cr\(^{3+}\), while Mn\(^{3+}\) is strongly oxidizing as it prefers to reduce to the more stable \( d^5 \) configuration in Mn\(^{2+}\).
Electronic Configurations
- Chromium (Cr):
\[ Cr^{2+} = [Ar] 3d^4 \]
\[ Cr^{3+} = [Ar] 3d^3 \quad (Stable t_{2g}^3 configuration) \]
- Manganese (Mn):
\[ Mn^{3+} = [Ar] 3d^4 \]
\[ Mn^{2+} = [Ar] 3d^5 \quad (Stable half-filled d^5 configuration) \]
Explanation
- Cr\(^{2+}\) loses an electron easily to form Cr\(^{3+}\) (stable \( d^3 \)), making it a strong reducing agent.
- Mn\(^{3+}\) readily gains an electron to form Mn\(^{2+}\) (stable \( d^5 \)), making it a strong oxidizing agent. Quick Tip: - Stable electronic configurations drive oxidation/reduction tendencies. - Cr\(^{2+}\) prefers oxidation to \( d^3 \), Mn\(^{3+}\) prefers reduction to \( d^5 \).
\( E^0 \) value of Cu\(^{2+}\)/Cu is \( +0.34 \) V.
View Solution
The high atomization enthalpy (\( \Delta H^0_{atom} \)) and low hydration enthalpy (\( \Delta H^0_{hydr} \)) of copper make its standard reduction potential (\( E^0 \)) positive.
Explanation of \( E^0 \) Value
- The electrode potential (\( E^0 \)) depends on:
- Atomization enthalpy (\( \Delta H^0_{atom} \)): The energy required to convert solid Cu to Cu\(^{2+}\) is high.
- Hydration enthalpy (\( \Delta H^0_{hydr} \)): Cu\(^{2+}\) has low hydration energy, making it less stable in aqueous solution.
Effect on \( E^0 \) Value
- Due to low hydration enthalpy, the reduction of Cu\(^{2+}\) to Cu is not highly favored.
- Hence, Cu\(^{2+}/\)Cu has a positive \( E^0 \) value of \( +0.34 \) V, indicating that Cu is less reactive than expected. Quick Tip: - High atomization enthalpy and low hydration enthalpy make Cu\(^{2+} \rightarrow\) Cu less favorable, giving a positive \( E^0 \) value.
Complete and balance the following chemical equations:
View Solution
CuCl\(_2\) is more stable than Cu\(_2\)Cl\(_2\) in aqueous solution because Cu\(^{2+}\) is more stable than Cu\(^+\) due to its higher hydration enthalpy (\(\Delta_{hyd} H^\circ\)).
In aqueous solution, Cu\(^+\) undergoes disproportionation, as shown in the equation: \[ 2Cu^+(aq) \rightarrow Cu^{2+}(aq) + Cu(s) \]
(ii) Write the general electronic configuration of f-block elements.
% Solution
Solution:
The general electronic configuration of f-block elements is: \[ (n-2)f^{1-14} (n-1)d^{0-1} ns^2 \]
(iii) Predict which of the following will be coloured in aqueous solution and why?
Sc\(^{3+}\), Fe\(^{3+}\), Zn\(^{2+}\)
[Atomic numbers: Sc = 21, Fe = 26, Zn = 30]
% Solution
Solution:
Among the given ions, Fe\(^{3+}\) is coloured in aqueous solution because it has unpaired electrons in its d-orbital, which allows d-d transitions.
On the other hand:
- Sc\(^{3+}\) has an empty d-orbital (d\(^0\) configuration), so no d-d transition occurs → Colourless.
- Zn\(^{2+}\) has a fully filled d-orbital (d\(^{10}\) configuration), so no d-d transition occurs → Colourless.
(iv) How can you obtain potassium dichromate from sodium chromate?
% Solution
Solution:
Potassium dichromate (K\(_2\)Cr\(_2\)O\(_7\)) can be obtained from sodium chromate (Na\(_2\)CrO\(_4\)) through the following reactions:
\[ 2Na_2CrO_4 + 2H^+ \rightarrow Na_2Cr_2O_7 + 2Na^+ + H_2O \]
\[ Na_2Cr_2O_7 + 2KCl \rightarrow K_2Cr_2O_7 + 2NaCl \]
(v) Why do transition metals and their compounds show catalytic activities?
% Solution
Solution:
Transition metals and their compounds show catalytic activity because:
- They can exhibit variable oxidation states, allowing them to form intermediate complexes during reactions.
- They can adsorb reactants onto their surface, increasing reaction rates.
- Their large surface area provides active sites for catalytic activity. Quick Tip: - KMnO\(_4\) decomposition is a key reaction in the production of oxygen in labs. - Dichromate-Iodide reaction is a classic redox reaction where Cr\(^{6+}\) is reduced, and I\(^-\) is oxidized to I\(_2\). - Always balance chemical equations by ensuring both mass and charge conservation.
At the same temperature, \( CO_2 \) gas is more soluble in water than \( O_2 \) gas. Which one of them will have a higher value of \( K_H \) and why?
View Solution
\( O_2 \) gas has a higher \( K_H \) value because a higher \( K_H \) means lower solubility of the gas in liquid.
Step 1: Understanding Henry’s Law Constant (\( K_H \))
Henry’s law states that: \[ C = K_H P \]
where \( C \) is the concentration of the gas in liquid, \( K_H \) is Henry’s law constant, and \( P \) is the partial pressure of the gas.
Step 2: Relationship Between \( K_H \) and Solubility \[ K_H \propto \frac{1}{Solubility of Gas} \]
Since \( CO_2 \) is more soluble in water than \( O_2 \), it has a lower \( K_H \) value.
Thus, \( O_2 \) has a higher \( K_H \). Quick Tip: - A higher \( K_H \) means lower solubility of the gas. - A lower \( K_H \) means higher solubility of the gas.
How does the size of blood cells change when placed in an aqueous solution containing more than 0.9% (mass/volume) sodium chloride?
View Solution
Blood cells shrink.
Step 1: Understanding Hypertonic Solutions
A solution with more than 0.9% NaCl is hypertonic compared to the fluid inside blood cells.
Step 2: Effect on Blood Cells
Due to osmosis, water moves out of the blood cells into the hypertonic solution to balance the concentration gradient.
This leads to shrinking (crenation) of blood cells. Quick Tip: - Hypertonic solution: Water leaves the cell, causing shrinkage. - Hypotonic solution: Water enters the cell, causing swelling.
1 molal aqueous solution of an electrolyte \( A_2B_3 \) is 60% ionized. Calculate the boiling point of the solution.
View Solution
\( T_b = 374.768 \) K (or 374.918 K if considering the boiling point of water as 373.15 K)
Step 1: Boiling Point Elevation Formula
The boiling point elevation is given by: \[ \Delta T_b = i K_b m \]
where, \( K_b = 0.52 \, K \cdot kg \cdot mol^{-1} \) (for water), \( m = 1 \) molal, \( i \) = van’t Hoff factor.
Step 2: Calculation of Van't Hoff Factor
The dissociation of \( A_2B_3 \) is: \[ A_2B_3 \rightarrow 2A^{+} + 3B^{-} \]
Total particles before dissociation = 1,
Total particles after dissociation = 5.
Degree of ionization \( \alpha \) is given as 60% (0.6).
\[ i = 1 + \alpha (n - 1) \] \[ i = 1 + 0.6 (5 - 1) \] \[ i = 1 + 2.4 = 3.4 \]
Step 3: Calculate Boiling Point Elevation \[ \Delta T_b = 3.4 \times 0.52 \times 1 \] \[ \Delta T_b = 1.768 K \] \[ T_b = 373 + 1.768 = 374.768 K \]
If the boiling point of water is taken as 373.15 K, \[ T_b = 373.15 + 1.768 = 374.918 K \] Quick Tip: - The van’t Hoff factor (\( i \)) accounts for the number of particles formed in solution. - A higher \( i \) results in a greater elevation in boiling point. - Electrolytes with higher ionization lead to larger \( \Delta T_b \).
























Comments