NEET Re-Exam 2024 Question paper for June 23 is available for download here. NTA reconducted NEET exam 2024 on June 23 for 1563 candidates from 2 PM to 5.20 PM. NEET question paper has 200 MCQs- 180 to be attempted in 3 hours 20 minutes. NEET 2024 question paper 2024 PDF is divided into 4 sections- Zoology, Botany, Chemistry, and Physics. You can download NEET Re-exam 2024 question paper with answer key with solutions PDF in English using the links given below.
Download NEET 2025 Question Paper PDFs for all Codes
NEET Re-Exam 2024 Question Paper with Answer Key PDF
| NEET Re-Exam 2024 Question Paper with Solution PDF | Check Solution |
NEET Re-Exam 2024 Questions with Solutions
The magnetic potential energy, when a magnetic bar of magnetic moment \( \mathbf{m} \) is placed perpendicular to the magnetic field \( \mathbf{B} \), is:
A bob is whirled in a horizontal circle by means of a string at an initial speed of 10 rpm. If the tension in the string is quadrupled while keeping the radius constant, the new speed is:
A metal cube of side 5 cm is charged with 6 μC. The surface charge density on the cube is:
The incorrect relation for a diamagnetic material (all the symbols carry their usual meaning and \( \epsilon \) is a small positive number) is:
An ideal fluid is flowing in a non-uniform cross-sectional tube XY (as shown in the figure) from end X to end Y. If \( K_1 \) and \( K_2 \) are the kinetic energy per unit volume of the fluid at X and Y respectively, then the correct option is:
The escape velocity for Earth is \( v \). A planet having 9 times mass that of Earth and radius 16 times that of Earth, has the escape velocity of:
An electron and an alpha particle are accelerated by the same potential difference. Let \( \lambda_e \) and \( \lambda_\alpha \) denote the de-Broglie wavelengths of the electron and the alpha particle, respectively, then:
An object moving along horizontal x-direction with kinetic energy 10 J is displaced through \( x = (3\hat{i} + 3\hat{j}) \, m \) by the force \( \mathbf{F} = (-2\hat{i} + 3\hat{j}) \, N \). The kinetic energy of the object at the end of the displacement \( x \) is:
An object falls from a height of 10 m above the ground. After striking the ground it loses 50% of its kinetic energy. The height upto which the object can rebound from the ground is:
In the circuit shown below, the inductance \( L \) is connected to an ac source. The current flowing in the circuit is \( I = I_0 \sin \omega t \). The voltage drop \( V_L \) across \( L \) is:
A 12 pF capacitor is connected to a 50 V battery, the electrostatic energy stored in the capacitor in nJ is:
A uniform wire of diameter \( d \) carries a current of 100 mA when the mean drift velocity of electrons in the wire is \( v \). For a wire of diameter \( \frac{d}{2} \) of the same material to carry a current of 200 mA, the mean drift velocity of electrons in the wire is:
In an electrical circuit, the voltage is measured as \( V = (200 \pm 4) \) volt and the current is measured as \( I = (20 \pm 0.2) \) A. The value of the resistance is:
A step up transformer is connected to an ac mains supply of 220 V to operate at 11000 V, 88 watt. The current in the secondary circuit, ignoring the power loss in the transformer, is:
A particle is moving along x-axis with its position \( x \) varying with time \( t \) as \( x = \alpha t^4 + \beta t^3 + \gamma t^2 + \delta \). The ratio of its initial velocity to its initial acceleration, respectively, is:
The radius of gyration of a solid sphere of mass 5 kg about XY is 5 m as shown in the figure. The radius of the sphere is \( \frac{5}{\sqrt{7}} \, m \), then the value of \( x \) is:
The I-V characteristics shown above are exhibited by a:
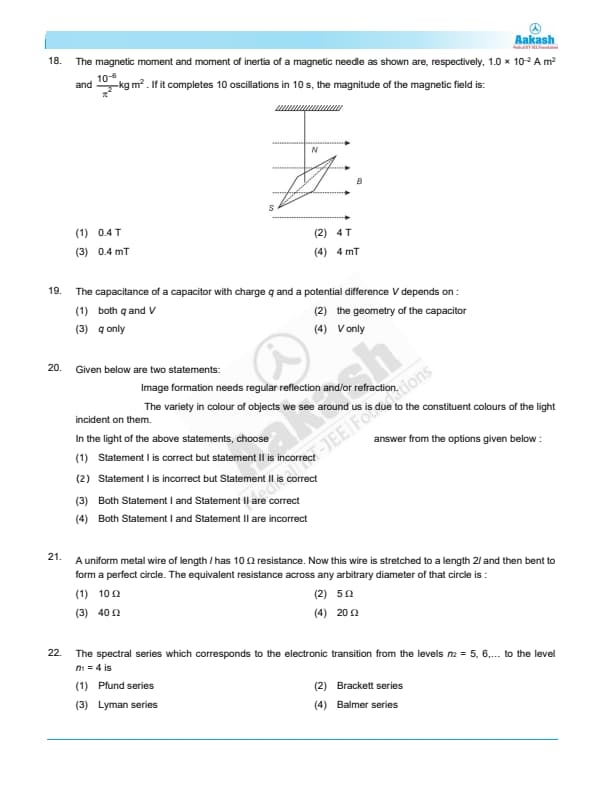
The magnetic moment and moment of inertia of a magnetic needle as shown are, respectively, \( 1.0 \times 10^{-2} \, A m^2 \) and \( 10^{-6} \, kg m^2 \). If it completes 10 oscillations in 10 s, the magnitude of the magnetic field is:
The capacitance of a capacitor with charge \( q \) and a potential difference \( V \) depends on:
Given below are two statements:
Statement I: Image formation needs regular reflection and/or refraction.
Statement II: The variety in the color of objects we see around us is due to the constituent colors of the light incident on them.
In light of the above statements, choose the most appropriate answer from the options given below:
A uniform metal wire of length \( l \) has 10 \( \Omega \) resistance. Now this wire is stretched to a length \( 2l \) and then bent to form a perfect circle. The equivalent resistance across any arbitrary diameter of that circle is:
The spectral series which corresponds to the electronic transition from the levels \( n_2 = 5, 6, \dots \) to the level \( n_1 = 4 \) is
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Houses made of concrete roofs overlaid with foam keep the room hotter during summer.
Reason R: The layer of foam insulation prohibits heat transfer, as it contains air pockets.
In the light of the above statements, choose the correct answer from the options given below.
A particle executing simple harmonic motion with amplitude A has the same potential and kinetic energies at the displacement
Two slits in Young's double slit experiment are 1.5 mm apart and the screen is placed at a distance of 1 m from the slits. If the wavelength of light used is 600 x \( 10^{-9} \) m, then the fringe separation is
Water is used as a coolant in a nuclear reactor because of its
The pitch of an error free screw gauge is 1 mm and there are 100 divisions on the circular scale. While measuring the diameter of a thick wire, the pitch scale reads 1 mm and 63rd division on the circular scale coincides with the reference line. The diameter of the wire is:
Let us consider two solenoids A and B, made from same magnetic material of relative permeability \( \mu_r \) and equal area of cross-section. Length of A is twice that of B and the number of turns per unit length in A is half that of B. The ratio of self inductances of the two solenoids, \( L_A : L_B \), is
When the output of an OR gate is applied as input to a NOT gate, then the combination acts as a
Interference pattern can be observed due to superposition of the following waves:
A. \( y = a \sin t \)
B. \( y = a \sin 2t \)
C. \( y = a \sin(\omega t - \varphi) \)
D. \( y = a \sin 3\omega t \)
Choose the correct answer from the options given below.
If \( \varphi \) is the work function of photosensitive material in eV and light of wavelength of numerical value \( \lambda = \frac{hc}{e} \) is incident on it with energy above its threshold value at an instant, then the maximum kinetic energy of the photo-electron ejected by it at that instant (in SI units) is:
The electromagnetic radiation which has the smallest wavelength are:
The equilibrium state of a thermodynamic system is described by:
A. Pressure
B. Total heat
C. Temperature
D. Volume
E. Work done
Choose the most appropriate answer from the options given below.
Some energy levels of a molecule are shown in the figure with their wavelengths of transitions. Then:
Choose the correct answer from the options given below.
A box of mass 5 kg is pulled by a cord, up along a frictionless plane inclined at 30° with the horizontal. The tension in the cord is 30 N. The acceleration of the box is (Take \( g = 10 \, m/s^2 \)):
If the ratio of relative permeability and relative permittivity of a uniform medium is 1 : 4. The ratio of the magnitudes of electric field intensity (E) to the magnetic field intensity (H) of an EM wave propagating in that medium is
Given that \( \mu_0 \varepsilon_0 = 120 \pi \):
The value of electric potential at a distance of 9 cm from the point charge \( 4 \times 10^{-7} \, C \) is
Given \( \frac{1}{4 \pi \varepsilon_0} = 9 \times 10^9 \, Nm^2 C^{-2} \):
The displacement of a travelling wave \( y = c \sin \left( \frac{2\pi}{\lambda} (at - x) \right) \) where \( t \) is time, \( x \) is distance and \( \lambda \) is the wavelength, all in S.I. units. Then the frequency of the wave is:
An object of mass 100 kg falls from point A to B as shown in the figure. The change in its weight, corrected to the nearest integer is (R\(_E\) is the radius of the earth):
The potential energy of a particle moving along x-direction varies as \( V = \frac{Ax^2}{\sqrt{x^2 + B}} \). The dimensions of \( \frac{A^2}{B} \) are:
The two-dimensional motion of a particle, described by \( \mathbf{r} = (i + 2j) A \cos \omega t \) is an:
(1) parabolic path
(2) elliptical path
(3) periodic motion
(4) simple harmonic motion
Choose the correct answer from the options given below:
A beam of unpolarized light of intensity \( I_0 \) is passed through a polaroid A, then through another polaroid B, oriented at 60° and finally through another polaroid C, oriented at 45° relative to B as shown. The intensity of emergent light is:
Select the correct statements among the following:
A. Slow neutrons can cause fission in \( ^{235}_{92} U \) than fast neutrons.
B. \( \alpha \)-rays are Helium nuclei.
C. \( \beta \)-rays are fast-moving electrons or positrons.
D. \( \gamma \)-rays are electromagnetic radiations of wavelengths larger than X-rays.
Choose the most appropriate answer from the options given below:
Let \( \omega_1, \omega_2 \) and \( \omega_3 \) be the angular speed of the second hand, minute hand and hour hand of a smoothly running analog clock, respectively. If \( x_1, x_2 \) and \( x_3 \) are their respective angular distances in 1 minute, then the factor which remains constant (k) is:
The magnetic moment of an iron bar is \( M \). It is now bent in such a way that it forms an arc of a circle subtending an angle of 60° at the centre. The magnetic moment of this arc is:
The given circuit shows a uniform straight wire \( AB \) of 40 cm length fixed at both ends. In order to get zero reading in the galvanometer \( G \), the free end of \( J \) is to be placed from \( B \) at:
According to the law of equipartition of energy, the number of vibrational modes of a polyatomic gas of constant \( \gamma = \frac{C_p}{C_v} \) is (where \( C_p \) and \( C_v \) are the specific heat capacities of the gas at constant pressure and constant volume, respectively):
The output \( Y \) for the inputs \( A \) and \( B \) of the given logic circuit is:
The amplitude of the charge oscillating in a circuit decreases exponentially as \( Q = Q_0 e^{-\frac{Rt}{2L}} \), where \( Q_0 \) is the charge at \( t = 0 \). The time at which charge amplitude decreases to 0.50 \( Q_0 \) is nearly:
The steady state current in the circuit shown below is:
The correct decreasing order of atomic radii (pm) of Li, Be, B, and C is:
Following data is for a reaction between reactants A and B:
\[ \begin{array}{|c|c|c|} \hline Rate & [A] & [B]
\hline 2 \times 10^{-3} & 0.1 \, M & 0.1 \, M
4 \times 10^{-3} & 0.2 \, M & 0.1 \, M
1.6 \times 10^{-2} & 0.2 \, M & 0.2 \, M
\hline \end{array} \]
The order of the reaction with respect to A and B, respectively, are:
Given below are two statements:
Statement I: Propene on treatment with diborane gives an addition product with the formula \(( (CH_3)_2 CH_3 B )\).
Statement II: Oxidation of \(( (CH_3)_2 CH_3 B )\) with hydrogen peroxide in the presence of NaOH gives propan-2-ol.
In the light of the above statements, choose the most appropriate answer from the options given below:
Baeyer's reagent is:
Which of the following molecules has "NON ZERO" dipole moment value?
The major product X formed in the following reaction sequence is:
Which indicator is used in the titration of sodium hydroxide against oxalic acid and what is the colour change at the end point?
Match List-I with List-II : \[ \begin{array}{|l|l|} \hline \textbf{List-I (Atom/Molecule)} & \textbf{List-II (Property)}
\hline A. Nitrogen atom & I. Paramagnetic
B. Fluorine molecule & II. Most reactive element in group 18
C. Oxygen molecule & III. Element with highest ionisation enthalpy in group 15
D. Xenon atom & IV. Strongest oxidising agent
\hline \end{array} \]
Identify the correct answer from the options given below:
From the following select the one which is not an example of corrosion.
Which of the following pairs of ions will have same spin only magnetic moment values within the pair?
At a given temperature and pressure, the equilibrium constant values for the equilibria are given below: \[ 3A_2 + B_2 \rightleftharpoons 2A_3B, \, K_1 \] \[ A_3B \rightleftharpoons \frac{3}{2} A_2 + \frac{1}{2} B_2, \, K_2 \]
The relation between \( K_1 \) and \( K_2 \) is :
Arrange the following compounds in increasing order of their solubilities in chloroform:
NaCl, CH\(_3\)OH, cyclohexane, CH\(_3\)CN
View Solution
Identify the incorrect statement about PCl\(_5\):
Choose the correct statement for the work done in the expansion and heat absorbed or released when 5 litres of an ideal gas at 10 atmospheric pressure isothermally expands into vacuum until the volume is 15 litres:
The correct IUPAC name of the compound is:
Which of the following set of ions act as oxidising agents?
Select the incorrect reaction among the following:
The UV-visible absorption bands in the spectra of lanthanoid ions are 'X', probably because of the excitation of electrons involving 'Y'. The 'X' and 'Y', respectively, are:
Ethylene diaminetetraacetate ion is a/an:
The amount of glucose required to prepare 250 mL of \( \frac{M}{20} \) aqueous solution is:
(Molar mass of glucose = 180 g mol\(^{-1}\))
Identify the incorrect statement from the following:
For the reaction in equilibrium:
N\(_2\)(g) + 3H\(_2\)(g) \(\rightleftharpoons\) 2NH\(_3\)(g), \(\Delta H = -Q\)
The reaction is favoured in forward direction by:
The major product D formed in the following reaction sequence is:
Match List-I with List-II:
\begin{tabular{|c|m{5cm|c|m{2cm|
\hline
List-I & (Block/group in periodic table) & List-II & (Element)
\hline
A. & Lanthanoid & I. & Ce
\hline
B. & d-block element & II. & As
\hline
C. & p-block element & III. & Cs
\hline
D. & s-block element & IV. & Mn
\hline
\end{tabular
Choose the correct answer from the options given below:
Which of the following is not an ambidentate ligand?
The quantum numbers of four electrons are given below:
I. \( n = 4; \, l = 2; \, m_l = -2; \, s = -\frac{1}{2} \)
II. \( n = 3; \, l = 2; \, m_l = 1; \, s = +\frac{1}{2} \)
III. \( n = 4; \, l = 1; \, m_l = 0; \, s = +\frac{1}{2} \)
IV. \( n = 3; \, l = 1; \, m_l = -1; \, s = +\frac{1}{2} \)
The correct decreasing order of energy of these electrons is:
The major product C in the below mentioned reaction is:
CH\(_3\)CH\(_2\)CH\(_2\)Br \( \xrightarrow{alc. KOH} \) A \( \xrightarrow{HBr} \) B \( \xrightarrow{aq. KOH} \) C
The compound that does not undergo Friedel-Crafts alkylation reaction but gives a positive carbylamine test is:
For an endothermic reaction:
(A) \( q_p \) is negative.
(B) \( \Delta H \) is positive.
(C) \( \Delta H \) is negative.
(D) \( q_p \) is positive.
Choose the correct answer from the options given below:
1.0 g of H\(_2\) has the same number of molecules as in:
Which of the following plot represents the variation of \(\ln k\) versus \(\frac{1}{T}\) in accordance with Arrhenius equation?
A steam volatile organic compound which is immiscible with water has a boiling point of 250°C. During steam distillation, a mixture of this organic compound and water will boil:
Given below are two statements:
Statement I: Glycogen is similar to amylose in its structure.
Statement II: Glycogen is found in yeast and fungi also.
In the light of the above statements, choose the correct answer from the options given below:
The oxidation states not shown by Mn in the given reaction is:
\(3 MnO_4^{2-} + 4 H^+ \rightarrow 2 MnO_4^- + MnO_2 + 2 H_2 O\)
(1) +6
(2) +2
(3) +4
(4) +7
Choose the most appropriate answer from the options given below:
Given below are two statements:
Statement I: The Balmer spectral line for H atom with the lowest energy is located at \( \frac{5}{36} R_H \, cm^{-1} \).
Statement II: When the temperature of a blackbody increases, the maxima of the curve (intensity and wavelength) shift to shorter wavelengths.
In light of the above statements, choose the correct answer from the options given below:
Identify D in the following sequence of reactions:
CH\(_3\)CH\(_2\)OH \( \xrightarrow{P + I_2} \) A \( \xrightarrow{Mg} \) B \( \xrightarrow{HCHO} \) C \( \xrightarrow{H_2O} \) D (major)
Identify the incorrect statement.
Match List-I with List-II:
Choose the correct answer from the options given below:
Match List-I with List-II:
List-I List-II
Molecule Bond enthalpy (kJ mol\(^{-1}\))
A. HCl I. 435.8
B. N\(_2\) II. 498
C. H\(_2\) III. 946.0
D. O\(_2\) IV. 431.0
Choose the correct answer from the options given below:
The standard cell potential of the following cell Zn|Zn\(^{2+}\) (aq)|Fe\(^{2+}\)(aq)|Fe is 0.32 V. Calculate the standard Gibbs energy change for the reaction:
Zn(s) + Fe\(^{2+}\)(aq) \( \rightarrow \) Zn\(^{2+}\)(aq) + Fe(s)
(Given: 1 F = 96487 C)
Match List-I with List-II:
Choose the correct answer from the options given below:
The ratio of solubility of AgCl in 0.1 M KCl solution to the solubility of AgCl in water is:
(Given: Solubility product of AgCl = \( 10^{-10} \))
On complete combustion, 0.3 g of an organic compound gave 0.2 g of CO\(_2\) and 0.1 g of H\(_2\)O. The percentage composition of carbon and hydrogen in the compound, respectively is:
The following reaction method is not suitable for the preparation of the corresponding haloarene products, due to the high reactivity of the halogen, when X is:
The alkane that can be oxidized to the corresponding alcohol by KMnO\(_4\) as per the equation:
is, when:
For the following reaction at 300 K:
\( A_2(g) + 3B_2(g) \to 2AB_3(g) \)
The enthalpy change is +15 kJ, then the internal energy change is:
Rate constants of a reaction at 500 K and 700 K are 0.04 s\(^{-1}\) and 0.14 s\(^{-1}\), respectively; then, activation energy of the reaction is:
(Given: \(\log{3.5} = 0.5441, R = 8.31 \, J K^{-1} mol^{-1}\))
Mass of glucose (\(C_6H_{12}O_6\)) required to be dissolved to prepare one litre of its solution which is isotonic with 15 g L\(^{-1}\) solution of urea (NH\(_4\)CONH\(_2\)) is:
(Given: Molar mass in g mol\(^{-1}\) C: 12, H: 1, O: 16, N: 14)
[Mn\(_2\)(CO)\(_{10}\)] and [Co\(_2\)(CO)\(_8\)] structures have:
(A) Metal-Metal linkage
(B) Terminal CO groups
(C) Bridging CO groups
(D) Metal in zero oxidation state
Choose the correct answer from the options given below:
Methyl group attached to a positively charged carbon atom stabilizes the carbocation due to:
The regions with high level of species richness, high degree of endemism and a loss of 70% of the species and habitat are identified as:
Which of the following simple tissues are commonly found in the fruit walls of nuts and pulp of pear?
In a chromosome, there is a specific DNA sequence, responsible for initiating replication. It is called:
Given below are two statements:
Statement I: When many alleles of a single gene govern a character, it is called polygenic inheritance.
Statement II: In polygenic inheritance, the effect of each allele is additive.
In the light of the above statements, choose the correct answer from the options given below:
Which of the following are required for the light reaction of Photosynthesis?
A. CO\(_2\) \quad B. O\(_2\) \quad C. H\(_2\)O \quad D. Chlorophyll \quad E. Light
Choose the correct answer from the options given below:
Match List-I with List-II:
Choose the correct answer from the options given below:
Match List-I with List-II:
Choose the correct answer from the options given below:
Which part of the ovule stores reserve food materials?
Which one of the following is not found in Gymnosperms?
Which one of the following is not included under in-situ conservation?
Given below are two statements:
Statement I: The Indian Government has set up GEAC, which will make decisions regarding the validity of GM research.
Statement II: Biopiracy is the term used to refer to the use of bio-resources by native people.
In the light of the above statements, choose the correct answer from the options given below:
Pollen grains remain preserved as fossils due to the presence of:
Identify the incorrect pair:
Which of the following is the correct match?
Given below are two statements regarding RNA polymerase in prokaryotes.
Statement I: In prokaryotes, RNA polymerase is capable of catalyzing the process of elongation during transcription.
Statement II: RNA polymerase associates transiently with ‘Rho’ factor to initiate transcription.
In the light of the above statements, choose the correct answer from the options given below:
Which of the following is a nucleotide?
Match List-I with List-II:
Choose the correct answer from the options given below:
Match List-I with List-II:
Choose the correct answer from the options given below:
Which of the following helps in maintenance of the pressure gradient in sieve tubes?
Mesosome in a cell is:
Match List-I with List-II:
Choose the correct answer from the options given below:
Match List-I with List-II:
Choose the correct answer from the options given below:
Match List-I with List-II:
Choose the correct answer from the options given below:
Cryopreservation technique is used for:
Which of the following are correct about cellular respiration?
A. Cellular respiration is the breaking of C-C bonds of complex organic molecules by oxidation.
B. The entire cellular respiration takes place in Mitochondria.
C. Fermentation takes place under anaerobic condition in germinating seeds.
D. The fate of pyruvate formed during glycolysis depends on the type of organism also.
E. Water is formed during respiration as a result of O\(_2\) accepting electrons and getting reduced.
Choose the correct answer from the options given below:
Given below are two statements:
Statement I: In eukaryotes there are three RNA polymerases in the nucleus in addition to the RNA polymerase found in the organelles.
Statement II: All the three RNA polymerases in eukaryotic nucleus have different roles.
In the light of the above statements, choose the correct answer from the options given below:
Match List-I with List-II:
Choose the correct answer from the options given below:
Given below are two statements:
Statement I: Failure of segregation of chromatids during cell cycle resulting in the gain or loss of a whole set of chromosomes in an organism is known as aneuploidy.
Statement II: Failure of cytokinesis after anaphase stage of cell division results in the gain or loss of a chromosome is called polyploidy.
In the light of the above statements, choose the correct answer from the options given below:
Recombination between homologous chromosomes is completed by the end of:
Match List-I with List-II:
Choose the correct answer from the options given below:
Ligases is a class of enzymes responsible for catalysing the linking together of two compounds.
Which of the following bonds is not catalysed by it?
Skoog observed that callus proliferated from the internodal segments of tobacco stem when auxin was supplied with one of the following except:
Given below are some statements about plant growth regulators:
A. All GAs are acidic in nature.
B. Auxins are antagonists to GAs.
C. Zeatin was isolated from coconut milk.
D. Ethylene induces flowering in Mango.
E. Abscisic acid induces parthenocarpy.
Choose the correct set of statements from the options given below:
Identify the incorrect statement related to gel electrophoresis.
Which of the following examples show monocarpellary, unilocular ovary with many ovules?
A. Sesbania
B. Brinjal
C. Indigofera
D. Tobacco
E. Asparagus
Choose the correct answer from the options given below:
Given below are two statements:
Statement I: In the lac operon, the z gene codes for beta-galactosidase which is primarily responsible for the hydrolysis of lactose into galactose and glucose.
Statement II: In addition to lactose, glucose or galactose can also induce lac operon.
In the light of the above statements, choose the correct answer from the options given below:
The part marked as 'x' in the given figure is:
Given below are two statements:
Statement I: In a dicotyledonous leaf, the adaxial epidermis generally bears more stomata than the abaxial epidermis.
Statement II: In a dicotyledonous leaf, the adaxially placed palisade parenchyma is made up of elongated cells, which are arranged vertically and parallel to each other.
In the light of the above statements, choose the correct answer from the options given below:
Which of the following are not fatty acids?
A. Glutamic acid
B. Arachidonic acid
C. Palmitic acid
D. Lecithin
E. Aspartic acid
Choose the correct answer from the options given below:
Consider the pyramid of energy of an ecosystem given below:
If \( T_4 \) is equivalent to 1000 J, what is the value at \( T_1 \)?
Which one of the following products diffuses out of the chloroplast during photosynthesis?
Recombinant DNA molecule can be created normally by cutting the vector DNA and source DNA respectively with:
The Bt toxin in genetically engineered Bt cotton kills the pest by:
Organisms Mode of Nutrition
A. Euglenoid I. Parasitic
B. Dinoflagellate II. Saprophytic
C. Slime mould III. Photosynthetic
D. Plasmodium IV. Switching between photosynthetic and heterotrophic mode
Choose the correct answer from the options given below:
Which of the following graphs depicts the effect of substrate concentration on velocity of enzyme catalysed reaction?
When will the population density increase, under special conditions?
When the number of:
When a tall pea plant with round seeds was selfed, it produced the progeny of:
(a) Tall plants with round seeds and
(b) Tall plants with wrinkled seeds.
Identify the genotype of the parent plant.
Match List-I with List-II:
Choose the correct answer from the options given below:
Observe the given figure. Identify the different stages labelled with alphabets by selecting the correct option.
Match List-I with List-II:
Choose the correct answer from the options given below:
Match List-I with List-II:
Choose the correct answer from the options given below:
Following are the steps involved in action of toxin in Bt. Cotton:
A. The inactive toxin converted into active form due to alkaline pH of gut of insect.
B. Bacillus thuringiensis produce crystals with toxic insecticidal proteins.
C. The alkaline pH solubilises the crystals.
D. The activated toxin binds to the surface of midgut cells, creates pores and causes death of the insect.
E. The toxin proteins exist as inactive protoxins in bacteria.
Choose the correct sequence of steps from the options given below:
Match List-I with List-II:
Choose the correct answer from the options given below:
Which evolutionary phenomenon is depicted by the sketch given in figure?
A person with blood group ARh+ can receive the blood transfusion from which of the following types?
Enzymes that catalyse the removal of groups from substrates by mechanisms other than hydrolysis leaving double bonds, are known as :
Match List-I with List-II:
List-I List-II
A. Chiasmata formation I. Pachytene
B. Crossing over II. Diakinesis
C. Synaptonemal complex formation III. Diplotene
D. Terminalisation of chiasmata IV. Zygotene
Match List-I with List-II:
Match List-I with List-II:
List-I List-II
A. Epinephrine I. Hyperglycemia
B. Thyroxine II. Smooth muscle contraction
C. Oxytocin III. Basal metabolic rate
D. Glucagon IV. Emergency hormone
Which of the following statements is correct about the type of junction and their role in our body?
Select the restriction endonuclease enzymes whose restriction sites are present for the tetracycline resistance (tet\textsuperscript{R}) gene in the pBR322 cloning vector.
Match List-I with List-II.
List-I List-II
A. Chondrichthyes I. Clarias
B. Cyclostomata II. Carcharodon
C. Osteichthyes III. Myxine
D. Amphibia IV. Ichthyophis
Choose the correct answer from the options given below:
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: During menstrual cycle, the ovulation takes place approximately on 14th day.
Reason R: Rapid secretion of LH in the middle of menstrual cycle induces rupture of Graafian follicle and thereby the release of ovum.
In the light of the above statements, choose the most appropriate answer from the options given below:
Match List-I with List-II with respect to convergent evolution:
List-I List-II
A. Lemur I. Flying phalanger
B. Bobcat II. Numbat
C. Anteater III. Spotted cuscus
D. Flying squirrels IV. Tasmanian tiger cat
Choose the correct answer from the options given below:
Match List-I with List-II.
List-I List-II
A. Cells are metabolically active and proliferate I. G2 phase
B. DNA replication takes place II. G1 phase
C. Proteins are synthesized III. G0 phase
D. Quiescent stage with metabolically active cells IV. S phase
Choose the correct answer from the options given below:
Match List-I with List-II.
List-I List-II
A. Migratory flamingoes and resident fish in
South American lakes I. Interference competition
B. Abingdon tortoise became extinct after introduction
of goats in their habitat II. Competitive release
C. Chathamalus expands its distributional range in
the absence of Balanus III. Resource Partitioning
D. Five closely related species of Warblers feeding in
different locations on same tree IV. Interspecific competition
Choose the correct answer from the options given below:
Match List-I with List-II relating to microbes and their products:
List-I (Microbes) List-II (Products)
A. Streptococcus I. Citric acid
B. Trichoderma polysporum II. Clot buster
C. Monascus purpureus III. Cyclosporin A
D. Aspergillus niger IV. Statins
Choose the correct answer from the options given below:
Match List-I with List-II.
List-I List-II
A. F1 Particles I. Chromosomes
B. Histones II. Cilia
C. Axoneme III. Golgi apparatus
D. Cisternae IV. Mitochondria
Choose the correct answer from the options given below:
Match List-I with List-II relating to examples of various kinds of IUDs and barrier:
List-I List-II
A. Copper releasing IUD I. Vaults
B. Non-medicated IUD II. Multiload 375
C. Contraceptive barrier III. LNG-20
D. Hormone releasing IUD IV. Lippes loop
Choose the correct answer from the options given below:
Given below are two statements:
Statement I: Antibiotics are chemicals produced by microbes that kill other microbes.
Statement II: Antibiotics are chemicals formed in the body that eliminate microbes.
In the light of the above statements, choose the most appropriate answer from the options given below:
Arrange the following parts in the human mammary gland, traversing the route of milk ejection.
A. Mammary duct
B. Lactiferous duct
C. Mammary alveolus
D. Ampulla
E. Mammary tubule
Choose the correct answer from the options given below:
Which of the following are correct about EcoRI?
A. Cut the DNA with blunt end
B. Cut the DNA with sticky end
C. Recognise a specific palindromic sequence
D. Cut the DNA between the base G and A when encounters the DNA sequence ‘GAATTC’
E. Exonuclease
Choose the correct answer from the options given below:
Which of the following is/are present in female cockroach?
A. Collateral gland
B. Mushroom gland
C. Spermatheca
D. Anal style
E. Phallic gland
Choose the most appropriate answer from the options given below:
Match List-I with List-II:
List-I List-II
A. Malignant tumors I. Destroy tumors
B. MALT II. AIDS
C. NACO III. Metastasis
D. \(\alpha\)-Interferons IV. Lymphoid tissue
Choose the correct answer from the options given below:
Open Circulatory system is present in:
Choose the correct answer from the options given below:
In which of the following connective tissues, the cells secrete fibres of collagen or elastin?
A. Cartilage
B. Done
C. Adipose tissue
D. Blood
E. Areolar tissue
Choose the most appropriate answer from the options given below:
Which of the following pairs is an incorrect match?
Match List-I with List-II:
List-I List-II
A. Residual Volume I. Maximum volume of air that can be breathed in after forced expiration
B. Vital Capacity II. Volume of air inspired or expired during normal respiration
C. Expiratory Capacity III. Volume of air remaining in lungs after forcible expiration
D. Tidal Volume IV. Total volume of air expired after normal inspiration
Choose the correct answer from the options given below:
Match List-I with List-II:
List-I List-II
A. Living Fossil I. Elongated canine teeth
B. Connecting Link II. Vermiform appendix
C. Vestigial Organ III. Echidna
D. Atavism IV. Latimeria
Choose the correct answer from the options given below:
Match List-I with List-II:
List-I List-II
A. Schwann cells I. Neurotransmitter
B. Synaptic knob II. Cerebral cortex
C. Bipolar neurons III. Myelin sheath
D. Multipolar neurons IV. Retina
Choose the correct answer from the options given below:
Diuresis is prevented by:
Choose the correct answer from the options given below:
Following is the list of STDs. Select the diseases which are not completely curable.
A. Genital warts
B. Genital herpes
C Syphilis
D Hepatitis-B
E Trichomoniasis
Choose the correct answer from the options given below:
What is the correct order (old to recent) of periods in the Paleozoic era?
Choose the correct answer from the options given below:
‘Lub’ sound of Heart is caused by the ........
Choose the correct answer from the options given below:
Match List-I with List-II relating to human female external genitalia.
List I (Structures) List II (Features)
A. Mons pubis I. A fleshy fold of tissue surrounding the vaginal opening
B. Clitoris II. Fatty cushion of cells covered by skin and hair
C. Hymen III. Tiny finger-like structure above labia minora
D. Labia majora IV. A thin membrane-like structure covering vaginal opening
Choose the correct answer from the options given below:
Aneuploidy is a chromosomal disorder where chromosome number is not the exact copy of its haploid set of chromosomes, due to:
A. Substitution
B. Addition
C. Deletion
D. Translocation
E. Inversion
Choose the most appropriate answer from the options given below:
Given below are two statements:
Statement I: RNA interference takes place in all Eukaryotic organisms as a method of cellular defense.
Statement II: RNA involves the silencing of a specific mRNA due to a complementary single-stranded RNA molecule that binds and prevents translation of mRNA.
In the light of the above statements, choose the correct answer from the options given below:
Identify the wrong statements:
(A) Erythropoietin is produced by juxtaglomerular cells of the kidney
(B) Leydig cells produce Androgens
(C) Atrial Natriuretic factor, a peptide hormone is secreted by the seminiferous tubules of the testes
(D) Cholecystokinin is produced by gastrointestinal tract
(E) Gastrin acts on intestinal wall and helps in the production of pepsinogen
Choose the most appropriate answer from the options given below:
Following are the steps involved in the process of PCR.
A. Annealing
B. Amplification (~1 billion times)
C. Denaturation
D. Treatment with Taq polymerase and deoxynucleotides
E. Extension
Choose the correct sequence of steps of PCR from the options given below:
Given below are two statements:
Statement I: Concentrated urine is formed due to counter current mechanism in nephron.
Statement II: Counter current mechanism helps to maintain osmotic gradient in the medullary interstitium.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements:
Statement I: Concentrically arranged cisternae of Golgi complex are arranged near the nucleus with distinct convex \textit{cis or maturing and concave \textit{trans or forming face.
Statement II: A number of proteins are modified in the cisternae of Golgi complex before they are released from \textit{cis face.
In the light of the above statements, choose the correct answer from the options given below:
Match List-I with List-II:
List I List II
A. Parturition I. Several antibodies for new-born babies
B. Placenta II. Collection of ovum after ovulation
C. Colostrum III. Foetal ejection reflex
D. Fimbriae IV. Secretion of the hormone hCG
Choose the correct answer from the options given below:
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Members of subphylum Vertebrata possess notochord during the embryonic period. The notochord is replaced by a cartilaginous or bony vertebral column in the adult.
Reason R: Thus all chordates are vertebrates; not all vertebrates are chordates.
In the light of the above statements, choose the correct answer from the options given below:
The mother has A+ blood group the father has B+ and the child is A+. What can be the possibility of genotypes of all three, respectively?
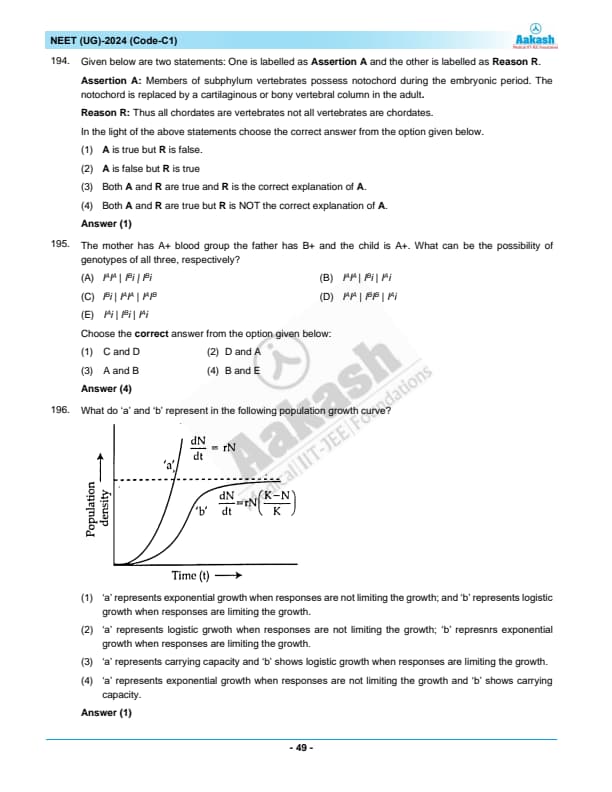
What do ‘a’ and ‘b’ represent in the following population growth curve?
Choose the correct answer from the options given below:
Select the correct statements regarding mechanism of muscle contraction.
A. It is initiated by a signal sent by CNS via sensory neuron.
B. Neurotransmitter generates action potential in the sarcolemma.
C. Increased Ca++ level leads to the binding of calcium with troponin on action filaments.
D. Masking of active site for actin is activated.
E. Utilising the energy from ATP hydrolysis to form cross bridge.
Choose the most appropriate answer from the options given below:
Match List I with List II:
List - I List - II
A. Squamous Epithelium I. Goblet cells of alimentary canal
B. Ciliated Epithelium II. Inner lining of pancreatic ducts
C. Glandular Epithelium III. Walls of blood vessels
D. Compound Epithelium IV. Inner surface of Fallopian tubes
Choose the correct answer from the options given below:
Match List I with List II:
List - I List - II
A. B-Lymphocytes I. Passive immunity
B. Interferons II. Cell-mediated immunity
C. T-Lymphocytes III. Produce an army of proteins in response to pathogens
D. Colostrum IV. Innate immunity
Choose the correct answer from the options given below:
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: During the transportation of gases, about 20-25 percent of CO\(_2\) is carried by Hemoglobin as carbamino-haemoglobin.
Reason R: This binding is related to high pCO\(_2\) and low pO\(_2\) in tissues.
In the light of the above statements, choose the correct answer from the options given below:
























































Comments