CBSE Class 12 Chemistry Question Paper 2024 PDF (Set 1 - 56/2/1) is available for download here. CBSE conducted the Chemistry exam on February 27, 2024, from 10:30 AM to 1:30 PM. The total marks for the theory paper are 70. The question paper contains 20% MCQ-based questions, 40% competency-based questions, and 40% short and long answer type questions.
CBSE Class 12 Chemistry Question Paper 2024 (Set 1 - 56/2/1) with Answer Key
| CBSE Class 12 2024 Chemistry Question Paper with Answer Key | Check Solution |
CBSE Class 12 Chemistry Question Paper 2024 with Solution

Section A
When MnO\(_2\) is fused with KOH in air, it gives:
Ligand EDTA\(^{4-}\) is an example of a:
Which of the following ligands forms a chelate complex?
Which of the following contains sp\(^2\) hybridized carbon bonded to X?
Which of the following is most acidic?
Anisole reacts with HI to give:
Ethanol on heating with conc. H\(_2\)SO\(_4\) at 413 K gives:
An azeotropic solution of two liquids has a boiling point lower than either of them when it:
The relative lowering of vapor pressure of an aqueous solution containing a non-volatile solute is 0.0225. The mole fraction of the non-volatile solute is:
During electrolysis of an aqueous solution of NaCl:
The addition of a catalyst during a chemical reaction alters which of the following quantities of the reaction?
For the elementary reaction \( P \to Q \), the rate of disappearance of ‘P’ increases by a factor of 8 upon doubling the concentration of ‘P’. The order of the reaction with respect to ‘P’ is:
Assertion (A): Aliphatic primary amines can be prepared by Gabriel phthalimide synthesis.
Reason (R): Alkyl halides undergo nucleophilic substitution with anion formed by phthalimide.
Assertion (A): Uracil base is present in DNA.
Reason (R): DNA undergoes self-replication.
Assertion (A): Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Reason (R): Diazonium salts of aliphatic amines show resonance.
Assertion (A): p-Nitroaniline is a weaker base than p-toluidine.
Reason (R): The electron-withdrawing effect of \(-NO_2\) group in p-nitroaniline makes it a weaker base.
A 6% solution of glucose (molar mass = 180 g mol\(^{-1}\)) is isotonic with a 2.5% solution of an unknown organic substance. Calculate the molecular weight of the unknown organic substance.
A first-order reaction has a rate constant \( 1.25 \times 10^{-3} \, s^{-1} \). How long will 5 g of this reactant take to reduce to 2.5 g?
\([ log 2 = 0·301, log 3 = 0·4771, log 4 = 0·6021 ]\)
(a) What is lanthanoid contraction? Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
(b) Why do transition metals have high enthalpy of atomization? Which element of the 3d-series has the lowest enthalpy of atomization?
(a) In the following pair of compounds, which compound undergoes an \(S_{N}\)2 reaction faster and why?
(b) Write the major product in the following reaction:
Define the following terms:
(a) Denaturation of protein
(b) Invert sugar
Write the structures of A, B, and C in the following reactions:
Write the reaction involved in the following:
Wolff-Kishner reduction
Give the equations of reactions for the preparation of:
Phenol from chlorobenzene
Give reasons for the following:
Chlorine is ortho/para directing in electrophilic aromatic substitution reactions, though chlorine is an electron withdrawing group.
The vapour pressure of a solvent at 283 K is 100 mm Hg. Calculate the vapour pressure of a dilute solution containing 1 mole of a strong electrolyte AB in 50 moles of the solvent at 283 K (assuming complete dissociation of solute AB).
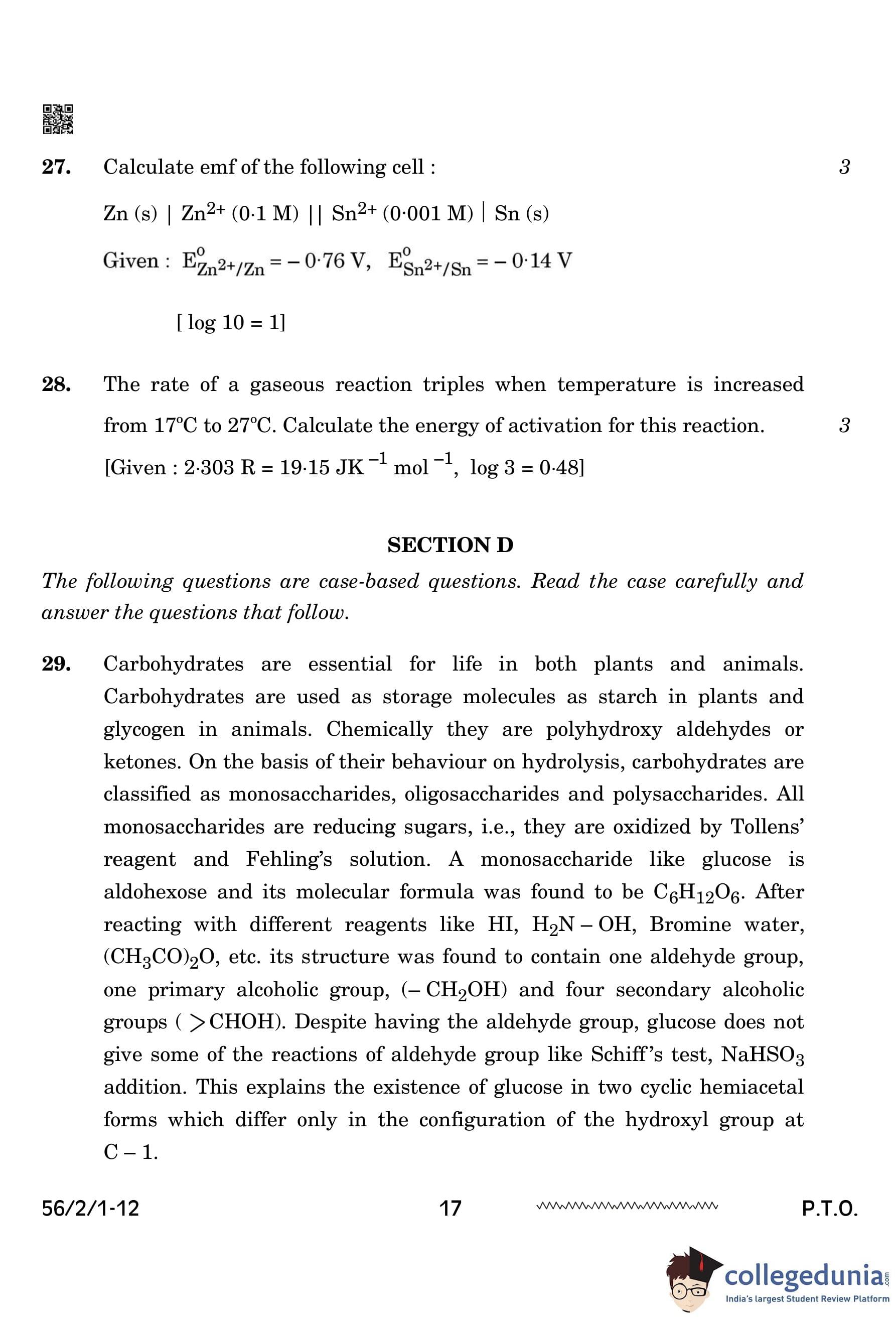
Calculate emf of the following cell: \[ Zn (s) | Zn^{2+} (0.1 M) || Sn^{2+} (0.001 M) | Sn (s) \]
Given: \[ E^\circ_{Zn^{2+}/Zn} = -0.76 \, V, \quad E^\circ_{Sn^{2+}/Sn} = -0.14 \, V \] \[ [\log 10 = 1] \]
The rate of a gaseous reaction triples when temperature is increased from 17°C to 27°C. Calculate the energy of activation for this reaction.
\([Given : 2 303 R = 19 15 JK^{-1} mol^{-1}, log 3 = 0 48]\)
(a) What are reducing sugars?
(b) Classify the following into monosaccharides and disaccharides: Fructose, Sucrose, Lactose, Galactose
(c) Name the polysaccharide which is known as ‘animal starch’. Why is it called ‘animal starch’?
(i) Name the isomers of glucose which in the cyclic form differ only in the configuration of the –OH group at C – 1.
(ii) Presence of which functional group was detected when glucose reacted with Br\(_2\) water?
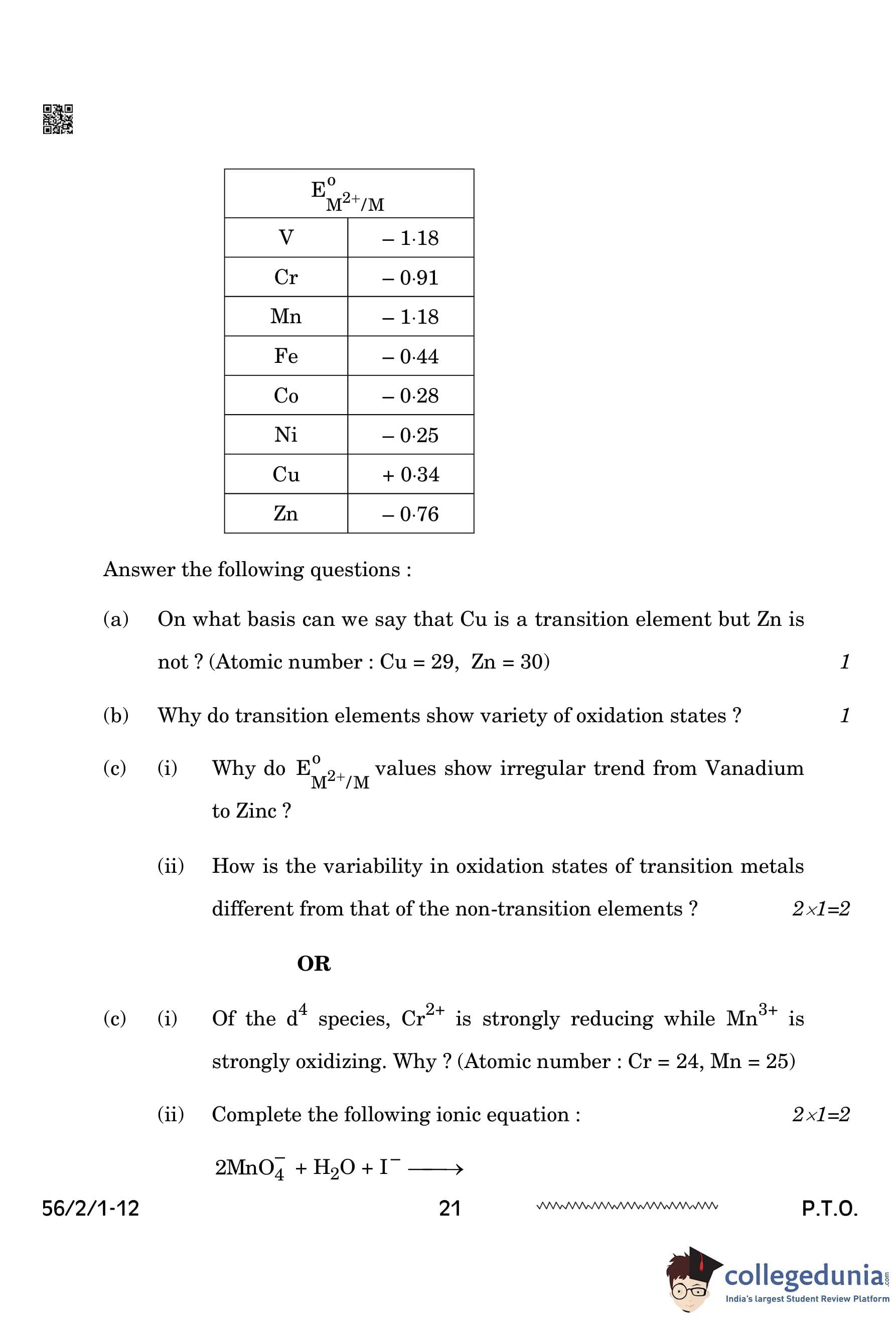
(a) On what basis can we say that Cu is a transition element but Zn is not? (Atomic number: Cu = 29, Zn = 30)
(b) Why do transition elements show a variety of oxidation states?
(c) (i) Why do \(\text{E\(^0\)_{M^{2+/M \) values show irregular trends from Vanadium to Zinc?
(c) (ii) How is the variability in oxidation states of transition metals different from that of non-transition elements?
(i) Of the d\(^4\) species, Cr\(^{2+}\) is strongly reducing while Mn\(^{3+}\) is strongly oxidizing. Why? (Atomic number: Cr = 24, Mn = 25)
(ii) Complete the following ionic equation:
(a) How is the crystal field splitting energy for octahedral complex (\(\Delta_o\)) related to that of tetrahedral complex (\(\Delta_t\))?
(b) Write the IUPAC name of the following complex: [PtCl\(_2\)(en)\(_2\)](NO\(_3\))\(_2\)
(c) Write the geometry and magnetic behaviour of the complex [Ni(CO)\(_4\)] on the basis of Valency Bond Theory (VBT).
(d) What type of isomerism is shown by the complex [Co(NH\(_3\))\(_6\)][Cr(CN)\(_6\)]?
(e) For the coordination compound on the basis of crystal field theory, write the electronic configuration for d\(^4\) ion if \(\Delta_o\) \(<\) P. Is the coordination compound a high spin or low spin complex?
(f) Out of [Co(NH\(_3\))\(_6\)]\(^{3+}\) and [Co(NH\(_3\))\(_4\)Cl\(_2\)]\(^+\), which complex is heteroleptic and why?
(g) Draw the structures of optical isomers of [PtCl\(_2\)(en)\(_2\)]\(^{2+}\).
(a) Account for the following:
(i) Oxidation of aldehydes is easier as compared to ketones.
The alpha (\(\alpha\)) hydrogen atoms of aldehydes are acidic in nature.
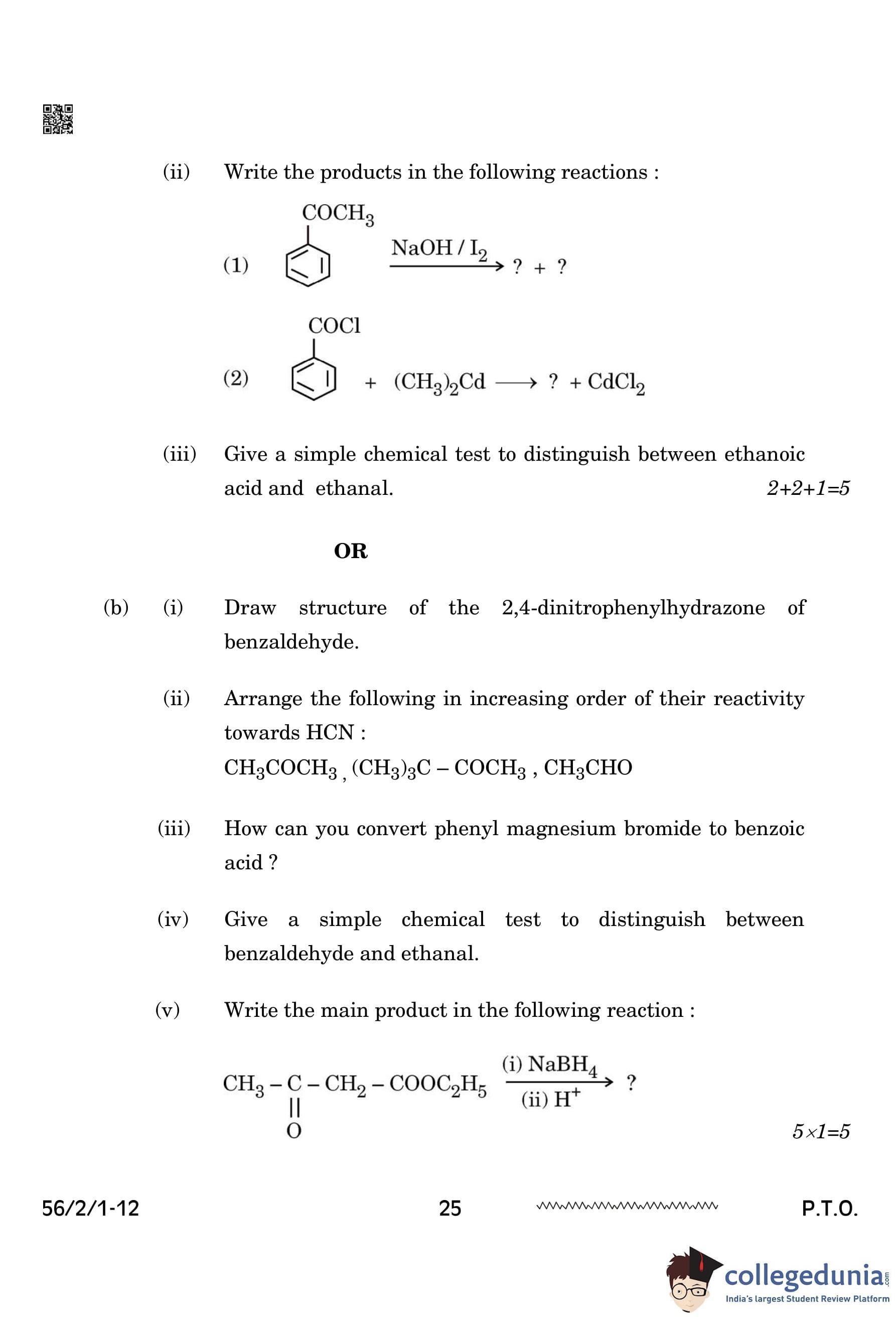
(ii) Write the products in the following reactions:
(iii) Give a simple chemical test to distinguish between ethanoic acid and ethanal.
(i) Draw structure of the 2,4-dinitrophenylhydrazone of benzaldehyde.
(ii) Arrange the following in increasing order of their reactivity towards HCN: \[ CH_3COCH_3, \, (CH_3)_3C - COCH_3, \, CH_3CHO \]
(iii) How can you convert phenyl magnesium bromide to benzoic acid?
(iv) Give a simple chemical test to distinguish between benzaldehyde and ethanal.
(v) Write the main product in the following reaction:
(i) The resistance of 0.05 M CH\(_3\)COOH solution is found to be 100 ohm. If the cell constant is 0.0354 cm\(^{-1}\), calculate the molar conductivity of the acetic acid solution.
(ii) State Faraday's first law of electrolysis. How much charge in Faraday is required for the reduction of 1 mol of MnO\(_4^-\) to Mn\(^{2+}\)?
(i) The conductivity of 0.0025 mol L\(^{-1}\) acetic acid is \(5.25 \times 10^{-5} \, S cm^{-1}\). Calculate its degree of dissociation if \(\Lambda_m^\circ\) for acetic acid is 390 S cm\(^2\) mol\(^{-1}\).
(ii) Write the anode, cathode, and overall reaction of lead storage battery.























CBSE Chemistry Class 12 Exam Review
As per students who took Chemistry paper today, the exam was moderate in terms of difficulty. MCQs were tricky while short and long answer questions were direct.
Important Questions
Anomers: Anomers are a special type of stereoisomers that are specific to carbohydrates, particularly in cyclic forms like pyranose or furanose structures. These isomers differ in the configuration around the hemiacetal or hemiketal carbon atom formed when a linear sugar molecule cyclizes. Anomers are classified based on the configuration of the anomeric carbon relative to the substituent group (usually a hydroxyl group) attached to it. The two main types of anomers are the alpha (α) and beta (β) anomers.
Explain why the electrophilic substitution reactions in haloarenes occur slowly and require more drastic conditions as compared to those in Benzene?
Electrophilic aromatic substitution reactions involve the substitution of an atom or group on an aromatic ring with an electrophile. In haloarenes (aromatic compounds containing halogen atoms), the presence of halogen atoms can significantly influence the reaction kinetics compared to benzene due to the electron-withdrawing nature of halogens.
Due to the following factors, electrophilic substitution reactions in haloarenes often require more drastic conditions such as higher temperatures or more concentrated reactant solutions to overcome the decreased reactivity compared to benzene.
- Electron-withdrawing effect of halogens
- Resonance stabilization
- Substituent effects
Phenol is less acidic than?
Phenol is less acidic than o-nitrophenol. In o-nitrophenol, the presence of an electron-withdrawing group (nitro group) at ortho position increases the acidic strength. On the other hand, in o-methylphenol and in o-methoxyphenol, electron-releasing group (-CH3, -OCH3) are present at ortho or para positions of phenol which decreases the acidic strength of phenols.
Nitrobenzene to Aniline:




Comments