Jasmine Grover Content Strategy Manager
Content Strategy Manager
Allosteric Enzymes are the regulatory enzymes which have an additional binding site other than the active site for modulators/ effectors to engage with and thus affect the overall catalytic activity performed by the enzyme. Cell division, hormone secretion, respiration, digestion, metabolism, etc – such activities are properly controlled by enzymes for survival and development purposes. Enzymes regulate these activities and they are the bio-catalysts of our body. Enzymes are made up of proteins. As proteins are made up of amino acids, enzymes are also made up of amino acids. One such regulatory enzyme is the allosteric enzyme.
| Table of Contents |
Key Takeaways: Allosteric enzymes, Enzymes, Amino acids, Proteins, Michaelis – Menten kinetic equation, sSubstrate, Active site, Inhibitor, Activator, Bio-catalysts
Enzymes
[Click Here for Sample Questions]
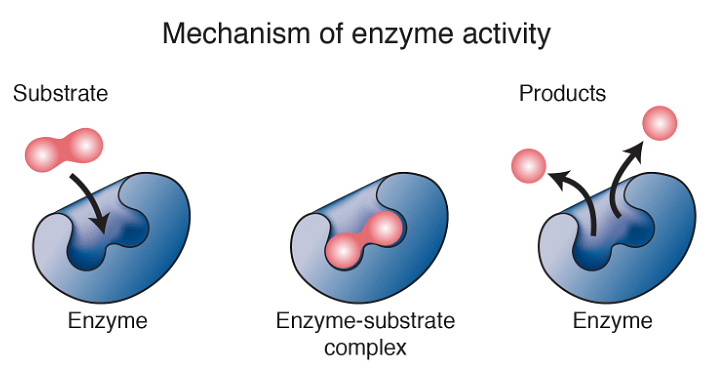
Enzymes are bio-catalysts of our body. They control the cellular activities in our body. They have amino acids which align themselves in a particular sequence to give a specific shape to the enzymes. The folding of such amino acids decides the three-dimensional structure of the enzyme. The shape or structure of an enzyme is crucial for its function because enzymes are able to speed up chemical reactions by physically interacting with the reactant/ reactants of a chemical reaction.
How Enzymes Work
The reactant on which the enzyme acts upon is called its substrate. An enzyme interacts with its substrate at a specific location called the active site of the enzyme. The shape of the active site is complementary to its substrate like a key to its lock. This complementarity makes an enzyme specific to its substrate, meaning the enzyme can only catalyse the reaction for its specific substrate. The interaction of the substrate and enzyme leads to the formation of an enzyme-substrate complex.

Enzyme and Substrate
When a substrate interacts with the enzyme at the active site, the shape of the active site changes slightly to accommodate the substrate due to the chemical interactions between the substrate and active site. This shape change allows the substrate to fit more tightly into the active site and is referred to as the Induced Fit Model of enzyme-substrate interaction.
Also Read:
| Topics Related Links | ||
|---|---|---|
| Enzyme Properties | Applications of enzymes | Digestive enzymes |
| Restriction Enzymes | Peptides | Digestive system |
Allosteric Enzymes
[Click Here for Previous Year Questions]
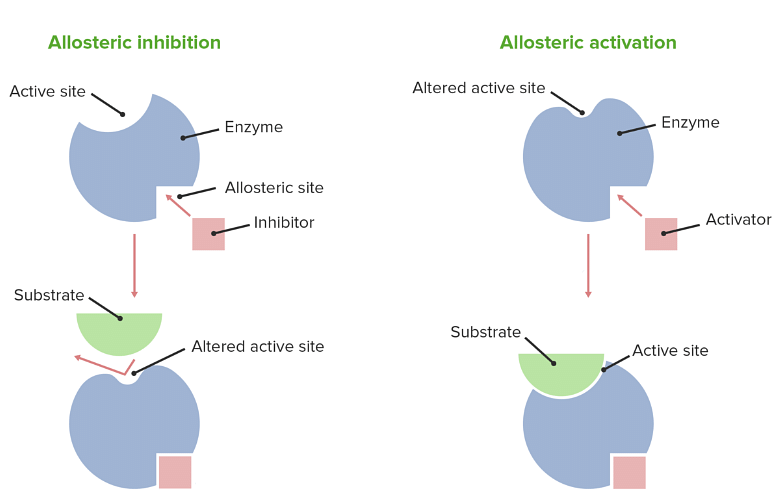
The term ‘allostery’ means ‘other site’ (allo - other, stereos – site/space). Allosteric enzymes are those enzymes which have an additional site apart from the active site. As seen in the case of enzyme-substrate complexes, the active site gets occupied with the substrate and later it results in product but for allosteric enzymes, the other site other than the active site is occupied by allosteric inhibitors or regulators which decides the result of the reaction.

Allosteric Enzyme
Thus these enzymes are responsible for the reaction determining step of the reaction as the binding of the allosteric inhibitor or activator can increase or decrease the reaction velocity. The presence of an allosteric inhibitor or activator brings about configurational/structural change in the enzyme. The allosteric inhibitor or activator binds with the allosteric site by reversible non-covalent interactions.
Read More: Difference between DNA and RNA
Allosteric Inhibition
Allosteric Inhibition is when an allosteric inhibitor binds at the allosteric site causing a negative change in the configuration of an enzyme. The change in the enzyme inhibits the interaction of the enzyme with the substrate thus no products are formed. It acts like a non-competitive inhibitor as it binds at another side other than the active site.
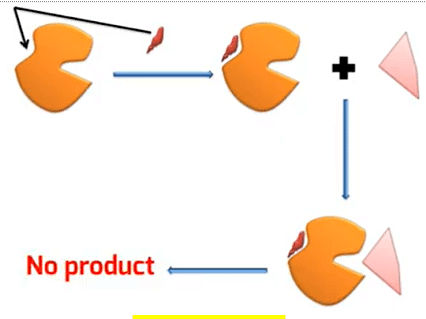
Allosteric Inhibition
Allosteric Activation
Allosteric Activation is when an allosteric activator (effector/regulator) binds at the allosteric site causing a positive change in the configuration of an enzyme. It enhances the activity of the enzyme. It promotes the interaction of the enzyme with the substrate thus products are formed.
Allosteric Activation
Read More: MCQ’s on biomolecules
Characteristics of Allosteric Enzymes
[Click Here for Sample Questions]
- Allosteric Enzymes have many units with a combination of sites for substrate and modulators like allosteric inhibitors or regulators.
- The activities done by the allosteric enzyme is controlled by its binding on its allosteric site. The binding decides the changes to happen to the enzyme and thus the overall rate of the reaction. It controls the catalytic activity of the enzyme.
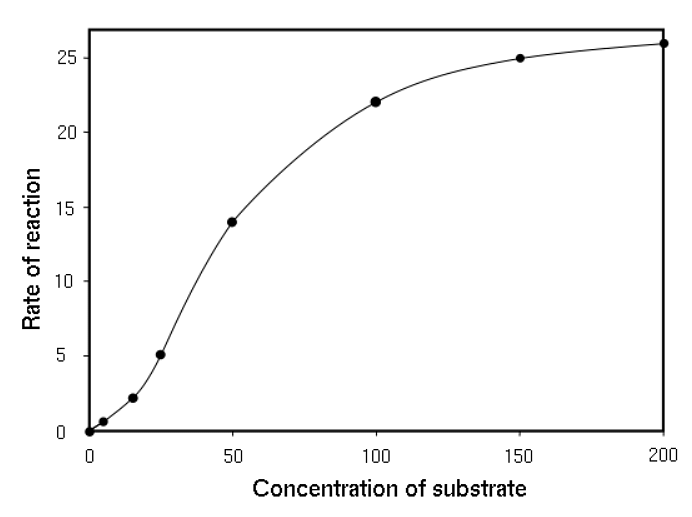
Relation between concentration of substrate and rate of reaction
- While studying the kinetics of the allosteric enzymes, it is observed that they do not follow the Michaelis – Menten equation. Instead, they form a sigmoid growth curve. At the initial stage of an allosteric enzyme-catalyzed reaction, the reaction rate increases exponentially as the substrate increases. Eventually, the influence of the substrate concentration on the reaction rate ceases when the enzyme molecules become saturated.
Read More: Saturated and Unsaturated Fats
Models of Allosteric Enzymes
[Click Here for Previous Year Questions]
For understanding the mechanism of regulation of allosteric enzymes, these models were given-
Symmetry or Concerted Model
Here there is simultaneous change throughout the units of the enzyme. The units signify the allosteric and active sites on the enzymes. These sites are present in either active form – R form or in inactive form – T form having less affinity to the substrate. The addition of an inhibitor or regulator is in concert with other subunits, aiming to maintain the symmetry of the enzyme. In this model the enzyme will always be in RR or TT state and not in an intermediate state like RT.

Concerted Model
Simple Sequential Model
Different from the symmetry model, the simple sequential model does not assume that the enzyme should have R and T in equilibrium. The addition of inhibitors and regulators does the resulting change in the configuration of the enzyme from T state to R state. The change causes change in the binding affinity of all units.
Read More: Proteins
Examples of Allosteric Enzymes
[Click Here for Sample Questions]
- Glucose – 6 – phosphate acts as an allosteric inhibitor of Hexokinase. This is also an example of feedback inhibition. Feedback inhibition is when after the saturation of the reaction with Glucose – 6 – phosphate, the reactant does not undergo further reaction. Here Hexokinase is the enzyme.
- Fructose – 6 – phosphate gives the product of Fructose – 1,6 – Bisphosphate with the enzyme Phosphofructokinase. There are two allosteric enzymes which participate in the reaction as an activator and as an inhibitor. The allosteric activator here is AMP – Adenosine Monophosphate, it encourages the enzyme to create more Fructose – 1,6 – Bisphosphate. The allosteric inhibitor here is ATP citrate – Adenosine Tri Phosphate citrate inhibits the further production of Fructose – 1,6 – Bisphosphate.
Also Read: Microbiology
Things to Remember
- Enzymes are sensitive to their surrounding temperature and pH. Thus they have an optimal temperature and pH at which they work the best.
- There is a possibility that one enzyme may have more than one allosteric sites
- Allosteric enzymes are multi-subunit proteins, which has one subunit that executes a catalytic function and at least, another subunit that executes a regulatory function.
- Enzymes lower the activation energy of the reaction they catalyse.
Also Read:
| Related Articles | ||
|---|---|---|
| Lipid Peroxidation | Proteolytic Enzyme | Enzymes Properties |
| Vitamin B12 | Peptide Bond | Fats and Oils |
Sample Questions
Ques. What are Inhibitors? Explain its types. [3 marks]
Ans. Enzyme function can be blocked by inhibitors. Inhibitors are of two categories, competitive inhibition, and non-competitive inhibition.
- Competitive inhibitors resemble the normal substrate for the enzyme and bind at the active site of the enzyme. They compete with the substrate for occupancy of the active site.
- Non-competitive inhibitors on another hand do not resemble the substrate and do not bind at the active site. They bind the enzyme at an alternate location, but the binding of non-competitive inhibitors alters the shape of the active site, preventing the substrate to bind.
Ques. What are Homotropic and Heterotropic enzymes? [3 marks]
Ans. Based on the nature of the modulator bonded at the allosteric site, homotropic and heterotropic allosteric enzymes are decided. If the modulator bonded at the allosteric site is the same as the substrate, then it is called the homotropic enzyme. Here the moderator affects the binding affinity of the same substrate on the active site of the enzyme (affects the same coordinate bond). e.g. binding of oxygen to haemoglobin.
If the modulator bonded at the allosteric site is other than the substrate, then it is called the heterotrophic enzyme. Here the modulator affects the binding affinity of the substrate’s other coordinate bond. e.g. binding of CO2 to haemoglobin.
Ques. Which of the following is not true for allosteric enzymes? [3 marks]
a) Greek word ‘allo’ means other and ‘steros’ means site
b) Enzymes having other site
c) Regulatory metabolites are called effector or modulator or modifier
d) Each of two or more enzymes with identical function but different structure
Ans. Answer: d
Explanation: “Each of two or more enzymes with identical function but a different structure.” This statement is not true for allosteric enzymes as it is defining isoenzymes. The following statements are true for allosteric enzymes:
• The Greek word ‘allo’ means other and ‘steros’ means site.
• Enzymes having other sites.
• Regulatory metabolites are called effector or modulator or modifier.
Ques. Explain the difference between the Inhibitor and Activator. [3 marks]
Ans.
| Enzyme Activator | Enzyme Inhibitor |
|---|---|
| A molecule that binds to an enzyme, increasing the activity | A molecule that binds to an enzyme, decreasing the activity |
| Can be either proteins, lipids, peptides, small organic molecules or ions | Two main types are reversible and irreversible inhibitors |
| Eg – calcium and magnesium ions, calmodulin, EDTA, EGTA, fructose 2,6 – bisulphate, and glucokinase | Eg – N-ethylmaleimide, DFMO, DFP, and most pharmaceutical drugs |
Ques. Why does an enzyme catalyse only one reaction? [3 marks]
Ans. Enzymes have an active site which is particular and unique for its kind of substrate. Its active site will reject the substrate which is of the same shape as that of the enzyme, instead the enzyme should possess a complementary shape to that of the substrate to have an induced fit enzyme-substrate complex. This complex is taken further to complete the reaction. The shape at which the amino acids shape themselves for the enzymes compliment a particular substrate. This makes it unique for its substrate. Thus the substrate involved in a reaction is only catalysed by one enzyme. Thus enzymes can only catalyse one reaction as per its complementary substrate.
Ques. What is allosteric inhibition and allosteric activation? [3 marks]
Ans. Allosteric Inhibition
Allosteric Inhibition is when an allosteric inhibitor binds at the allosteric site causing a negative change in the configuration of an enzyme. The change in the enzyme inhibits the interaction of the enzyme with the substrate thus no products are formed. It acts like a non-competitive inhibitor as it binds at another side other than the active site.
Allosteric Activation: Allosteric Activation is when an allosteric activator (effector / regulator) binds at the allosteric site causing a positive change in the configuration of an enzyme. It enhances the activity of the enzyme. It promotes the interaction of the enzyme with the substrate thus products are formed.
Ques. Compare reversible enzyme inhibition and irreversible enzyme inhibition. [3 marks]
Ans.
| Reversible Enzyme Inhibition | Irreversible Enzyme Inhibition |
|---|---|
| The process of binding inhibitors to the enzymes through monovalent interactions so that, once removed they allow the restoring of the enzyme function | The process of binding inhibitors to the enzyme through covalent interactions so that their dissociation takes a long time, permanently removing the enzyme action. |
| Reversible inhibitors bind to the enzyme through non-covalent interactions such as hydrogen bonds, hydrophobic interactions, and ionic bonds in reversible enzyme inhibition | Irreversible inhibitors bind to the enzyme through covalent interactions, which modify amino acids residues by reactive functional group in irreversible enzyme inhibition |
| The enzyme-inhibitor complex dissociates quickly | Dissociates very slowly |
| Enzymatic action can be restored | It takes a long time to restore the enzymatic action |
| Types – competitive, uncompetitive, and mixed inhibition | Occurs through the covalent inactivation of the active site of the enzyme. |
Ques. Effectors that inhibit enzyme activity are termed as ____________ [1 mark]
a) positive effectors
b) modulators
c) negative effectors
d) inhibitor
Ans. Answer: c
Explanation: Effectors that inhibit enzyme activity are termed as negative effectors. Whereas those that increase the enzyme activity are referred to as positive effectors. Regulatory metabolites are called modulator or modifier or effector. Substances which cause reduction in the rate of an enzyme catalyzed reaction are known as inhibitors.
Ques. Describe competitive and non-competitive inhibition of an enzyme. [3 marks]
Ans. Inhibitors aim to restrict or ‘inhibit’ the formation of the enzyme-substrate complex.
Competitive inhibitors – Competitive Inhibitors have similar shape to their substrate, and they compete with their substrate to achieve the active site of the enzyme. In its competition, by having a greater number of substrates, the inhibitor's actions can be restricted.
Non-competitive inhibitors – Non-competitive inhibitors bind to a different site other than the active site. This results in complete changes in the shape of the enzyme. As the substrate is sensitive to shape, the changed shape enzyme does not form an enzyme-substrate complex thus inhibiting its interaction with the substrate. Here the inhibitor does not compete with the substrate to occupy the active site of the enzyme. Non-competitive inhibitors have an additional advantage over competitive inhibitors that they cannot be overcomed by having a greater number of substrates.
Ques. Distinguish between the active site and binding site. [3 marks]
Ans.
| Active site | Binding Site |
|---|---|
| A region on an enzyme to which the substrates of a chemical reaction bind in order to undergo a catalysed chemical reaction | A region on a protein, DNA or RNA to which ligands can bind |
| Exists on enzymes | Exists on proteins, DNA or RNA |
| Responsible for the reduction of activation energy of a chemical reaction: thereby, increase the reaction rate | Responsible for the binding of a specific substrate or ligand with a molecule |
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:




Comments