
Jasmine Grover Content Strategy Manager
Content Strategy Manager
Bohr's model of the atom is a model of the atom proposed by Danish physicist Niels Bohr in 1913. It was a significant development in the field of atomic physics, and it helped to explain the observed spectra of elements. Here are the main points of the Bohr model of the atom:
- Electrons in atoms are confined to specific energy levels or shells, which are designated by the quantum number n.
- These orbits or shells are represented by K, L, M, N or the numbers n = 1, 2, 3, 4
- Electrons can move between energy levels by absorbing or emitting photons of specific energies.
- The energy of an electron in an atom is quantized, meaning it can only have certain discrete values.
- The electrons in the outermost energy level (valence electrons) are responsible for chemical bonding.
- The positively charged nucleus is located at the centre of the atom, and the electrons orbit around it in circular paths.
- The energy of the electron in a particular energy level is related to the radius of the electron's orbit.
- Electrons in lower energy levels have less energy and are closer to the nucleus, while electrons in higher energy levels have more energy and are farther away from the nucleus.
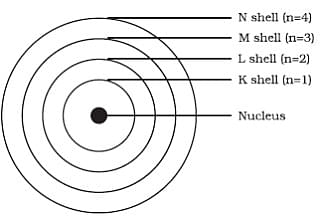
Bohr’s atomic model
- The model successfully explains the spectral lines of hydrogen and other atoms.
- Certain special orbits are allowed inside the atom and are known as discrete orbits of electrons.
- Electrons don’t radiate energy while revolving in discrete orbits.
The Bohr model is a stepping stone to more modern models, such as the quantum mechanical model, which provides a more accurate description of the atom.
Also Read:





Comments