NEET 2024 Question paper with answer key pdf T6 is available for download. NEET 2024 T6 question paper has been conducted by the NTA on May 5, 2024, in pen-paper mode. NEET 2024 question paper code T6 consists of 200 MCQs- 180 to be attempted in 200 minutes. Each of the 4 subjects (Zoology, Botany, Chemistry, Physics) in NEET T6 question paper 2023 have 50 MCQs (45 to be attempted). You can download NEET 2024 question paper with answer key with solutions PDF for T6 using the links given below.
Related Links:
- Download NEET Previous Year Question Papers PDF with Solutions
- Download NEET 2024 Question Paper for all Shifts
NEET 2024 Question Paper with Answer Key PDF T6 in English
| NEET 2024 Question Paper with Answer Key | Check Solutions |
NEET 2024 Question Paper With Solution
In a uniform magnetic field of \(0.049 \, T\), a magnetic needle performs 20 complete oscillations in 5 seconds as shown. The moment of inertia of the needle is \(9.8 \times 10^{-6} \, kg \, m^2\). If the magnitude of magnetic moment of the needle is \(x \times 10^{-5} \, Am^2\), then the value of 'x' is:
View Solution
Step 1: Calculate the angular frequency \(\omega\) of the needle's oscillations.
Given that the needle completes 20 oscillations in 5 seconds, the frequency \(f\) is: \[ f = \frac{20 \, oscillations}{5 \, s} = 4 \, Hz \]
The angular frequency \(\omega\) is given by: \[ \omega = 2\pi f = 2\pi \times 4 = 8\pi \, rad/s \]
Step 2: Using the formula for the period \(T\) of a magnetic dipole in a magnetic field.
The period \(T\) is given by: \[ T = \frac{2\pi}{\omega} \]
Since \(\omega = 8\pi\), \[ T = \frac{2\pi}{8\pi} = \frac{1}{4} \, s \]
Step 3: Relate the moment of inertia and magnetic moment to the angular frequency.
From the equation for the oscillation of a magnetic dipole: \[ \omega^2 = \frac{\mu B}{I} \]
where \(\mu\) is the magnetic moment, \(B\) is the magnetic field, and \(I\) is the moment of inertia. Plugging in the values we get: \[ (8\pi)^2 = \frac{\mu \times 0.049}{9.8 \times 10^{-6}} \] \[ \mu = \frac{(8\pi)^2 \times 9.8 \times 10^{-6}}{0.049} \]
Step 4: Calculate \(\mu\).
\[ \mu = \frac{64\pi^2 \times 9.8 \times 10^{-6}}{0.049} \approx 1280 \pi^2 \times 10^{-5} \, Am^2 \]
Therefore, \(x = 1280\pi^2\). Quick Tip: For oscillations of a magnetic dipole in a magnetic field, remember that the angular frequency \(\omega\) is directly proportional to the square root of the magnetic moment \(\mu\) and inversely proportional to the square root of the moment of inertia \(I\).
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: The potential (V) at any axial point, at 2 m distance (r) from the centre of the dipole of dipole moment vector \( \vec{P} \) of magnitude, \( 4 \times 10^{-6} \) C m, is \( \pm 9 \times 10^3 \) V.
(Take \( \frac{1}{4 \pi \epsilon_0} = 9 \times 10^9 \) SI units)
Reason R: \( V = \pm \frac{2P}{4 \pi \epsilon_0 r^2} \), where \( r \) is the distance of any axial point, situated at 2 m from the centre of the dipole.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the formula for the potential at an axial point.
The potential at an axial point (distance \( r \) from the center of the dipole) is given by the formula:
\[ V = \frac{1}{4 \pi \epsilon_0} \cdot \frac{2P}{r^3}. \]
Here, \( P \) is the dipole moment and \( r \) is the distance from the center of the dipole.
Step 2: Substituting the given values.
We are given \( P = 4 \times 10^{-6} \) C m, \( r = 2 \) m, and \( \frac{1}{4 \pi \epsilon_0} = 9 \times 10^9 \) SI units. Substituting these values into the equation:
\[ V = \frac{9 \times 10^9 \times 2 \times 4 \times 10^{-6}}{(2)^3} = \frac{9 \times 10^9 \times 8 \times 10^{-6}}{8} = 9 \times 10^3 V. \]
Thus, Assertion A is true.
Step 3: Verifying the reason.
The formula given in the reason is incorrect. The correct formula for the potential at an axial point is \( V = \frac{1}{4 \pi \epsilon_0} \cdot \frac{2P}{r^3} \), not \( V = \pm \frac{2P}{4 \pi \epsilon_0 r^2} \). Hence, Reason R is false. Quick Tip: For dipoles, remember the correct formula for the potential at an axial point is \( V = \frac{1}{4 \pi \epsilon_0} \cdot \frac{2P}{r^3} \). This is crucial in avoiding errors in calculations.
Match List I with List II.
View Solution
Step 1: Identify the transitions and corresponding wavelengths
The spectral lines of hydrogen for transitions from higher energy levels \( n_2 \) to \( n_1 = 2 \) correspond to the following wavelengths:
\[ For n_2 = 3 to n_1 = 2, \, \lambda = 656.3 \, nm \quad (H_\alpha) \] \[ For n_2 = 4 to n_1 = 2, \, \lambda = 486.1 \, nm \quad (H_\beta) \] \[ For n_2 = 5 to n_1 = 2, \, \lambda = 434.1 \, nm \quad (H_\gamma) \] \[ For n_2 = 6 to n_1 = 2, \, \lambda = 410.2 \, nm \quad (H_\delta) \]
Step 2: Match the spectral lines with the wavelengths from List II
\( n_2 = 3 to n_1 = 2 \) corresponds to the wavelength 656.3 nm (\( H_\alpha \)), so it matches with option III.
\( n_2 = 4 to n_1 = 2 \) corresponds to 486.1 nm (\( H_\beta \)), so it matches with option IV.
\( n_2 = 5 to n_1 = 2 \) corresponds to 434.1 nm (\( H_\gamma \)), so it matches with option II.
\( n_2 = 6 to n_1 = 2 \) corresponds to 410.2 nm (\( H_\delta \)), so it matches with option I.
Thus, the correct matching is: \[ A-III, B-IV, C-II, D-I. \] Quick Tip: For hydrogen spectral lines, use the Balmer series formula to identify the wavelength corresponding to transitions from higher to lower energy levels.
The maximum elongation of a steel wire of 1 m length if the elastic limit of steel and its Young’s modulus, respectively, are \( 8 \times 10^8 \) N/m\(^{-2}\) and \( 2 \times 10^{11} \) N/m\(^{-2}\) is:
View Solution
N/A Quick Tip: When dealing with problems on material properties like elasticity, converting all units to match (e.g., converting meters to millimeters) before performing calculations is crucial to ensure accuracy.
Two bodies A and B of same mass undergo completely inelastic one dimensional collision. The body A moves with velocity \(v_1\) while body B is at rest before collision. The velocity of the system after collision is \(v_2\). The ratio \(v_1 : v_2\) is:
View Solution
Conservation of momentum in inelastic collision.
Momentum before collision:
\[ m v_1 + 0 = m v_2 \]
Momentum after collision: \[ (m + m) v_2 = 2m v_2 \]
Thus, \(v_2 = \frac{v_1}{2}\) and the ratio \(v_1 : v_2\) is \(2:1\). Quick Tip: In completely inelastic collisions, remember that the bodies stick together, and the velocity of the system is the same for both bodies after the collision. Use the conservation of momentum to find the final velocity.
In a vernier callipers, (N + 1) divisions of vernier scale coincide with N divisions of main scale. If 1 MSD represents 0.1 mm, the vernier constant (in cm) is:
View Solution
Understanding the vernier scale.
One main scale division (MSD) is 0.1 mm. Since (N+1) vernier divisions (VD) coincide with N MSDs, each VD is:
\[ VD = \frac{N \times 0.1 \, mm}{N+1} \]
Calculate the vernier constant.
The vernier constant (VC) is the difference between one MSD and one VD:
\[ VC = 0.1 \, mm - \frac{N \times 0.1 \, mm}{N+1} = \frac{0.1 \, mm}{N+1} \]
Converting to cm: \[ VC = \frac{0.1 \, cm}{10(N+1)} = \frac{1}{100(N+1)} \, cm \] Quick Tip: To calculate the Vernier constant, subtract the value of one Vernier scale division from one main scale division. The result gives the precision of the Vernier caliper.
A thermodynamic system is taken through the cycle abcda. The work done by the gas along the path bc is:
View Solution
N/A Quick Tip: For cycles involving different thermodynamic processes, it's essential to consider the type of each segment to determine overall work done and energy changes. Recall that the area enclosed by a process path on a P-V diagram represents the work done during the cycle.
A wheel of a bullock cart is rolling on a level road as shown in the figure below. If its linear speed is \( v \) in the direction shown, which one of the following options is correct (P and Q are any highest and lowest points on the wheel, respectively)?
View Solution
N/A Quick Tip: Remember in rolling motion, points on the rim of the wheel have varying speeds depending on their position relative to the point of contact with the ground. The highest point moves the fastest, while the point in contact with the ground has zero speed.
A bob is whirled in a horizontal plane by means of a string with an initial speed of \( \omega \) rpm. The tension in the string is T. If the speed becomes \( 2\omega \) while keeping the same radius, the tension in the string becomes:
View Solution
N/A Quick Tip: Remember that the tension needed for circular motion is directly proportional to the square of the angular velocity. Doubling the speed quadruples the required centripetal force.
A light ray enters through a right-angled prism at point \( P \) with the angle of incidence \( 30^\circ \) as shown in the figure. It travels through the prism parallel to its base \( BC \) and emerges along the face \( AC \). The refractive index of the prism is:
View Solution
Step 1: Analyze the Geometry
The prism is right-angled at B. The light ray enters at P with an angle of incidence 30°, travels parallel to BC, and exits at Q along AC. Let the angle at A be \(\theta\).
Step 2: Apply Snell's Law at P
Let the refractive index of the prism be \(n\). Applying Snell's Law at point P:
\[ 1 \cdot \sin(30^\circ) = n \cdot \sin(r_1) \]
where \(r_1\) is the angle of refraction at P.
Step 3: Angles and Relationships
Since the ray travels parallel to BC, the angle of refraction at P, \(r_1\), is related to the angle at A (\(\theta\)) by:
\[ r_1 + \theta = 90^\circ \Rightarrow r_1 = 90^\circ - \theta \]
Step 4: Apply Snell's Law at Q
At point Q, the angle of incidence is \(\theta\) and the angle of refraction is 90°. Applying Snell's Law:
\[ n \cdot \sin(\theta) = 1 \cdot \sin(90^\circ) = 1 \]
Step 5: Combine and Solve
From the equation at P: \[ \sin(30^\circ) = n \sin(r_1) = n \sin(90^\circ - \theta) = n \cos(\theta) \]
Since \(\sin(30^\circ) = \frac{1}{2}\), we have: \[ \frac{1}{2} = n \cos(\theta) \]
From the equation at Q: \[ n \sin(\theta) = 1 \]
We can write \(\cos(\theta) = \sqrt{1 - \sin^2(\theta)}\). Substituting this into the equation from P: \[ \frac{1}{2} = n \sqrt{1 - \sin^2(\theta)} \]
Substitute \(\sin(\theta) = \frac{1}{n}\) from the equation at Q: \[ \frac{1}{2} = n \sqrt{1 - \frac{1}{n^2}} \] \[ \frac{1}{4} = n^2 \left(1 - \frac{1}{n^2}\right) = n^2 - 1 \] \[ n^2 = \frac{5}{4} \] \[ n = \frac{\sqrt{5}}{2} \]
% Quick tip
\begin{quicktipbox
For a ray to travel parallel inside a prism, the refractive index is calculated using Snell’s law at the first interface.
\end{quicktipbox Quick Tip: For a ray to travel parallel inside a prism, the refractive index is calculated using Snell’s law at the first interface.
Match List-I with List-II.
View Solution
Understanding magnetic susceptibility \( \chi \)
Diamagnetic materials have small negative susceptibility (\( \chi < 0 \)).
Ferromagnetic materials have very high positive susceptibility.
Paramagnetic materials have small positive susceptibility.
Non-magnetic materials have zero susceptibility. Quick Tip: Magnetic materials are classified based on their susceptibility \( \chi \):
- \( \chi < 0 \) for diamagnetic,
- \( 0 < \chi < \epsilon \) for paramagnetic,
- \( \chi \gg 1 \) for ferromagnetic.
If \( x = 5 \sin \left( \pi t + \frac{\pi}{3} \right) \) m represents the motion of a particle executing simple harmonic motion, the amplitude and time period of motion, respectively, are:
View Solution
Step 1: Identifying amplitude and time period
- The given equation is in standard SHM form: \[ x = A \sin (\omega t + \phi) \]
Comparing with \( x = 5 \sin \left( \pi t + \frac{\pi}{3} \right) \), we identify: \[ A = 5 m, \quad \omega = \pi \]
Time period is given by: \[ T = \frac{2\pi}{\omega} = \frac{2\pi}{\pi} = 2 s \] Quick Tip: The amplitude is the coefficient of the sine function, and the time period is given by \( T = \frac{2\pi}{\omega} \).
Consider the following statements A and B and identify the correct answer:
A. For a solar-cell, the I-V characteristics lie in the IV quadrant of the given graph.
B. In a reverse biased \textit{pn junction diode, the current measured in \( \mu A \), is due to majority charge carriers.
View Solution
Step 1: Analyzing Statement A (Solar Cell I-V Characteristics).
- A solar cell operates by converting light energy into electrical energy.
- The I-V characteristics of a solar cell are plotted in the IV quadrant of the voltage-current graph because it supplies power instead of consuming it.
- Thus, Statement A is correct.
Step 2: Analyzing Statement B (Reverse Bias in \( pn \)-Junction Diode).
In reverse bias, the current that flows is minority carrier current (not majority carriers).
Majority carriers do not contribute significantly in reverse bias, and the current measured in \( \mu A \) is due to minority carriers.
Thus, Statement B is incorrect.
Step 3: Conclusion.
Since A is correct and B is incorrect, the correct answer is Option (3). Quick Tip: - A solar cell works as a power-generating device, so its I-V characteristics appear in the IV quadrant. - In a reverse-biased \( pn \)-junction diode, the current is mainly due to minority carriers.
In the following circuit, the equivalent capacitance between terminal A and terminal B is:
View Solution
We have four capacitors in the circuit, each of \( 2 \, \mu F \). First, we simplify the capacitors in series and parallel.
1. The two capacitors in series on the left-hand side (each of \( 2 \, \mu F \)) have an equivalent capacitance \( C_1 \) given by: \[ \frac{1}{C_1} = \frac{1}{2 \, \mu F} + \frac{1}{2 \, \mu F} = 1 \, \mu F \quad \Rightarrow \quad C_1 = 1 \, \mu F. \]
2. The two capacitors in series on the right-hand side (each of \( 2 \, \mu F \)) also have an equivalent capacitance \( C_2 \) given by: \[ \frac{1}{C_2} = \frac{1}{2 \, \mu F} + \frac{1}{2 \, \mu F} = 1 \, \mu F \quad \Rightarrow \quad C_2 = 1 \, \mu F. \]
3. Now, the two series capacitors \( C_1 \) and \( C_2 \) are in parallel with each other, so the total equivalent capacitance \( C_{eq} \) is: \[ C_{eq} = C_1 + C_2 = 1 \, \mu F + 1 \, \mu F = 2 \, \mu F. \]
Thus, the equivalent capacitance between terminals A and B is \( 2 \, \mu F \). Quick Tip: For capacitors in series, the equivalent capacitance is found using \( \frac{1}{C_{eq}} = \sum \frac{1}{C_i} \), and for capacitors in parallel, \( C_{eq} = \sum C_i \).
In an ideal transformer, the turns ratio is \( \frac{N_P}{N_S} = \frac{1}{2} \). The ratio \( V_S : V_P \) is equal to (the symbols carry their usual meaning):
View Solution
Understanding Transformer Voltage Relation
The voltage transformation equation for an ideal transformer is: \[ \frac{V_S}{V_P} = \frac{N_S}{N_P} \]
- Given \( \frac{N_P}{N_S} = \frac{1}{2} \), we take the reciprocal: \[ \frac{N_S}{N_P} = 2 \]
Thus, \[ \frac{V_S}{V_P} = 2 \]
\(\Rightarrow\) The voltage ratio \( V_S : V_P \) is \( 2:1 \).
% Quick tip
\begin{quicktipbox
In an ideal transformer, voltage is directly proportional to the turns ratio: \[ \frac{V_S}{V_P} = \frac{N_S}{N_P} \]
More secondary turns result in a step-up transformer, while fewer secondary turns result in a step-down transformer.
\end{quicktipbox Quick Tip: In an ideal transformer, voltage is directly proportional to the turns ratio: \[ \frac{V_S}{V_P} = \frac{N_S}{N_P} \] More secondary turns result in a step-up transformer, while fewer secondary turns result in a step-down transformer.
A thin flat circular disc of radius 4.5 cm is placed gently over the surface of water. If the surface tension of water is \(0.07 \, N/m^{-1}\), then the excess force required to take it away from the surface is:
View Solution
N/A Quick Tip: When calculating forces related to surface tension, always ensure the linear measurement (such as circumference in this case) is calculated accurately to get precise results. The surface tension force calculation essentially involves a linear force distribution along the periphery of the object in contact with the fluid.
A wire of length ‘l’ and resistance 100 \(\Omega\) is divided into 10 equal parts. The first 5 parts are connected in series while the next 5 parts are connected in parallel. The two combinations are again connected in series. The resistance of this final combination is:
View Solution
N/A Quick Tip: Remember, when combining resistances in series and parallel, always ensure calculations follow the correct formula for each type of connection. This will ensure accurate results in complex circuits.
The quantities which have the same dimensions as those of solid angle are:
View Solution
N/A Quick Tip: Solid angle, like planar angle and strain, is a dimensionless quantity. When identifying dimensionally consistent quantities, always consider the fundamental nature of each quantity's measurement and units.
In the above diagram, a strong bar magnet is moving towards solenoid-2 from solenoid-1. The direction of induced current in solenoid-1 and that in solenoid-2, respectively, are through the directions:
View Solution
Step 1: Applying Lenz’s Law.
According to Lenz’s Law, the induced current in a solenoid opposes the change in magnetic flux.
Step 2: Determining the current direction in Solenoid-1.
Since the bar magnet is moving towards Solenoid-2, the flux through Solenoid-1 is decreasing.
To oppose this decrease, the solenoid induces a current that maintains the original north polarity near the magnet.
Using the right-hand rule, the current in Solenoid-1 circulates in the direction \( AB \).
Step 3: Determining the current direction in Solenoid-2.
The approaching magnet increases the flux in Solenoid-2.
To oppose this increase, Solenoid-2 induces a current to create a south pole at the nearest end.
Using the right-hand rule, the induced current flows in the \( DC \) direction.
Step 4: Conclusion.
Thus, the correct directions of the induced currents are \( AB \) in Solenoid-1 and \( DC \) in Solenoid-2. Quick Tip: Lenz’s Law states that the induced current opposes the change in magnetic flux. Always apply the right-hand rule to determine the direction of induced current.
The terminal voltage of the battery, whose emf is 10 V and internal resistance 1 \( \Omega \), when connected through an external resistance of 4 \( \Omega \) as shown in the figure is:
View Solution
The total resistance in the circuit is the sum of the internal resistance \( r = 1 \, \Omega \) and the external resistance \( R = 4 \, \Omega \), so the total resistance is \( R_{total} = 5 \, \Omega \).
The current \( I \) in the circuit is given by: \[ I = \frac{emf}{R_{total}} = \frac{10}{5} = 2 \, A. \]
The terminal voltage \( V_{terminal} \) is the emf minus the voltage drop across the internal resistance: \[ V_{terminal} = emf - I \times r = 10 - 2 \times 1 = 8 \, V. \] Quick Tip: To calculate the terminal voltage, subtract the voltage drop across the internal resistance from the emf of the battery: \( V_{terminal} = emf - I \times r \).
The graph which shows the variation of \( \frac{1}{\lambda^2} \) and its kinetic energy, \( E \), where \( \lambda \) is de Broglie wavelength of a free particle:
View Solution
N/A Quick Tip: Understanding the relationship between kinetic energy and wavelength is crucial in quantum mechanics and helps in analyzing particle behavior at quantum scales.
If \( c \) is the velocity of light in free space, the correct statements about photons among the following are:
A. The energy of a photon is \( E = h\nu \).
B. The velocity of a photon is \( c \).
C. The momentum of a photon, \( p = \frac{h\nu}{c} \).
D. In a photon-electron collision, both total energy and total momentum are conserved.
E. Photon possesses positive charge.
Choose the correct answer from the options given below:
View Solution
Understanding the photon properties.
Statement A is correct because photon energy is given by \( E = h\nu \).
Statement B is correct because photons travel at the speed of light \( c \).
Statement C is correct as photon momentum is given by \( p = \frac{h\nu}{c} \).
Statement D is correct as photon-electron collisions obey conservation of energy and momentum.
Statement E is incorrect because photons are neutral (no charge).
Final Answer: A, B, C, and D are correct. Quick Tip: Photon has no mass, always moves at speed \( c \), and follows energy-momentum relations.
In the nuclear emission stated above, the mass number and atomic number of the product \( Q \) respectively, are:
View Solution
N/A Quick Tip: In nuclear reactions, each type of decay affects the atomic and mass numbers differently. Alpha decay reduces both by 2 and 4, respectively. Positron emission and electron capture lower the atomic number by 1, while beta minus decay increases it by 1.
At any instant of time \( t \), the displacement of any particle is given by \( x = 2t - 1 \) (SI unit) under the influence of force of \( 5 \) N. The value of instantaneous power is (in SI unit):
View Solution
Step 1: Finding velocity
Velocity is given by differentiating displacement:
\[ v = \frac{dx}{dt} = \frac{d}{dt} (2t - 1) = 2 m/s \]
Step 2: Calculating Instantaneous Power
Power is given by:
\[ P = F \cdot v \]
Substituting \( F = 5 \) N and \( v = 2 \) m/s,
\[ P = 5 \times 2 = 10 W \]
% Quick tip
\begin{quicktipbox
Instantaneous power is calculated as \( P = Fv \), where \( v \) is the instantaneous velocity of the particle.
\end{quicktipbox Quick Tip: Instantaneous power is calculated as \( P = Fv \), where \( v \) is the instantaneous velocity of the particle.
The moment of inertia of a thin rod about an axis passing through its mid point and perpendicular to the rod is 2400 g cm\(^2\). The length of the 400 g rod is nearly:
View Solution
N/A Quick Tip: Always check units and conversion factors when dealing with physical constants and measurements. This ensures the accuracy of the calculated results.
If the monochromatic source in Young’s double slit experiment is replaced by white light, then:
View Solution
Step 1: Understanding the Effect of White Light in Young’s Experiment
When a monochromatic source is used, the fringes are of uniform colour and spacing.
When white light is used, all wavelengths interfere, resulting in a central bright white fringe.
Surrounding fringes have different colours because different wavelengths interfere at different positions.
Step 2: Elimination of Incorrect Options
Option (2) is incorrect because different wavelengths result in different fringe widths.
Option (3) is incorrect because interference still occurs but with colour variation.
Option (4) is incorrect because the central fringe remains bright, not dark.
Quick Tip: For Young’s experiment with white light, remember:
The central fringe is always white.
Coloured fringes appear due to wavelength-dependent interference.
The fringe width varies for different wavelengths.
A thin spherical shell is charged by some source. The potential difference between the two points \( C \) and \( P \) (in V) shown in the figure is:
(Take \( \frac{1}{4\pi\epsilon_0} = 9 \times 10^9 \) SI units)
View Solution
Step 1: Understanding the properties of a charged spherical shell
A charged spherical shell behaves such that the electric potential inside and on the surface of the shell remains constant.
The potential at any point inside the shell is the same as the potential on its surface and is given by:
\[ V = \frac{1}{4\pi\epsilon_0} \cdot \frac{q}{R} \]
Step 2: Applying the concept to given points \( C \) and \( P \)
Both \( C \) (center) and \( P \) (on the shell) experience the same potential.
Since the potential difference is given by:
\[ V_C - V_P \]
and \( V_C = V_P \), we conclude:
\[ V_C - V_P = 0 \]
% Quick tip
\begin{quicktipbox
For a charged conducting spherical shell, the electric potential remains constant everywhere inside the shell, meaning the potential difference between any two internal points is always zero.
\end{quicktipbox Quick Tip: For a charged conducting spherical shell, the electric potential remains constant everywhere inside the shell, meaning the potential difference between any two internal points is always zero.
The output (Y) of the given logic gate is similar to the output of an/a:
View Solution
Step 1: Identifying the logic gates in the circuit.
The first gate is a NAND gate with both inputs as \( A \), giving:
\[ Output = \neg(A \cdot A) = \neg A. \]
The second gate is an OR gate with inputs \( A \) and \( B \), giving:
\[ Output = A + B. \]
The final gate is an AND gate that takes the outputs of the previous two gates as inputs:
\[ Y = (\neg A) \cdot (A + B). \]
Step 2: Simplifying the expression.
Expanding using Boolean algebra:
\[ Y = \neg A \cdot A + \neg A \cdot B. \]
Since \( A \cdot \neg A = 0 \), we get:
\[ Y = \neg A \cdot B. \]
This is the same as an AND gate operation where one input is \( B \) and the other is \( \neg A \), meaning the circuit functions like an AND gate. Quick Tip: When analyzing logic circuits, break them down into individual logic gates and use Boolean algebra to simplify step by step.
A logic circuit provides the output \( Y \) as per the following truth table:
The expression for the output Y is :
View Solution
N/A Quick Tip: In logic circuits, if the output is dependent on one variable and independent of another, analyzing the truth table closely can simplify finding the correct logical expression.
A tightly wound 100-turns coil of radius 10 cm carries a current of 7 A. The magnitude of the magnetic field at the centre of the coil is (Take permeability of free space as \( 4\pi \times 10^{-7} \) SI units):
View Solution
N/A Quick Tip: The formula \( B = \frac{\mu_0 N I}{2R} \) is applicable for a tightly wound coil, ensuring uniform field distribution.
The mass of a planet is \( \frac{1}{10} \) that of the Earth and its diameter is half that of the Earth. The acceleration due to gravity on that planet is:
View Solution
Step 1: Calculate the radius of the planet.
Given that the diameter of the planet is half that of Earth, the radius of the planet \( r \) will also be half of Earth's radius \( R \). If \( R \) is the radius of Earth, then:
\[ r = \frac{R}{2} \]
Step 2: Use the formula for gravitational acceleration.
The formula for gravitational acceleration \( g \) on the surface of a planet is given by:
\[ g = \frac{G M}{r^2} \]
where \( G \) is the gravitational constant, \( M \) is the mass of the planet, and \( r \) is the radius of the planet. The gravitational acceleration on Earth \( g_{Earth} \) is \( 9.8 \, m/s^{-2} \).
Step 3: Apply the ratios of mass and radius to the formula.
The mass of the planet is \( \frac{1}{10} \) of Earth's mass \( M_{Earth} \), and the radius is \( \frac{R}{2} \). Plugging these into the formula for \( g \) gives: \[ g_{planet} = \frac{G \left(\frac{1}{10} M_{Earth}\right)}{\left(\frac{R}{2}\right)^2} = \frac{G \left(\frac{1}{10} M_{Earth}\right)}{\frac{R^2}{4}} = \frac{4G \left(\frac{1}{10} M_{Earth}\right)}{R^2} = \frac{4}{10} g_{Earth} \] \[ g_{planet} = \frac{4}{10} \times 9.8 \, m/s^{-2} = 3.92 \, m/s^{-2} \] Quick Tip: When calculating gravitational acceleration on other planets, remember to account for both changes in mass and radius, as gravity is sensitive to both these factors. This problem highlights the square law nature of gravitational force relative to distance (radius).
A particle moving with uniform speed in a circular path maintains:
View Solution
Understanding circular motion
Velocity changes because the direction changes continuously.
Acceleration also changes due to the changing direction of velocity (centripetal acceleration).
Quick Tip: In uniform circular motion, speed is constant but velocity and acceleration continuously change due to direction changes.
A horizontal force of 10 N is applied to block A as shown in the figure. The mass of blocks A and B are 2 kg and 3 kg respectively. The blocks slide over a frictionless surface. The force exerted by block A on block B is:
View Solution
N/A Quick Tip: Always isolate the component or part of the system you are analyzing to simplify force calculations in systems with multiple objects.
An unpolarised light beam strikes a glass surface at Brewster’s angle. Then:
View Solution
Understanding Brewster’s angle
At Brewster’s angle, the reflected light is completely polarised perpendicular to the plane of incidence, while the refracted light remains partially polarised. Quick Tip: At Brewster’s angle \( \theta_B \), the reflected light is completely polarised perpendicular to the incident plane. The refracted light is not fully polarised.
Given below are two statements:
Statement I: Atoms are electrically neutral as they contain equal numbers of positive and negative charges.
Statement II: Atoms of each element are stable and emit their characteristic spectrum.
View Solution
N/A Quick Tip: Understanding atomic structure and behavior involves knowing when and why atoms emit light. The emission of light in the form of a spectrum generally indicates a transition between energy levels, not the inherent stability of atoms.
If the plates of a parallel plate capacitor connected to a battery are moved closer to each other, then:
A. the charge stored in it, increases.
B. the energy stored in it, decreases.
C. its capacitance increases.
D. the ratio of charge to its potential remains the same.
E. the product of charge and voltage increases.
Choose the most appropriate answer from the options given below:
View Solution
N/A Quick Tip: Understanding how changes in physical dimensions of a capacitor affect its electrical properties is crucial in both theoretical and practical applications in circuits.
The following graph represents the \( T \)-\( V \) curves of an ideal gas (where \( T \) is the temperature and \( V \) the volume) at three pressures \( P_1 \), \( P_2 \), and \( P_3 \), compared with those of Charles’s law represented as dotted lines.
Then the correct relation is:
View Solution
Step 1: Understanding the Graph
The equation governing isobaric processes is Charles’s Law:
\[ V \propto T \quad (for constant pressure) \]
Higher pressure results in a less steep slope in the \( T \)-\( V \) graph.
Step 2: Identifying the Correct Order
Since \( P_1 \) corresponds to the least steep curve, it represents the highest pressure.
\( P_3 \) has the steepest slope, indicating the lowest pressure.
Thus, the correct order is:
\[ P_1 > P_2 > P_3 \]
% Quick tip
\begin{quicktipbox
For an ideal gas, the steeper the isobaric curve in a \( T \)-\( V \) graph, the lower the pressure.
\end{quicktipbox Quick Tip: For an ideal gas, the steeper the isobaric curve in a \( T \)-\( V \) graph, the lower the pressure.
Choose the correct circuit which can achieve the bridge balance:
View Solution
Analysis of Circuits:
In a balanced Wheatstone bridge:
\[ \frac{R_1}{R_2} = \frac{R_3}{R_4} \]
where \( R_1 \) and \( R_2 \) are resistances on one side of the bridge, and \( R_3 \) and \( R_4 \) are on the opposite side.
Circuit Examination:
Circuit 1: \( \frac{10\Omega}{15\Omega} \neq \frac{15\Omega}{10\Omega} \)
Circuit 2: \( \frac{10\Omega}{15\Omega} \neq \frac{15\Omega}{10\Omega} \)
Circuit 3: \( \frac{15\Omega}{10\Omega} = \frac{15\Omega}{10\Omega} \), satisfying the balance condition.
Circuit 4: \( \frac{10\Omega}{15\Omega} \neq \frac{15\Omega}{10\Omega} \)
Conclusion:
Circuit 3 is correctly configured to achieve a bridge balance, as the ratios of the resistances on opposite arms are equal. Thus, Circuit 3 is the correct answer, aligning with the solution provided. Quick Tip: For a Wheatstone bridge to be balanced, the ratio of the resistances in one diagonal must equal the ratio in the other diagonal.
A sheet is placed on a horizontal surface in front of a strong magnetic pole. A force is needed to:
Statements:
A. Hold the sheet there if it is magnetic.
B. Hold the sheet there if it is non-magnetic.
C. Move the sheet away from the pole with uniform velocity if it is conducting.
D. Move the sheet away from the pole with uniform velocity if it is both, non-conducting and non-polar.
Choose the correct statement(s) from the options given below:
View Solution
If the sheet is magnetic, it will experience attraction towards the magnetic pole, requiring a force to hold it in place (Statement A is correct).
If the sheet is conducting, eddy currents are induced, which generate a repulsive force moving the sheet away from the pole (Statement C is correct).
A non-magnetic sheet does not experience attraction, so Statement B is incorrect.
A non-conducting, non-polar sheet does not interact with the field significantly, so Statement D is incorrect.
% Quick tip
\begin{quicktipbox
Eddy currents in a conductor generate a repulsive force when placed in a time-varying magnetic field.
\end{quicktipbox Quick Tip: Eddy currents in a conductor generate a repulsive force when placed in a time-varying magnetic field.
A parallel plate capacitor is charged by connecting it to a battery through a resistor. If \( I \) is the current in the circuit, then in the gap between the plates:
View Solution
N/A Quick Tip: Displacement current is crucial for the symmetry of Maxwell's equations and explains phenomena like electromagnetic waves where no actual charge carriers are present.
A small telescope has an objective of focal length 140 cm and an eyepiece of focal length 5.0 cm. The magnifying power of the telescope for viewing a distant object is:
View Solution
N/A Quick Tip: The magnifying power of a telescope increases with the increase of the objective lens's focal length or the decrease of the eyepiece's focal length. This is crucial for achieving high-resolution images of distant celestial objects.
The velocity (v)–time (t) plot of the motion of a body is shown below. The acceleration (a)–time (t) graph that best suits this motion is:
The acceleration (a) – time (t) graph that best suits this motion is :
View Solution
From the given velocity-time graph:
Initially, velocity increases linearly, implying constant acceleration.
Then, velocity remains constant, meaning zero acceleration.
Finally, velocity decreases linearly, implying constant negative acceleration.
Thus, the correct acceleration-time graph will have a positive constant value initially, followed by zero acceleration, and then a negative constant value. Quick Tip: Acceleration is the slope of the velocity-time graph. A constant velocity means zero acceleration, while a linear velocity change means constant acceleration.
A metallic bar of Young’s modulus \(0.5 \times 10^{11} \, N/m^{-2}\) and coefficient of linear thermal expansion \(10^{-5} \, °C^{-1}\), length 1 m and area of cross-section \(10^{-3} \, m^2\) is heated from 0°C to 100°C without expansion or bending. The compressive force developed in it is:
View Solution
N/A Quick Tip: When dealing with thermal expansion and stress calculations, always verify that units are consistent across all terms to avoid calculation errors.
A 10 \( \mu \)F capacitor is connected to a 210 V, 50 Hz source as shown in the figure. The peak current in the circuit is nearly (\( \pi = 3.14 \)):
View Solution
The capacitive reactance is given by: \[ X_C = \frac{1}{\omega C} = \frac{1}{2 \pi f C}. \]
Substituting \( f = 50 \) Hz and \( C = 10 \mu F = 10^{-5} \) F: \[ X_C = \frac{1}{2 \times 3.14 \times 50 \times 10^{-5}} \approx 318 \Omega. \]
The peak current is given by: \[ I_0 = \frac{V_0}{X_C} = \frac{210 \times \sqrt{2}}{318} \approx 0.93 A. \] Quick Tip: For AC circuits with capacitors, use \( X_C = \frac{1}{2\pi f C} \) and \( I_0 = \frac{V_0}{X_C} \) to find peak current.
Two heaters A and B have power ratings of 1 kW and 2 kW, respectively. Those two are first connected in series and then in parallel to a fixed power source. The ratio of power outputs for these two cases is:
View Solution
N/A Quick Tip: When calculating power in series and parallel circuits, remember to first find equivalent resistances and then use the power formula accordingly. This helps avoid calculation errors in complex circuits.
A force defined by \( F = \alpha t^2 + \beta t \) acts on a particle at a given time \( t \). The factor which is dimensionless, if \( \alpha \) and \( \beta \) are constants, is:
View Solution
N/A Quick Tip: When determining dimensionality in physical equations, match units across terms carefully to identify dimensionless combinations.
The property which is not of an electromagnetic wave travelling in free space is that:
View Solution
N/A Quick Tip: Recall that electromagnetic waves are generated by accelerating charges, not just moving charges. The change in acceleration is key to changing the electromagnetic fields.
An iron bar of length \( L \) has a magnetic moment \( M \). It is bent at the middle of its length such that the two arms make an angle of 60° with each other. The magnetic moment of this new magnet is:
View Solution
N/A Quick Tip: When dealing with vector quantities like magnetic moments, the angle between components and the method of their vector addition play critical roles in determining the resultant vector's magnitude and direction.
If the mass of the bob in a simple pendulum is increased to thrice its original mass and its length is made half its original length, then the new time period of oscillation is \( \frac{x}{2} \) times its original time period. Then the value of \( x \) is:
View Solution
N/A Quick Tip: In mechanics, understanding the dependencies of oscillatory motion such as the pendulum's period on length but not on mass is crucial. This conceptual clarity helps in problem-solving across various topics in physics.
The minimum energy required to launch a satellite of mass \( m \) from the surface of Earth of mass \( M \) and radius \( R \) in a circular orbit at an altitude of \( 2R \) from the surface of the Earth is:
View Solution
N/A Quick Tip: In launching satellites, it is crucial to consider both the energy required to overcome Earth's gravitational pull to the desired altitude and the kinetic energy needed to achieve orbital velocity. Both energies significantly contribute to the total launch energy requirement.
Among Group 16 elements, which one does NOT show –2 oxidation state?
View Solution
Step 1: Analyze the chemical properties and trends within Group 16.
Oxygen (\( O \)) is known for almost exclusively showing a \(-2\) oxidation state due to its high electronegativity.
Sulfur (\( S \)), Selenium (\( Se \)), and Tellurium (\( Te \)) can also commonly exhibit the \(-2\) oxidation state, reflecting their non-metallic nature and ability to gain electrons.
Polonium (\( Po \)), however, being a metalloid and more metallic than its predecessors, does not typically show a \(-2\) oxidation state. Polonium is more likely to exhibit positive oxidation states, such as \(+2\) or \(+4\), which are typical for metals and metalloids.
Quick Tip: Understanding periodic trends, such as increasing metallic character and decreasing electronegativity from oxygen to polonium, helps in predicting typical oxidation states of elements.
The highest number of helium atoms is in:
View Solution
Step 1: Convert each option to the number of atoms using Avogadro's number.
4 g of helium: The molar mass of helium is approximately 4 g/mol.
Thus, 4 g of helium is 1 mol, which contains \(6.022 \times 10^{23}\) atoms.
2.271098 L of helium at STP: At STP, 1 mol of any gas occupies 22.4 L. Therefore, \(2.271098 \, L\) of helium corresponds to \(\frac{2.271098}{22.4} \approx 0.1014\) mol, which is about \(6.1 \times 10^{22}\) atoms.
4 mol of helium: 4 mol of helium contains \(4 \times 6.022 \times 10^{23} = 2.409 \times 10^{24}\) atoms.
4 u of helium: Since 4 u corresponds to 1 mol of helium (4 g/mol), it represents \(6.022 \times 10^{23}\) atoms.
Step 2: Conclusion.
The option with the highest number of helium atoms is 4 mol of helium, containing \(2.409 \times 10^{24}\) atoms. Quick Tip: Remember that Avogadro's number (\(6.022 \times 10^{23}\) atoms/mol) is key to converting moles to atoms.
Given below are two statements:
Statement I: The boiling point of three isomeric pentanes follows the order \(n\)-pentane \(>\) isopentane \(>\) neopentane.
Statement II: When branching increases, the molecule attains a shape of sphere. This results in smaller surface area for contact, due to which the intermolecular forces between the spherical molecules are weak, thereby lowering the boiling point.
In the light of the above statements, choose the most appropriate answer from the options given below:
View Solution
Statement I: The boiling points of alkanes generally decrease with increased branching because branched molecules have less surface area for intermolecular forces to act upon, which lowers the boiling point. Thus, \(n\)-pentane (straight-chain) has the highest boiling point, followed by isopentane (branched), and neopentane (more highly branched) has the lowest boiling point.
Statement II: This is correct because, as branching increases, the molecule becomes more spherical in shape, leading to reduced surface area and weaker intermolecular forces (van der Waals forces), thus lowering the boiling point.
Quick Tip: When comparing boiling points, remember that more branching usually leads to lower boiling points due to decreased surface area and weaker intermolecular forces.
Which plot of \( \ln k \) vs \( \frac{1}{T} \) is consistent with Arrhenius equation?
View Solution
Understand the Arrhenius Equation.
The Arrhenius plot, specifically \(\ln k\) vs \( \frac{1}{T} \), provides critical insights into the reaction mechanism:
Slope (\(-\frac{E_a}{R}\)): This value directly indicates the activation energy required to initiate the reaction. A steeper negative slope implies a higher activation energy, indicating a more temperature-sensitive process.
Intercept (\(\ln A\)): The intercept on the \( \ln k \) axis provides the natural logarithm of the pre-exponential factor, which is a measure of the frequency of collisions and the orientation factor conducive to the reaction.
These parameters are crucial for chemists to understand the temperature dependence of reaction rates and to estimate the feasibility and speed of chemical reactions under different conditions.
Quick Tip: Always remember that a plot of \( \ln k \) vs \( \frac{1}{T} \) in the context of the Arrhenius equation should be a straight line decreasing as \( \frac{1}{T} \) increases, due to the negative relationship.
The most stable carbocation among the following is:
View Solution
Analyze the stability of the carbocations based on structural features.
The most stable carbocation typically has the most alkyl groups attached to the positively charged carbon, providing greater electron-donating effects through hyperconjugation and inductive effects. Among the given options, the structure with extensive alkyl substitution around the carbocation (as described for option (2)) would be the most stable. Quick Tip: Carbocations are stabilized by alkyl groups through hyperconjugation and inductive effects. The more alkyl groups directly attached, the more stable the carbocation.
The \( E^\circ \) value for the \( Mn^{3+}/Mn^{2+} \) couple is more positive than that of \( Cr^{3+}/Cr^{2+} \) or \( Fe^{3+}/Fe^{2+} \) due to a change of:
View Solution
N/A Quick Tip: When analyzing redox couples and their electrode potentials, consider how the electron configurations before and after the redox change contribute to the overall stability and energy of the ions. This can significantly affect the observed \( E^\circ \) values.
Arrange the following elements in increasing order of first ionization enthalpy:
Li, Be, B, C, N
Choose the correct answer from the options given below:
View Solution
Understanding the trend in ionization energies across the periodic table.
The first ionization energy generally increases across a period from left to right due to increasing nuclear charge and decreasing atomic radius. However, there are exceptions due to electron configuration stability:
Li (1s\(^2\) 2s\(^1\)) has the lowest ionization energy because it has only one electron in the outermost shell.
B (1s\(^2\) 2s\(^2\) 2p\(^1\)) is lower than Be due to the p-orbital having a higher energy level than the s-orbital.
Be (1s\(^2\) 2s\(^2\)) has higher ionization energy due to a fully filled s-orbital, which is more stable.
C (1s\(^2\) 2s\(^2\) 2p\(^2\)) and N (1s\(^2\) 2s\(^2\) 2p\(^3\)) have progressively higher ionization energies, with nitrogen being the highest due to half-filled p-orbital stability.
Quick Tip: Recall the effects of electronic configurations and orbital types (s vs. p) on ionization energies to predict trends accurately.
Match List I with List II.
Choose the correct answer from the options given below:
View Solution
A. [Co(NH\(_3\))\(_5\)(NO\(_2\))]Cl\(_2\): The NO\(_2\) ligand can bind through either nitrogen (nitro) or oxygen (nitrito), exhibiting linkage isomerism. Match: A - II
B. [Co(NH\(_3\))\(_5\)(SO\(_4\))]Br: The SO\(_4^{2-}\) ion can be inside or outside the coordination sphere, leading to ionization isomerism. Match: B - III
C. [Co(NH\(_3\))\(_6\)][Cr(CN)\(_6\)]: This complex has two complex ions. The ligands on the metal centers can be exchanged, resulting in coordination isomerism. Match: C - IV
D. [Co(H\(_2\)O)\(_6\)]Cl\(_3\): This complex can exist as different hydrates, with varying numbers of water molecules coordinated to the cobalt ion. This is a form of solvate isomerism (specifically, hydrate isomerism). Match: D - I
Therefore, the correct matching is A-II, B-III, C-IV, and D-I. The correct answer is (3). Quick Tip: Review each complex's structure and the nature of its ligands to identify the possible isomerism types effectively.
Which reaction is NOT a redox reaction?
View Solution
Option (1): This is a redox reaction where hydrogen and chlorine are both reduced and oxidized, respectively.
Option (3): This is a redox reaction where zinc is oxidized and copper is reduced.
Option (4): This is a redox reaction where potassium chlorate is reduced and iodine is oxidized.
Option (2) is a double displacement reaction, where no change in oxidation states occurs. Therefore, it is not a redox reaction. Quick Tip: In redox reactions, the oxidation states of elements change. In non-redox reactions, there is no change in oxidation states.
Given below are two statements:
Statement I: Aniline does not undergo Friedel-Crafts alkylation reaction.
Statement II: Aniline cannot be prepared through Gabriel synthesis.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Analyze Statement I.
Aniline, being an aromatic amine, has a lone pair of electrons on the nitrogen atom that can interact with Lewis acid catalysts used in the Friedel-Crafts alkylation, leading to complex formation that deactivates the catalyst. Thus, it does not effectively undergo Friedel-Crafts reactions.
Step 2: Analyze Statement II.
Gabriel synthesis is typically used for synthesizing primary amines from primary alkyl halides. Aniline, which involves an aromatic ring, cannot be prepared using Gabriel synthesis because it requires conditions that would lead to the destruction of the aromatic ring. Quick Tip: Understanding the chemical properties and reactivity of compounds helps in predicting their behavior in different chemical reactions.
Match List I with List II.
Choose the correct answer from the options given below:
View Solution
NH3 (A) has a trigonal pyramidal shape due to the lone pair on nitrogen, corresponding to I.
BrF5 (B) has a square pyramidal shape due to 5 bonding pairs and 1 lone pair on the central atom, matching with IV.
XeF4 (C) has a square planar shape due to 4 bonding pairs and 2 lone pairs, aligning with II.
SF6 (D) has an octahedral shape with 6 bonding pairs, corresponding to III.
Quick Tip: For molecular geometries, consider the number of bonding pairs and lone pairs around the central atom using VSEPR theory.
Which one of the following alcohols reacts instantaneously with Lucas reagent?
View Solution
Lucas reagent, a mixture of zinc chloride and concentrated hydrochloric acid, is employed to classify low molecular weight alcohols based on their reactivity. The nature of the reaction is influenced by the stability of the carbocation formed during the substitution process:
Primary alcohols (like n-butyl alcohol) form a primary carbocation, which is least stable and reacts very slowly, often showing no visible change at room temperature.
Secondary alcohols (like isobutyl alcohol) form a more stable secondary carbocation and react at a moderate rate.
Tertiary alcohols (like tert-butyl alcohol), form a highly stable tertiary carbocation almost instantaneously, leading to a rapid reaction evident by the quick formation of a cloudy solution or precipitate. Quick Tip: Lucas reagent is used to classify alcohols based on their reactivity. Tertiary alcohols react instantaneously, secondary alcohols react within minutes, and primary alcohols do not react significantly at room temperature.
The Henry’s law constant (\(K_H\)) values of three gases (A, B, C) in water are 145, \(2 \times 10^{-5}\), and 35 kbar, respectively. The solubility of these gases in water follow the order:
View Solution
According to Henry's Law, solubility is inversely proportional to the Henry’s law constant (\(K_H\)). A higher value of \(K_H\) corresponds to a lower solubility. Based on the given constants:
Gas A has the highest \(K_H\), so it has the lowest solubility.
Gas C has a medium \(K_H\) value, so its solubility is intermediate.
Gas B has the lowest \(K_H\), so it has the highest solubility.
Thus, the order of solubility is \(B > C > A\). Quick Tip: For gases, solubility in a liquid is inversely proportional to the Henry’s law constant. A higher constant means lower solubility.
The reagents with which glucose does not react to give the corresponding tests/products are:
A. Tollen’s reagent
B. Schiff’s reagent
C. HCN
D. NH2OH
E. NaHSO3
Choose the correct options from the given below:
View Solution
Tollen’s reagent (A) and Schiff’s reagent (B) react with aldehydes; glucose has an aldehyde group and thus reacts with both.
HCN (C) reacts with glucose, forming cyanohydrin.
NH2OH (D) reacts with glucose to form an oxime derivative.
NaHSO3 (E) reacts with aldehydes, but glucose does not typically react with sodium bisulfite under normal conditions.
Quick Tip: Glucose, with its aldehyde group, can react with reagents like Tollen's and Schiff's, but not all reagents with aldehyde-specific reactions will work with glucose.
Identify the correct reagents that would bring about the following transformation.
View Solution
N/A Quick Tip: Understanding the sequence and specificity of reagent actions in organic synthesis is crucial for designing effective synthetic routes and achieving the desired chemical transformations.
Fehling's solution 'A' is:
View Solution
Step 1: Understanding Fehling's solution.
Fehling's solution is used as a chemical test to differentiate between water-soluble carbohydrate and ketone functional groups, and more specifically, it detects the presence of aldehyde groups which are oxidizable.
Step 2: Composition of Fehling's solution.
Fehling's solution is typically made up of two parts:
Fehling's 'A': This part contains aqueous copper(II) sulphate, which provides the Cu\(^{2+}\) ions necessary for the oxidation reaction.
Fehling's 'B': This part contains an alkaline solution of sodium potassium tartrate (Rochelle salt), which acts to keep the copper ions in solution.
Since the correct answer for Fehling's solution 'A' is aqueous copper sulphate, the best choice is (3). Quick Tip: Remember the specific roles of each component in Fehling's solution: 'A' supplies the copper ions, and 'B' maintains these ions in an alkaline and complexed form.
Match List I with List II.
Choose the correct answer from the options given below:
View Solution
Step 1: Analyze each quantum number and its information.
\(m_l\) (A): Magnetic quantum number, which indicates the orientation of the orbital in space, thus matching with III.
\(m_s\) (B): Spin quantum number, indicating the orientation of the electron's spin, which is IV.
\(l\) (C): Azimuthal quantum number, related to the shape of the orbital, thus matching with I.
\(n\) (D): Principal quantum number, which influences the size and energy level of the orbital, corresponding to II. Quick Tip: Memorize the functions of each quantum number: principal (\(n\)), azimuthal (\(l\)), magnetic (\(m_l\)), and spin (\(m_s\)) to easily solve quantum mechanics problems.
1 gram of sodium hydroxide was treated with 25 mL of 0.75 M HCl solution, the mass of sodium hydroxide left unreacted is equal to:
View Solution
Step 1: Calculate the moles of HCl used.
The molarity (M) of HCl is 0.75 M, and the volume (V) used is 25 mL (or 0.025 L). The moles of HCl used are given by:
\[ n = M \times V = 0.75 \, mol/L \times 0.025 \, L = 0.01875 \, mol \]
Step 2: Stoichiometry of the reaction.
The reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) is:
\[ NaOH + HCl \rightarrow NaCl + H_2O \]
This reaction is a 1:1 molar reaction. Therefore, 0.01875 mol of NaOH will react with 0.01875 mol of HCl.
Step 3: Calculate the mass of NaOH that reacts.
The molar mass of NaOH is approximately 40 g/mol. Therefore, the mass of NaOH that reacts is:
\[ Mass = 0.01875 \, mol \times 40 \, g/mol = 0.75 \, g \]
Step 4: Calculate the mass of NaOH left unreacted.
Initially, there was 1 gram of NaOH. The amount of NaOH left unreacted after the reaction is: \[ 1 \, g - 0.75 \, g = 0.25 \, g = 250 \, mg \] Quick Tip: When dealing with stoichiometry problems, always check the mole ratio in the balanced chemical equation to ensure correct calculations for reactants and products.
On heating, some solid substances change from solid to vapour state without passing through liquid state. The technique used for the purification of such solid substances based on the above principle is known as:
View Solution
N/A Quick Tip: Sublimation is particularly useful for purifying substances that are unstable or decompose at temperatures near their melting points. It can also be used to separate volatile substances from non-volatile impurities.
In which of the following processes entropy increases?
A. A liquid evaporates to vapour.
B. Temperature of a crystalline solid lowered from 130 K to 0 K.
C. \(2NaHCO_3(s) \rightarrow Na_2CO_3(s) + CO_2(g) + H_2O(g)\)
D. \(Cl_2(g) \rightarrow 2Cl(g)\)
Choose the correct answer from the options given below:
View Solution
N/A Quick Tip: Entropy, a measure of disorder or randomness in a system, tends to increase in processes where gases form from liquids or solids, or where more particles are produced from fewer, especially at higher temperatures or when bonds are broken.
Match List I with List II.
Choose the correct answer from the options given below:
View Solution
Determine the correct conditions for each process.
Isothermal process (A) occurs at a constant temperature, matching with II.
Isochoric process (B) occurs at constant volume, matching with III.
Isobaric process (C) occurs at constant pressure, matching with IV.
Adiabatic process (D) occurs with no heat exchange, matching with I.
Quick Tip: Associate each thermodynamic process with its defining characteristic to quickly identify correct matching in problems.
Arrange the following elements in increasing order of electronegativity:
N, O, F, C, Si
Choose the correct answer from the options given below:
View Solution
Understanding the trend in electronegativity across the periodic table.
Electronegativity generally increases across a period from left to right and decreases down a group. Therefore, among the given elements:
Silicon (Si) being a group 14 element and further down the period compared to carbon, has the lowest electronegativity.
Carbon (C) has a higher electronegativity than Si.
Nitrogen (N) is more electronegative than C due to its position in the periodic table and having a smaller atomic radius.
Oxygen (O) is more electronegative than N, attributed to its higher effective nuclear charge.
Fluorine (F), being the most electronegative element in the periodic table, ranks highest among the options.
Quick Tip: Remember that electronegativity helps predict how atoms share electrons in chemical bonds, with higher values indicating stronger attraction for bonding electrons.
Given below are two statements:
Statement I: Both \([Co(NH_3)_6]^{3+}\) and \([CoF_6]^{3-}\) complexes are octahedral but differ in their magnetic behaviour.
Statement II: \([Co(NH_3)_6]^{3+}\) is diamagnetic whereas \([CoF_6]^{3-}\) is paramagnetic.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Statement I: Both \([Co(NH_3)_6]^{3+}\) and \([CoF_6]^{3-}\) are octahedral complexes with similar coordination numbers but differ in their magnetic properties.
Statement II: \([Co(NH_3)_6]^{3+}\) is diamagnetic due to the low-spin configuration of Co\((^{3+})\) (\(3d^6\)), while \([CoF_6]^{3-}\) is paramagnetic due to the weak field fluoride ligands, leading to unpaired electrons.
Quick Tip: Check the type of ligands and the electron configuration of the central metal ion to determine the magnetic behavior of coordination complexes.
Match List I with List II.
Choose the correct answer from the options given below:
View Solution
Matching molecules with bond types.
Ethane (A) has a single \(\sigma\)-bond between carbons.
Ethene (B) has one \(\sigma\)-bond and one \(\pi\)-bond.
The carbon molecule \( C_2 \) (C) typically has two \(\pi\)-bonds.
Ethyne (D) has one \(\sigma\)-bond and two \(\pi\)-bonds.
Quick Tip: Visualizing the molecular structure and counting the types of bonds between atoms are crucial for correctly identifying molecular bonding.
Activation energy of any chemical reaction can be calculated if one knows the value of:
View Solution
The activation energy \( E_a \) can be calculated using the Arrhenius equation, which involves the rate constants at two different temperatures: \[ k = A \exp\left(-\frac{E_a}{RT}\right) \]
where \( k \) is the rate constant, \( A \) is the frequency factor, \( E_a \) is the activation energy, \( R \) is the gas constant, and \( T \) is temperature. Quick Tip: Use the Arrhenius equation to relate the rate constant at two different temperatures to determine the activation energy.
In which of the following equilibria, \( K_p \) and \( K_c \) are NOT equal?
View Solution
Understand the relationship between \( K_p \) and \( K_c \).
The equilibrium constants \( K_p \) and \( K_c \) are related by the equation \( K_p = K_c(RT)^{\Delta n} \) where \( \Delta n \) is the change in moles of gas.
In Reaction (3), \( \Delta n = 1 - 2 = -1 \), therefore \( K_p \neq K_c \). Quick Tip: When \( \Delta n \neq 0 \), \( K_p \) and \( K_c \) will differ due to the contribution of the gas constant and temperature to the equation.
Match List I with List II.
Choose the correct answer from the options given below:
View Solution
A. 1 mol of H\(_2\)O to O\(_2\): The balanced equation is 2H\(_2\)O \(\rightarrow\) O\(_2\) + 4H\(^+\) + 4e\(^-\). 2 moles of H\(_2\)O require 4 moles of electrons, so 1 mole of H\(_2\)O requires 2 moles of electrons (2F). Match: A - II
B. 1 mol of MnO\(_4^-\) to Mn\(^{2+}\): The balanced equation is MnO\(_4^-\) + 8H\(^+\) + 5e\(^-\) \(\rightarrow\) Mn\(^{2+}\) + 4H\(_2\)O. 1 mole of MnO\(_4^-\) requires 5 moles of electrons (5F). Match: B - IV
C. 1.5 mol of Ca from molten CaCl\(_2\): The balanced equation is Ca\(^{2+}\) + 2e\(^-\) \(\rightarrow\) Ca. 1 mole of Ca requires 2 moles of electrons. 1.5 moles of Ca require 1.5 \(\times\) 2 = 3 moles of electrons (3F). Match: C - I
D. 1 mol of FeO to Fe\(_2\)O\(_3\): The balanced equation is 2FeO + \(\frac{1}{2}\)O\(_2\) \(\rightarrow\) Fe\(_2\)O\(_3\). The oxidation state of Fe changes from +2 in FeO to +3 in Fe\(_2\)O\(_3\). For 1 mole of FeO, the change is 1 mole of electrons (1F). Match: D - III
Therefore, the correct matching is A-II, B-IV, C-I, and D-III. The correct answer is (3). Quick Tip: In electrochemical processes, one Faraday (F) of charge corresponds to the charge of one mole of electrons, about \( 96,485 \) coulombs.
For the reaction \(2A \rightleftharpoons B + C\), \(K_c = 4 \times 10^{-3}\). At a given time, the composition of the reaction mixture is: \([A] = [B] = [C] = 2 \times 10^{-3} \, M\). Then, which of the following is correct?
View Solution
N/A Quick Tip: When analyzing whether a reaction will proceed in the forward or reverse direction, always calculate and compare the reaction quotient \(Q_c\) to the equilibrium constant \(K_c\). This will indicate whether the system needs to adjust toward more products or more reactants to achieve equilibrium.
Intramolecular hydrogen bonding is present in
View Solution
Identify structures capable of intramolecular hydrogen bonding.
Intramolecular hydrogen bonding typically occurs when hydrogen-bonding groups are positioned to form a stable five- or six-membered ring. Option (3) allows for such a configuration, with the OH and NO2 on the same carbon.
Quick Tip: Intramolecular hydrogen bonds are stronger and more stable when they form smaller rings, typically five- or six-membered.
The compound that will undergo \( S_N1 \) reaction with the fastest rate is:
View Solution
Evaluate the substrate stability for \( S_N1 \) reactions.
Compound 1: Benzyl Bromide - Forms a benzyl carbocation, which is stabilized by resonance with the phenyl ring.
Compound 2: para-Methylbenzyl Bromide - Forms a para-methylbenzyl carbocation, which is further stabilized by electron-donating effects of the methyl group through resonance, making it more stable than the benzyl carbocation.
Compound 3: Secondary Alkyl Bromide - Forms a secondary carbocation, which lacks the additional resonance stabilization seen in benzyl carbocations.
Compound 4: Cyclohexyl Bromide - Forms a cyclohexyl carbocation, which is not resonance stabilized and is generally less stable.
Conclusion:
Compound 2 will undergo an \( S_N1 \) reaction the fastest due to the enhanced stability of the carbocation intermediate by both resonance and inductive effects from the methyl group. The correct answer is 2. Quick Tip: Benzyl and allyl halides are excellent substrates for \( S_N1 \) reactions due to carbocation stabilization.
Match List I with List II.
Choose the correct answer from the options given below:
View Solution
Let's analyze and match List I (Reactions) with List II (Reagents/Conditions):
Reaction A: The reaction shows the cleavage of a benzyl group into two benzaldehyde molecules. This is characteristic of ozonolysis using \(O_3/Zn-H_2O\).
\(\Rightarrow\) Matches with IV
Reaction B: The reaction results in the formation of a benzophenone-like structure. This suggests a Friedel-Crafts acylation reaction, which requires benzoyl chloride and anhydrous AlCl\(_3\).
\(\Rightarrow\) Matches with I
Reaction C: The oxidation of a benzylic alcohol to a ketone suggests the use of chromium trioxide (CrO\(_3\)).
\(\Rightarrow\) Matches with II
Reaction D: The conversion of an alkyl benzene into a carboxylate group suggests a strong oxidizing agent like KMnO\(_4\)/KOH, \(\Delta\).
\(\Rightarrow\) Matches with III
Correct Answer: (1) A-IV, B-I, C-II, D-III Quick Tip: When identifying reagents for specific organic reactions, consider the common oxidation agents (CrO\(_3\), KMnO\(_4\)) and electrophilic substitution reagents like AlCl\(_3\).
'Spin only' magnetic moment is same for which of the following ions?
A. Ti\(^{3+}\)
B. Cr\(^{2+}\)
C. Mn\(^{2+}\)
D. Fe\(^{2+}\)
E. Sc\(^{3+}\)
Choose the most appropriate answer from the options given below.
View Solution
Step 1: Calculate the 'spin only' magnetic moment for each ion.
The formula for the 'spin only' magnetic moment (\(\mu\)) is \(\mu = \sqrt{n(n+2)}\) Bohr magnetons, where \(n\) is the number of unpaired electrons.
Ti\(^{3+}\) has 1 unpaired electron, \(\mu = \sqrt{1(1+2)} = 1.73\) Bohr magnetons.
Cr\(^{2+}\) has 4 unpaired electrons, \(\mu = \sqrt{4(4+2)} = 4.90\) Bohr magnetons.
Mn\(^{2+}\) has 5 unpaired electrons, \(\mu = \sqrt{5(5+2)} = 5.92\) Bohr magnetons.
Fe\(^{2+}\) also has 4 unpaired electrons, \(\mu = \sqrt{4(4+2)} = 4.90\) Bohr magnetons.
Sc\(^{3+}\) has no unpaired electrons, \(\mu = \sqrt{0(0+2)} = 0\) Bohr magnetons.
Step 2: Match the ions with the same magnetic moments.
Cr\(^{2+}\) and Fe\(^{2+}\) both have the same magnetic moment of 4.90 Bohr magnetons. Quick Tip: Use the number of unpaired electrons to quickly estimate the magnetic properties of transition metal ions.
The energy of an electron in the ground state (n = 1) for \( He^+ \) ion is \(-x\) J, then that for an electron in n = 2 state for \( Be^{3+} \) ion in J is:
View Solution
N/A Quick Tip: When comparing the energies of electrons in hydrogen-like ions across different atomic numbers and principal quantum numbers, the square of the charge \( Z^2 \) and the inverse square of the principal quantum number \( n^2 \) are key to understanding the energy scaling.
Given below are two statements:
Statement I: The boiling point of hydrides of Group 16 elements follow the order \( H_2O > H_2Te > H_2Se > H_2S \).
Statement II: On the basis of molecular mass, \( H_2O \) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \( H_2O \), it has higher boiling point.
In the light of the above statements, choose the correct answer from the options given below:
Choose the correct answer from the options given below:
View Solution
Step 1: Analyze Statement I.
Statement I is true as \( H_2O \) has a higher boiling point than other Group 16 hydrides due to hydrogen bonding, which is stronger than in \( H_2Te \), \( H_2Se \), and \( H_2S \).
Step 2: Analyze Statement II.
Statement II is also true. While the molecular mass of \( H_2O \) is lower compared to other hydrides, which suggests a lower boiling point, the extensive hydrogen bonding in \( H_2O \) significantly raises its boiling point above those of its heavier congeners. Quick Tip: Remember that hydrogen bonding can significantly affect physical properties like boiling points beyond what would be expected based purely on molecular mass.
Mass in grams of copper deposited by passing 9.6487 A current through a voltmeter containing copper sulphate solution for 100 seconds is (Given: Molar mass of Cu = 63 g mol\(^{-1}\), 1 F = 96487 C)
View Solution
To calculate the mass of copper deposited, we can use the formula for electrolysis:
\[ Mass of Cu = \frac{I \times t \times M}{n \times F} \]
Where:
\( I = 9.6487 \, A \) (current),
\( t = 100 \, seconds \) (time),
\( M = 63 \, g/mol \) (molar mass of copper),
\( n = 2 \) (valency of copper, \( Cu^{2+} \)),
\( F = 96487 \, C/mol \) (Faraday's constant).
Now, substituting the values into the formula:
\[ Mass of Cu = \frac{9.6487 \times 100 \times 63}{2 \times 96487} \]
Thus, the mass of copper deposited is \( 0.315 \, grams \). Quick Tip: To calculate the mass of a metal deposited during electrolysis, use the formula: \[ Mass = \frac{I \times t \times M}{n \times F} \] where \( I \) is the current, \( t \) is the time, \( M \) is the molar mass, \( n \) is the valency of the metal, and \( F \) is Faraday's constant.
A compound X contains 32% of A, 20% of B and remaining percentage of C. Then, the empirical formula of X is:
(Given atomic masses of A = 64; B = 40; C = 32 u)
View Solution
To determine the empirical formula, we calculate the moles of A, B, and C based on their percentages:
Moles of A = \( \frac{32}{64} = 0.5 \) mol
Moles of B = \( \frac{20}{40} = 0.5 \) mol
Moles of C = \( \frac{48}{32} = 1.5 \) mol
Now, dividing each by the smallest number of moles (0.5), the ratio of A : B : C is 1 : 1 : 3. Therefore, the empirical formula is ABC\(_3\). Quick Tip: To find the empirical formula, first convert the mass percentages into moles, then simplify the ratios to get the simplest whole-number formula.
Consider the following reaction in a sealed vessel at equilibrium with concentrations of
\(N_2 = 3.0 \times 10^{-3} \, M\), \(O_2 = 4.2 \times 10^{-3} \, M\), and \(NO = 2.8 \times 10^{-3} \, M\).
The reaction is: \(2NO(g) \rightleftharpoons N_2(g) + O_2(g)\)
If 0.1 mol L\(^{-1}\) of NO(g) is taken in a closed vessel, what will be the degree of dissociation (\(\alpha\)) of NO(g) at equilibrium?
View Solution
Let the initial concentration of NO be 0.1 M, and \(\alpha\) be the degree of dissociation. At equilibrium, the concentration of NO will be:
\[ [NO] = 0.1 - 2\alpha \]
The concentration of \(N_2\) and \(O_2\) formed will be \(\alpha\). Using the equilibrium concentrations and applying the reaction stoichiometry, we can set up the equilibrium expression and solve for \(\alpha\). After solving, we find that \(\alpha = 0.717\). Quick Tip: For dissociation problems, use the stoichiometry of the reaction to set up the concentration changes and solve for \(\alpha\).
The work done during reversible isothermal expansion of one mole of hydrogen gas at 25°C from pressure of 20 atmosphere to 10 atmosphere is:
(Given \(R = 2.0 \, cal K^{-1} mol^{-1}\))
View Solution
The work done during reversible isothermal expansion is given by: \[ W = -nRT \ln \left( \frac{P_1}{P_2} \right) \]
Given:
\( n = 1 \, mol \)
\( R = 2.0 \, cal K^{-1} mol^{-1} \)
\( T = 25^\circ C = 298 \, K \)
\( P_1 = 20 \, atm \)
\( P_2 = 10 \, atm \)
Step 1: Substitute the values into the formula. \[ W = - (1 \, mol) \cdot (2.0 \, cal K^{-1} mol^{-1}) \cdot (298 \, K) \cdot \ln \left( \frac{20 \, atm}{10 \, atm} \right) \]
Step 2: Simplify the pressure ratio. \[ \frac{20 \, atm}{10 \, atm} = 2 \]
\subsection{Step 3: Calculate the natural logarithm. \[ \ln(2) \approx 0.6931 \]
Step 4: Calculate the work done. \[ W = - (1) \cdot (2.0) \cdot (298) \cdot (0.6931) \] \[ W = - 413.14 \, cal \]
The negative sign indicates that work is done by the system (gas) during expansion.
Final Answer: \[ \boxed{-413.14 \, calories} \] Quick Tip: For isothermal expansion, use the equation \(W = -nRT \ln \left(\frac{P_2}{P_1}\right)\) to calculate work done by the gas.
The products A and B obtained in the following reactions, respectively, are:
\( 3ROH + PCl_3 \rightarrow 3RCl + A \)
\( ROH + PCl_5 \rightarrow RCl + HCl + B \)
View Solution
In the first reaction with PCl\(_3\), alcohol reacts with phosphorus trichloride (PCl\(_3\)) to produce alkyl chloride (RCl) and phosphorous acid (H\(_3\)PO\(_3\)) as product A.
In the second reaction, alcohol reacts with phosphorus pentachloride (PCl\(_5\)) to produce alkyl chloride (RCl), hydrogen chloride (HCl), and POCl\(_3\) as product B.
Thus, the products are H\(_3\)PO\(_3\) and POCl\(_3\). Quick Tip: Phosphorus trichloride reacts with alcohol to form phosphorous acid, while phosphorus pentachloride yields POCl\(_3\) and releases HCl.
Given below are two statements:
Statement I: \([Co(NH_3)_6]^{3+}\) is a homoleptic complex whereas \([Co(NH_3)_4Cl_2]^+\) is a heteroleptic complex.
Statement II: Complex \([Co(NH_3)_6]^{3+}\) has only one kind of ligands but \([Co(NH_3)_4Cl_2]^+\) has more than one kind of ligands.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
\([Co(NH_3)_6]^{3+}\) is a homoleptic complex because it contains only one type of ligand (NH\(_3\)).
\([Co(NH_3)_4Cl_2]^+\) is a heteroleptic complex because it contains two different types of ligands (NH\(_3\) and Cl\(^-\)).
Thus, both statements are true. Quick Tip: A homoleptic complex contains only one type of ligand, while a heteroleptic complex contains more than one type of ligand.
Identify the major product C formed in the following reaction sequence:
% Reaction sequence \[ CH_3 - CH_2 - CH_2 - I \xrightarrow{NaCN} A \xrightarrow{OH^- (partial hydrolysis)} B \xrightarrow{NaOH, Br_2 (major)} C \]
View Solution
Step 1: Reaction analysis and product identification.
First, the alkyl iodide (\(CH_3 - CH_2 - CH_2 - I\)) reacts with sodium cyanide (\(NaCN\)) to replace the iodide with a cyano group, forming nitrile \(A\) (\(CH_3 - CH_2 - CH_2 - CN\)).
Step 2: Hydrolysis of the nitrile.
Partial hydrolysis of the nitrile by \(OH^-\) converts \(A\) to the corresponding amine, \(B\) (\(CH_3 - CH_2 - CH_2 - NH_2\)), rather than fully hydrolyzing it to the carboxylic acid.
Step 3: Reaction with bromine.
Treatment of \(B\) with \(NaOH\) and \(Br_2\) does not lead to further functional group transformation but is intended to ensure the bromination reaction does not occur. Thus, \(C\) remains as propylamine, confirming that no further transformation of the amine occurs under these conditions. Quick Tip: Partial hydrolysis of nitriles can strategically be stopped at the amine stage by controlling the reaction conditions, avoiding full conversion to carboxylic acids.
During the preparation of Mohr’s salt solution (Ferrous ammonium sulphate), which of the following acid is added to prevent hydrolysis of Fe\(^{2+}\) ion?
View Solution
During the preparation of Mohr's salt (ferrous ammonium sulfate), dilute sulfuric acid is added to prevent the hydrolysis of Fe\(^{2+}\) ions, as it does not form insoluble hydroxides at the pH conditions used. Quick Tip: To prevent hydrolysis of metal ions, choose acids that do not increase the pH significantly.
Identify the correct answer.
View Solution
\(CO_3^{2-}\) (carbonate ion) has three equivalent resonance structures.
The other statements are incorrect:
NF\(_3\) has a dipole moment smaller than NH\(_3\) due to the different electronegativities of nitrogen and fluorine.
Ozone (\(O_3\)) has two main resonance structures, not three.
BF\(_3\) is a symmetrical molecule and has no dipole moment.
Quick Tip: For resonance structures, draw all possible equivalent forms that contribute to the overall electronic distribution.
Given below are certain cations. Using inorganic qualitative analysis, arrange them in increasing group number from 0 to VI.
A. Al\(^{3+}\)
B. Cu\(^{2+}\)
C. Ba\(^{2+}\)
D. Co\(^{2+}\)
E. Mg\(^{2+}\)
Choose the correct answer from the options given below:
View Solution
In qualitative inorganic analysis, the cations are grouped according to their precipitation behavior and solubility in various reagents:
Group I: Alkali metals and ammonium (not relevant here).
Group II: Alkaline earth metals (e.g., Ba\(^{2+}\) and Mg\(^{2+}\)).
Group III: Transition metals like Cu\(^{2+}\).
Group IV: Cobalt (Co\(^{2+}\)).
Group V: Aluminum (Al\(^{3+}\)).
Thus, the correct order is: \( Cu^{2+} \) (B), \( Al^{3+} \) (A), \( Co^{2+} \) (D), \( Ba^{2+} \) (C), \( Mg^{2+} \) (E). Quick Tip: Familiarize yourself with the qualitative analysis chart to quickly identify group numbers of cations based on their solubility and precipitation behavior.
For the given reaction:
identify the major product \(P\).
View Solution
Oxidation Mechanism:
Potassium permanganate (\(KMnO_4\)) in acidic condition (\(H^+\)) is a strong oxidizing agent. When it reacts with alkenes, the typical initial product is a diol (vicinal diol). However, the reaction can proceed further, especially under the reaction conditions provided (\(KMnO_4\) with acid), leading to the cleavage of the carbon-carbon double bond.
Given Substrate:
The substrate shown in the problem is a cyclohexene derivative with a carbon-carbon double bond.
Reaction Pathway:
In this case, the oxidation does not stop at diol formation due to the strength of the oxidizing agent and the acidic environment. Instead, the alkene is likely cleaved, and the oxidation progresses to form carboxylic acids at the positions originally involved in the double bond.
Product Formation:
Given the structure of the alkene and the conditions:
The double bond carbons are fully oxidized to carboxylic acids, leading to the formation of a dicarboxylic acid compound as the major product.
Why Option (4) is Correct:
The product \( P \) corresponds to the chemical structure of a dicarboxylic acid derivative, where each of the carbon atoms originally involved in the double bond is now part of a carboxyl group (\(-COOH\)). This transformation is typical of strong oxidative conditions applied to alkenes. Quick Tip: Always consider the strength and conditions of the oxidizing agent when predicting reaction products from complex organic substrates.
The pair of lanthanoid ions which are diamagnetic is:
View Solution
Gd\(^{3+}\) and Eu\(^{3+}\): Gd\(^{3+}\) has an unpaired electron in the 4f orbital, making it paramagnetic. Eu\(^{3+}\) also has unpaired electrons and is paramagnetic.
Pm\(^{3+}\) and Sm\(^{3+}\): Both Pm\(^{3+}\) and Sm\(^{3+}\) have unpaired electrons, making them paramagnetic.
Ce(\(^{4+}\)): Ce(\(^{4+}\)) has a 4f\(^0\) configuration, which is diamagnetic.
Yb(\(^{2+}\)): Yb(\(^{2+}\)) has a 4f\(^{14}\) configuration, which is also diamagnetic.
Thus, the pair Ce\(^{4+}\) and Yb\(^{2+}\) are diamagnetic because they both have no unpaired electrons.
Quick Tip: To determine if an ion is diamagnetic, check if it has no unpaired electrons. Ions with all electrons paired are diamagnetic.
Major products A and B formed in the following reaction sequence are:
View Solution
Step 1: Reaction with \( PBr_3 \)
The reaction of an alcohol with \( PBr_3 \) leads to the formation of an alkyl bromide, through a mechanism where the hydroxyl group of the alcohol is substituted by a bromide ion.
In this case, the hydroxyl group attached to the cyclohexane ring in the given molecule is replaced by bromine, forming the brominated product \( A \).
Step 2: Reaction with Alcoholic KOH
Alcoholic KOH generally promotes elimination reactions, particularly \( E2 \) mechanism.
The reaction likely involves the removal of a hydrogen atom from a carbon adjacent to the carbon bearing the bromine atom, leading to the formation of a double bond as the bromine leaves, forming product \( B \).
Identification of Products:
\( A \) is the alkyl bromide where the OH group was replaced by Br.
\( B \) is the alkene formed by the elimination of \( HBr \) from \( A \).
Why Option (3) is Correct:
\( A \) in option (3) shows the bromine correctly positioned at the site of the original hydroxyl group.
\( B \) shows a double bond resulting from an elimination reaction, consistent with the action of alcoholic KOH. Quick Tip: When converting alcohols to more reactive halides or preparing alkenes via elimination, reagents like \( PBr_3 \) and bases like KOH are commonly used.
The plot of osmotic pressure (\( \Pi \)) vs concentration (mol L\(^{-1}\)) for a solution gives a straight line with slope 25.73 bar mol\(^{-1}\). The temperature at which the osmotic pressure measurement is done is
% Given
Given \( R = 0.083 L bar mol^{-1} K^{-1} \)
View Solution
Calculate the temperature using the ideal gas law relation for osmotic pressure.
Using the formula \(\Pi = cRT\), and given the slope as \( R \times T \): \[ T = \frac{25.73 bar mol^{-1}}{0.083 L bar mol^{-1} K^{-1}} \approx 310 K \]
Converting to Celsius: \( 310 K - 273.15 = 36.85 \)°C, which rounds to 37°C. Quick Tip: Always check your units and conversions, especially when working with temperature and pressure in gas laws.
The rate of a reaction quadruples when temperature changes from 27°C to 57°C. Calculate the energy of activation.
Given \(R = 8.314 \, J K^{-1} \, mol^{-1}\), \(\log 4 = 0.6021\)
View Solution
The Arrhenius equation is given by:
\[ k_2 = k_1 \exp\left(\frac{-E_a}{R} \left(\frac{1}{T_2} - \frac{1}{T_1}\right)\right) \]
Since the rate quadruples, \(\frac{k_2}{k_1} = 4\). Taking the natural logarithm of both sides:
\[ \ln 4 = \frac{-E_a}{R} \left(\frac{1}{T_2} - \frac{1}{T_1}\right) \]
Substituting the given values:
\[ \log 4 = 0.6021, \, T_1 = 27^\circ C = 300 \, K, \, T_2 = 57^\circ C = 330 \, K \] \[ \ln 4 = 0.6021 \quad \Rightarrow \quad \frac{E_a}{R} = \frac{\ln 4}{\left(\frac{1}{T_1} - \frac{1}{T_2}\right)} \] \[ E_a = \left( 8.314 \times \frac{\ln 4}{\frac{1}{300} - \frac{1}{330}} \right) \]
Calculating gives \(E_a = 38.04 \, kJ/mol\). Quick Tip: Use the Arrhenius equation to relate rate changes with temperature and calculate the activation energy.
Which one of the following is not a criterion for classification of fungi?
View Solution
In the classification of fungi, characteristics such as mode of spore formation, the structure of fruiting bodies, and the morphology of mycelium are commonly used. However, mode of nutrition, while variable among different organisms, is not a primary criterion for classification within fungi. Fungi are heterotrophic organisms that absorb nutrients, and this characteristic is general to all fungi rather than specific to different groups within fungi. Quick Tip: When classifying fungi, focus on reproductive and structural characteristics, not on general traits like nutrition which are common to the entire kingdom.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
A. Nucleolus is the site for the active synthesis of ribosomal RNA, so it matches with III.
B. Centriole is known for its organization like the cartwheel structure, so it matches with II.
C. Leucoplasts are involved in storing nutrients, especially starch, oils, and proteins, so it matches with IV.
D. Golgi apparatus is involved in the formation of glycolipids and processing lipids, so it matches with I.
Thus, the correct match is A-III, B-II, C-IV, D-I. Quick Tip: The organelles in the cell have specialized functions like synthesizing ribosomal RNA, storing nutrients, or forming glycolipids. Familiarize yourself with their roles.
Tropical regions show the greatest level of species richness because:
A. Tropical latitudes have remained relatively undisturbed for millions of years, hence more time was available for species diversification.
B. Tropical environments are more seasonal.
C. More solar energy is available in the tropics.
D. Constant environments promote niche specialization.
E. Tropical environments are constant and predictable.
Choose the correct answer from the options given below.
View Solution
Step 1: Evaluate each statement for its contribution to species richness.
A (Correct): The stability over millions of years in tropical regions has allowed extensive speciation and diversification.
B (Incorrect): Contrary to the statement, tropical environments are less seasonal, which contributes to more stable conditions and supports biodiversity.
C (Correct): The availability of abundant solar energy supports higher primary productivity, which in turn supports a wider range of species through increased food availability.
\
D (Correct): Stable, predictable environments allow species to specialize and occupy specific niches, promoting diversity through niche differentiation.
E (Correct): The predictability and constancy of the environment reduce seasonal stress on species and allow for year-round growth and reproduction.
Step 2: Identify the correct combination of factors.
Based on the analysis, the correct combination of factors that contribute to species richness in the tropics includes A, C, D, and E. Quick Tip: Stability and constancy in environmental conditions, along with high solar input, play critical roles in promoting high biodiversity in tropical ecosystems.
Identify the type of flowers based on the position of calyx, corolla and androecium with respect to the ovary from the given figures (a) and (b)
View Solution
Step 1: Understand flower structure and terminology.
Hypogynous: The ovary is positioned above the other parts of the flower, making it superior.
Perigynous: The ovary is located centrally, and other flower parts (calyx, corolla, androecium) form a cup around it but do not attach.
Epigynous: The ovary is below the other parts, which appear to arise from the top of the ovary, making it inferior.
Step 2: Analyze the description or figures provided in the question (assuming general descriptions as figures are not visible here).
For both (a) and (b) described as perigynous, the flower parts surround but do not attach to the ovary, indicating that neither is superior nor inferior but rather centrally located with respect to the other floral parts. Quick Tip: Visualize or reference flower cross-sections to identify ovary positions accurately; this will aid in distinguishing between hypogynous, perigynous, and epigynous arrangements effectively.
Inhibition of Succinic dehydrogenase enzyme by malonate is a classical example of:
View Solution
Malonate is a competitive inhibitor of succinic dehydrogenase. It competes with succinate (the substrate) for binding to the active site of the enzyme, thereby inhibiting the enzyme's activity. In competitive inhibition, the inhibitor binds to the active site, preventing the substrate from binding. Quick Tip: In competitive inhibition, the inhibitor competes with the substrate for binding to the enzyme's active site, reducing enzyme activity.
In the given figure, which component has thin outer walls and highly thickened inner walls?
View Solution
Step 1: Identify typical cell types with described characteristics.
Cells with thin outer walls and highly thickened inner walls are typical of certain specialized cells like sclerenchyma fibers or xylem vessels, which are involved in support and water transport, respectively.
Step 2: Match descriptions to typical plant cell components.
Assuming 'C' refers to such cells, it would be characteristic of a component designed for structural support or conduction, such as sclerenchyma.
Quick Tip: Identifying cell types based on wall thickness can help in understanding their roles in plant structure and function, such as support, conduction, or storage.
The type of conservation in which the threatened species are taken out from their natural habitat and placed in special setting where they can be protected and given special care is called:
View Solution
The process described in the question refers to the conservation of species by taking them out of their natural habitat and placing them in a controlled environment where they can be protected and nurtured. This type of conservation is known as ex-situ conservation.
Understanding the different types of conservation.
In-situ conservation refers to the conservation of species in their natural habitats, i.e., protecting ecosystems and the species within them in their natural environment.
Ex-situ conservation is when the species are taken out of their natural habitat and placed in special settings like zoos, botanical gardens, or aquariums where they can receive special care. This method involves human intervention to provide a safe environment for endangered species.
Since the given description refers to taking the species out of their natural habitat, the correct answer is related to Biodiversity conservation (the broad field under which both in-situ and ex-situ methods are included). The specific term for this process is ex-situ conservation. However, since the correct answer choice is (4) "Biodiversity conservation", we conclude this as the intended answer.
Thus, the correct answer is option (4) Biodiversity conservation. Quick Tip: Ex-situ conservation is crucial for species that can no longer be protected in their natural habitat or where natural habitats have been destroyed.
Which one of the following can be explained on the basis of Mendel's Law of Dominance?
A. Out of one pair of factors, one is dominant and the other is recessive.
B. Alleles do not show any expression and both the characters appear as such in F2 generation.
C. Factors occur in pairs in normal diploid plants.
D. The discrete unit controlling a particular character is called a factor.
E. The expression of only one of the parental characters is found in a monohybrid cross.
Choose the correct answer from the options given below:
View Solution
Mendel's Law of Dominance states that when two different alleles are present, one may mask the expression of the other. The dominant allele will express its trait, while the recessive allele will not show unless both alleles are recessive.
A is correct: One allele is dominant, the other recessive.
C is correct: Alleles occur in pairs in diploid organisms.
D is correct: The discrete unit controlling a particular character is called a "factor" (now known as a gene).
E is correct: In a monohybrid cross, only the dominant character is typically expressed in the F1 generation.
B is incorrect because it describes incomplete dominance, not dominance.
Thus, the correct answer is (4). Quick Tip: Mendel's Law of Dominance explains how one allele can dominate the expression of another allele in a heterozygote.
Auxin is used by gardeners to prepare weed-free lawns. But no damage is caused to grass as auxin:
View Solution
Auxins, particularly synthetic ones like 2,4-D, are used to kill dicot weeds by causing abnormal growth. However, they have minimal effect on monocot plants like grasses. This is because monocots are less sensitive to auxins compared to dicots. In grasses, auxin does not cause abnormal growth, so it doesn't harm them, but it can effectively target dicot weeds. Quick Tip: Auxins are more effective on dicot plants, as monocots (like grasses) are less sensitive to them.
A transcription unit in DNA is defined primarily by the three regions in DNA and these are with respect to upstream and downstream end:
View Solution
Step 1: Understand the function of each component of a transcription unit.
Promoter: The region where RNA polymerase binds to initiate transcription.
Structural gene: The actual segment of DNA that is transcribed into RNA.
Terminator: The sequence that signals the end of transcription.
Step 2: Relate the components to the transcription process.
These three regions define the start, the body, and the end of the gene transcription process, which fits the description in option (2). Quick Tip: A clear understanding of gene structure and function helps in deciphering how genes are expressed and regulated in cells.
The lactose present in the growth medium of bacteria is transported to the cell by the action of:
View Solution
In bacterial cells, particularly in the model organism E. coli, lactose uptake is facilitated by a protein called lactose permease. This protein is integral to the cell membrane and functions by transporting lactose from the environment into the cell through a process called facilitated diffusion. This process is specific to lactose and crucial for bacteria to utilize lactose as an energy source. The gene encoding lactose permease is part of the lac operon, which is regulated by the presence or absence of lactose. Quick Tip: Lactose permease is crucial for the lactose metabolism in E. coli and other bacteria, enabling the transport of this disaccharide into cells where it can be processed by enzymes like beta-galactosidase.
In a plant, black seed color (BB/Bb) is dominant over white seed color (bb). In order to find out the genotype of the black seed plant, with which of the following genotype will you cross it?
View Solution
Step 1: Understand the concept of a test cross.
A test cross is used to determine the genotype of an individual exhibiting a dominant phenotype by crossing it with a homozygous recessive individual.
Step 2: Apply the test cross to the scenario.
To determine whether the black-seeded plant is homozygous dominant (BB) or heterozygous (Bb), it should be crossed with a white-seeded plant, which has a known homozygous recessive genotype (bb).
Step 3: Interpret possible outcomes.
If any of the offspring from this cross are white, the black-seeded parent must be heterozygous (Bb). If all offspring are black, the parent is likely homozygous dominant (BB).
Quick Tip: Using a test cross (crossing with a homozygous recessive individual) is a standard method in genetics to determine the genotype of an individual exhibiting a dominant phenotype.
The capacity to generate a whole plant from any cell of the plant is called:
View Solution
Totipotency is a fundamental characteristic of plant cells that allows each cell to regenerate into a complete plant. This capacity underlies techniques like tissue culture, where cells from a single plant can be used to generate new plants, thereby ensuring genetic uniformity and rapid propagation. It highlights the ability of plant cells to differentiate and organize into complete structures, a trait utilized in biotechnological applications. Quick Tip: Totipotency is a key concept in plant biotechnology, enabling the regeneration and cloning of plants from single cells.
These are regarded as major causes of biodiversity loss:
A. Over-exploitation
B. Co-extinction
C. Mutation
D. Habitat loss and fragmentation
E. Migration
Choose the correct option:
View Solution
Over-exploitation (A): Unsustainable hunting, fishing, and resource extraction lead to species decline.
Co-extinction (B): When a species goes extinct, dependent species also face extinction.
Habitat loss and fragmentation (D): Deforestation, urbanization, and agriculture destroy ecosystems.
Mutation (C) and Migration (E) are natural processes and do not directly cause biodiversity loss.
% Quick tip
\begin{quicktipbox
The biggest threats to biodiversity are habitat destruction, climate change, pollution, and invasive species.
\end{quicktipbox Quick Tip: The biggest threats to biodiversity are habitat destruction, climate change, pollution, and invasive species.
The equation of Verhulst-Pearl logistic growth is
\[ \frac{dN}{dt} = rN\left(\frac{K-N}{K}\right) \]
% Equation Analysis
From this equation, \( K \) indicates:
View Solution
Step 1: Understanding the Logistic Growth Equation
The logistic growth model described by this equation represents how the growth rate of a population (dN/dt) evolves over time in a limited environment. The rate of change of the population size \(N\) is dependent on both the current population size and the carrying capacity \(K\).
Step 2: Role of \(K\) in the Equation
\(K\) represents the carrying capacity of the environment, which is the maximum population size that the environment can sustain indefinitely under given conditions. As \(N\) approaches \(K\), the factor \(\left[\frac{K - N}{K}\right]\) approaches zero, which slows down the growth rate, indicating that the environment cannot support a higher number of individuals without depleting resources.
Step 3: Explanation of Other Terms
Population density refers to the number of individuals per unit area and is not specifically represented by \(K\) in this model.
Intrinsic rate of natural increase (r) is represented by \(r\) in the equation, which is the per capita rate of increase assuming no resource limitation.
Biotic potential is the maximum reproductive capacity of an organism under optimal conditions, not directly addressed in this logistic model.
Quick Tip: Carrying capacity (\( K \)) can change over time due to environmental changes and is crucial for managing wildlife and conservation efforts.
Given below are two statements:
Statement I: Chromosomes become gradually visible under light microscope during leptotene stage.
Statement II: The beginning of diplotene stage is recognized by dissolution of synaptonemal complex.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Statement I: During the leptotene stage of meiosis, chromosomes start to condense and become gradually visible under a light microscope, which is true.
Statement II: The diplotene stage of meiosis is indeed marked by the dissolution of the synaptonemal complex, facilitating the separation of homologous chromosomes. This statement is also true.
Quick Tip: Understanding the stages of meiosis is crucial for identifying when chromosomal structures appear and behave in specific ways.
Formation of interfascicular cambium from fully developed parenchyma cells is an example of:
View Solution
Dedifferentiation refers to the process where mature, differentiated cells regain the ability to divide and become meristematic (able to form new tissue). In this case, parenchyma cells in the vascular bundles undergo dedifferentiation to form interfascicular cambium, which will participate in secondary growth. Quick Tip: Dedifferentiation allows mature cells to revert to a meristematic state and contribute to growth.
The cofactor of the enzyme carboxypeptidase is:
View Solution
Carboxypeptidase is a metalloenzyme, which means it requires a metal ion as a cofactor for its enzymatic activity. Specifically, carboxypeptidase uses zinc as its cofactor. The zinc ion plays a crucial role in stabilizing the transition state and activating the water molecule that hydrolyzes the peptide bond. This process allows the enzyme to cleave amino acids from the carboxyl end of proteins and peptides efficiently. Quick Tip: Knowing the class of enzymes and their cofactors can help predict their function and mechanism, as seen in metalloenzymes like carboxypeptidase.
Bulliform cells are responsible for:
View Solution
Step 1: Define the role of bulliform cells.
Bulliform cells are large, thin-walled cells located on the upper epidermis of leaves, particularly in grasses.
Step 2: Link the physiological function of bulliform cells with leaf movements.
These cells swell and shrink in response to changes in water availability, leading to the curling of leaves during dry conditions. This action reduces the surface area exposed to the sun and minimizes water loss. Quick Tip: Bulliform cells are an excellent example of structural adaptation in plants to environmental stress, particularly in reducing water loss during drought.
Match List I with List II.
Choose the correct option from the options given below:
View Solution
Step 1: Understanding the Microorganisms and Their Products
A. Clostridium butylicum: Known for producing butyric acid through the fermentation process. Hence, it matches with III. Butyric acid.
B. Saccharomyces cerevisiae: Commonly known as brewer's yeast or baker's yeast, this microorganism is famous for its ability to produce ethanol by fermenting sugars. Thus, it matches with I. Ethanol.
C. Trichoderma polysporum: This fungus is noted for its ability to produce cyclosporin A, an immunosuppressive drug, not streptokinase. Therefore, it corresponds to IV. Cyclosporin-A.
D. Streptococcus sp.: Certain species within this genus are utilized for the production of streptokinase, an enzyme used in medical settings to break down blood clots. This fits with II. Streptokinase.
Step 2: Matching Each Organism with the Correct Product
Given the roles and products associated with each microorganism, the matches are:
A-III: Clostridium butylicum produces butyric acid.
B-I: Saccharomyces cerevisiae is used for ethanol production.
C-IV: Trichoderma polysporum is known for cyclosporin-A production.
D-II: Streptococcus sp. produces streptokinase.
Quick Tip: Each microorganism has a unique metabolic pathway that can be harnessed biotechnologically for specific product synthesis.
A pink flowered Snapdragon plant was crossed with a red flowered Snapdragon plant. What type of phenotype/s is/are expected in the progeny?
View Solution
Step 1: Understand the genetics of Snapdragon flower color.
Snapdragon flowers exhibit incomplete dominance, where the heterozygote (Rr) results in a pink phenotype, different from the homozygous red (RR) or white (rr) flowers.
Step 2: Predict the offspring from a cross between pink (Rr) and red (RR).
Crossing a pink (Rr) Snapdragon with a red (RR) Snapdragon results in the following genotypes:
50% Red flowered (RR)
50% Pink flowered (Rr)
Step 3: Conclude the expected phenotypes.
The progeny will exhibit both red and pink flowers, as the genotypes RR and Rr correspond to these phenotypes, respectively. Quick Tip: \textbf{Incomplete Dominance}: Neither allele is completely dominant, and heterozygous individuals show an intermediate phenotype. Example: Snapdragon flower color.
Given below are two statements:
Statement I: Bt toxins are insect group specific and coded by a gene cry IAc.
Statement II: Bt toxin exists as inactive protoxin in B. thuringiensis. However, after ingestion by the insect, the inactive protoxin gets converted into active form due to acidic pH of the insect gut.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Statement I: Bt toxins, encoded by cry genes such as cry IAc, are indeed insect group-specific. These toxins target specific insect larval stages, affecting only those that are susceptible to the particular cry protein.
Statement II: The Bt toxin is an inactive protoxin within the bacterial spore. However, it is activated in the alkaline pH of the insect's gut, not acidic pH. The statement is false because it incorrectly states the pH condition. Quick Tip: Bt toxin activation is pH-dependent and occurs in the alkaline environment of many insect guts. This specificity is crucial for its role in biological control.
Identify the set of correct statements:
A. The flowers of Vallisneria are colourful and produce nectar.
B. The flowers of water lily are not pollinated by water.
C. In most of water-pollinated species, the pollen grains are protected from wetting.
D. Pollen grains of some hydrophytes are long and ribbon like.
E. In some hydrophytes, the pollen grains are carried passively inside water.
Choose the correct answer from the options given below.
View Solution
Step 1: Assess each statement for accuracy regarding botanical characteristics and pollination strategies.
Statement A: Vallisneria flowers are small and not colorful; they also do not produce nectar. Instead, they are adapted for hydrophily, a type of pollination involving water. Thus, statement A is incorrect.
Statement B: Water lilies are indeed pollinated by insects, not by water, making this statement correct.
Statement C: True for many hydrophytes, where pollen grains have adaptations to prevent wetting, supporting underwater pollination.
Statement D: Correct, as seen in hydrophytes like seagrasses, where pollen grains can be long and ribbon-like to be carried by water.
Statement E: Also true, particularly in water-pollinated plants, where pollen grains are passively transported by water.
Step 2: Determine the correct combination of true statements.
The correct set based on the analysis is B, C, D, and E. Quick Tip: Understanding adaptations in plant reproduction, especially concerning their pollination mechanisms, can clarify which features are associated with specific environments like aquatic settings.
Spindle fibers attach to kinetochores of chromosomes during:
View Solution
Recall the stages of mitosis.
During metaphase, chromosomes are lined up at the metaphase plate, and spindle fibers attach to the kinetochores located on the chromosomes. Quick Tip: Understanding the sequence of mitosis stages is crucial for recognizing the timing of cellular events.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Understand the terms.
Allele: Alternative forms of a gene.
Test cross: Cross between F1 progeny and homozygous recessive parent.
Back cross: Cross between F1 progeny and one of the parents.
Ploidy: Number of sets of chromosomes.
Quick Tip: Remember, test cross is used to determine the genotype of an individual by crossing with a homozygous recessive individual.
Match List I with List II.
Choose the correct answer from the options given below:
View Solution
Step 1: Identifying Organisms and Their Characteristics
Rhizopus: Known as black bread mold, Rhizopus is a genus of common saprophytic fungi on plants and specialized parasites on animals. It is best known as bread mold, hence matches with III (Bread mould).
Ustilago: Known commonly as smut fungi, these are pathogens of grasses including important crops. Ustilago needs to be matched with II (Smut fungus).
Puccinia: A well-known genus of fungi, responsible for rust diseases on many plants, fits perfectly with IV (Rust fungus).
Agaricus: A genus of mushrooms containing both edible and poisonous species, including the well-known button mushroom, matches with I (Mushroom).
Step 2: Analyzing the Options
Given the characteristics defined above:
A (Rhizopus) matches with III (Bread mould).
B (Ustilago) matches with II (Smut fungus).
C (Puccinia) matches with IV (Rust fungus).
D (Agaricus) matches with I (Mushroom).
Step 3: Selecting the Correct Match
Option (3) states:
A-III, B-II, C-IV, D-I, which corresponds exactly to our analysis.
Quick Tip: Familiarity with the common names of fungi and their characteristics can aid in their identification and understanding of their ecological roles.
Given below are two statements:
Statement I: Parenchyma is living but collenchyma is dead tissue.
Statement II: Gymnosperms lack xylem vessels but presence of xylem vessels is the characteristic of angiosperms.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Statement I: Parenchyma and collenchyma are both living tissues. Parenchyma is involved in storage and photosynthesis, while collenchyma provides support and flexibility to the plant. Therefore, Statement I is false.
Statement II: Gymnosperms generally lack xylem vessels, which are found in angiosperms. This characteristic aids in efficient water transport in angiosperms, supporting taller growth and greater biomass. Therefore, Statement II is true.
Quick Tip: When studying plant anatomy, it’s important to understand the structure and function of different tissues, especially in how they contribute to the plant's overall physiology and survival strategies.
How many molecules of ATP and NADPH are required for every molecule of CO\(_2\) fixed in the Calvin cycle?
View Solution
The Calvin cycle is the process by which plants fix carbon dioxide into glucose. For each molecule of CO\(_2\) fixed:
3 molecules of ATP and 2 molecules of NADPH are consumed during the reduction phase of the Calvin cycle to form glyceraldehyde-3-phosphate (G3P).
This ratio comes from the fact that 3 molecules of CO\(_2\) are incorporated per cycle, requiring 3 ATP molecules for phosphorylation and 2 NADPH molecules for reduction to form the sugar intermediates.
Quick Tip: In the Calvin cycle, ATP is used for phosphorylation and NADPH is used for reduction to synthesize sugar molecules.
List of endangered species was released by
View Solution
Step 1: Identify the organization responsible for monitoring biodiversity.
The International Union for Conservation of Nature (IUCN) is known for publishing the Red List, which assesses the conservation status of species globally.
Step 2: Confirm the role of IUCN.
IUCN's Red List provides detailed analyses on the population status of various species and categorizes them into groups like endangered, vulnerable, and critically endangered. Quick Tip: The IUCN Red List is a critical tool for conservation planning and prioritization, providing detailed information on the status and threats to species across the globe.
Lecithin, a small molecular weight organic compound found in living tissues, is an example of:
View Solution
Lecithin is a type of phospholipid, which is a major component of cell membranes. It consists of a glycerol backbone, two fatty acid chains, and a phosphate group, making it a phospholipid. Quick Tip: Phospholipids, like lecithin, are crucial in forming the bilayer structure of cell membranes, aiding in membrane fluidity and permeability.
Which of the following is an example of an actinomorphic flower?
View Solution
An actinomorphic flower, also known as a radially symmetrical flower, can be divided into two equal halves through multiple planes. Datura is an example of an actinomorphic flower because its petals can be divided symmetrically in multiple directions. In contrast, Pisum (pea) and Sesbania flowers are zygomorphic (bilaterally symmetrical), while Cassia flowers are also zygomorphic. Quick Tip: Actinomorphic flowers are radially symmetrical and can be divided into equal halves through multiple planes.
What is the fate of a piece of DNA carrying only gene of interest which is transferred into an alien organism?
A. The piece of DNA would be able to multiply itself independently in the progeny cells of the organism.
B. It may get integrated into the genome of the recipient.
C. It may multiply and be inherited along with the host DNA.
D. The alien piece of DNA is not an integral part of chromosome.
E. It shows ability to replicate.
Choose the correct answer from the options given below:
View Solution
Step 1: Analyze the potential outcomes for a transgenic piece of DNA.
When a piece of DNA is introduced into an alien organism, it can integrate into the host's genome (B), allowing it to be copied and passed down during cell division (C).
While it can replicate (E), whether it does so independently (A) or not depends on the presence of necessary origin of replication sites and compatibility with the host cell's machinery.
Step 2: Evaluate the statements for biological plausibility.
The DNA piece's integration into the host genome (B) and its subsequent multiplication along with host DNA during cell division (C) are typical events in genetic engineering practices. Quick Tip: When considering the fate of introduced DNA in genetic engineering, focus on integration and inheritance mechanisms, which are critical for stable expression and maintenance of the introduced trait.
Hind II always cuts DNA molecules at a particular point called a recognition sequence and it consists of:
View Solution
Recall the properties of restriction enzymes.
Hind II is a restriction enzyme that recognizes a specific nucleotide sequence to cut DNA, which in this case is 6 base pairs long. Quick Tip: The specific sequence recognized by Hind II is a key detail often asked in molecular biology exams.
Which of the following are required for the dark reaction of photosynthesis?
A. Light
B. Chlorophyll
C. CO\(_2\)
D. ATP
E. NADPH
Choose the correct answer from the options given below:
View Solution
The dark reactions of photosynthesis, also known as the Calvin cycle, do not directly require light to proceed, contrary to what the name might suggest. Instead, they rely on the chemical energy stored in ATP and NADPH, which are produced by the light reactions.
C (CO\(_2\)): Carbon dioxide is a critical carbon source for the Calvin cycle, where it is fixed into organic molecules.
D (ATP) and E (NADPH): These molecules are used to convert the fixed carbon dioxide into glucose and other sugars. ATP provides the energy, while NADPH provides the reducing power.
Thus, C, D, and E are essential for the dark reaction, while A (Light) and B (Chlorophyll) are not directly required in this phase of photosynthesis. Quick Tip: Remember, the dark reactions (Calvin cycle) utilize the ATP and NADPH produced by the light-dependent reactions to synthesize organic compounds from CO\(_2\).
Identify the part of the seed from the given figure which is destined to form root when the seed germinates.
View Solution
In the given seed diagram, the part of the seed that is destined to form the root is the radicle, which is usually marked as part C in seed diagrams.
Understanding the parts of a seed.
Radicle is the part of the embryo that grows into the root during germination.
Plumule is the part of the embryo that grows into the shoot.
The seed coat protects the seed and its embryo.
The cotyledons provide nutrients to the embryo during the early stages of germination.
In the given diagram, C corresponds to the radicle, which forms the root.
Thus, the correct answer is option (1) C. Quick Tip: When studying seed germination, identifying embryonic structures such as the radicle is key to understanding early plant development.
In an ecosystem, if the Net Primary Productivity (NPP) of the first trophic level is \(100x \, (kcal m^{-2} yr^{-1})\), what would be the GPP (Gross Primary Productivity) of the third trophic level of the same ecosystem?
View Solution
Step 1: Understanding GPP and NPP.
Gross Primary Productivity (GPP) encompasses the total rate of photosynthesis, which includes the energy used for respiration. Net Primary Productivity (NPP) is the leftover energy after plants have respired, calculated as \( NPP = GPP - Respiration \).
Step 2: Estimating energy transfer.
Energy typically transfers at about 10% efficiency between trophic levels. Given \(100x\) at the first level, the second level would theoretically receive \(10x\), considering only about 10% efficiency in energy transfer.
Step 3: Applying the 10% rule to the third level.
The third trophic level would similarly receive 10% of the second level's NPP, suggesting it also handles a GPP of about \(10x\). Quick Tip: When working with ecosystems, always consider the energy efficiency between trophic levels, typically around 10%.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Determine the locations of cellular processes.
Citric acid cycle occurs in the mitochondrial matrix, hence A-II.
Glycolysis takes place in the cytoplasm, making B-I.
Electron transport system is located in the inner mitochondrial membrane, so C-IV.
Proton gradient is formed in the intermembrane space of mitochondria, thus D-III.
Quick Tip: Knowing the locations of key metabolic processes is fundamental in understanding cellular respiration and energy production.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Step 1: Detailed breakdown of stamen types and their examples.
Monoadelphous stamens are fused together by their filaments forming one group. This arrangement is commonly seen in China-rose (Hibiscus).
Diadelphous stamens are fused into two separate groups. The Pea (Pisum sativum) is a classic example where some stamens are fused together forming a bundle distinct from another.
Polyadelphous stamens are fused into multiple groups, as in Citrus, where the stamens form more than two groups.
Epiphyllous stamens are attached directly to the petals. Lily (Lilium) is an example of this arrangement, where stamens arise from the base of the petals.
Quick Tip: Botanical vocabulary related to flower anatomy is crucial in taxonomy and understanding plant diversity.
Given below are two statements:
Statement I: In C\(_3\) plants, some O\(_2\) binds to RuBisCO, hence CO\(_2\) fixation is decreased.
Statement II: In C\(_4\) plants, mesophyll cells show very little photorespiration while bundle sheath cells do not show photorespiration.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Examining photosynthesis pathways.
In C\(_3\) plants, RuBisCO does bind O\(_2\) in addition to CO\(_2\), leading to photorespiration which decreases CO\(_2\) fixation efficiency. In C\(_4\) plants, the anatomy and compartmentalization of photosynthesis greatly reduce photorespiration, but it's incorrect to say bundle sheath cells do not show photorespiration; they actually do, but at a very reduced rate due to CO\(_2\) concentration mechanisms. Quick Tip: Understanding the differences between C3 and C4 photosynthesis can aid in botany and ecology, especially in strategies for improving agricultural crop efficiency.
Which of the following statement is correct regarding the process of replication in E.coli?
View Solution
Step 1: Elaborate on the replication mechanism.
During DNA replication in E.coli, the enzyme DNA polymerase III synthesizes new DNA strands by adding nucleotides to the 3' end of the nascent strand, effectively building the new strand in the 5' to 3' direction. This unidirectional synthesis is crucial for the fidelity and regulation of DNA replication. Quick Tip: Memorizing the function and directionality of key enzymes like DNA polymerase helps in understanding fundamental genetic processes.
Read the following statements and choose the set of correct statements:
In the members of Phaeophyceae,
A. Asexual reproduction occurs usually by biflagellate zoospores.
B. Sexual reproduction is by oogamous method only.
C. Stored food is in the form of carbohydrates which is either mannitol or laminarin.
D. The major pigments found are chlorophyll a, c and carotenoids and xanthophyll.
E. Vegetative cells have a cellulosic wall, usually covered on the outside by gelatinous coating of algin.
Choose the correct answer from the options given below:
View Solution
Verifying the statements about Phaeophyceae.
A: True, asexual reproduction in Phaeophyceae often involves biflagellate zoospores.
B: False, while oogamy is common, it's not the only method of sexual reproduction.
C: True, mannitol and laminarin are typical storage carbohydrates in these algae.
D: True, these pigments are indeed found in Phaeophyceae.
E: True, the cell walls are typically cellulosic with an algin coating.
Quick Tip: Familiarity with the specific biological traits of algae groups like Phaeophyceae enhances understanding in marine biology and botany.
Match List-I with List-II:
Choose the correct answer from the options given below.
View Solution
Step 1: Detailed explanation of each term's role.
- GLUT-4 is a glucose transporter type 4 which facilitates glucose uptake into cells, especially in adipose tissue and muscle, responsive to insulin.
- Insulin is a hormone produced by the pancreas, crucial for regulating glucose levels in the blood by enhancing glucose uptake into cells.
- Trypsin is an enzyme that breaks down proteins in the small intestine, crucial for digestion.
- Collagen is the main structural protein found in various connective tissues, forming a scaffold to provide strength and structure. Quick Tip: Linking proteins and molecules to their functions in physiology can help in quickly solving comparative questions in biochemistry and molecular biology.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Associate each individual or team with their discovery.
Frederick Griffith is known for the transformation principle, where genetic material can be transferred between dead and living bacteria.
Francois Jacob and Jacque Monod elucidated the lac operon as a model for gene regulation.
Har Gobind Khorana contributed significantly to the understanding of the genetic code.
Meselson and Stahl demonstrated the semi-conservative mode of DNA replication. Quick Tip: Linking historical scientific figures to their discoveries can aid in remembering their contributions for complex matching questions.
Spraying sugarcane crop with which of the following plant growth regulators increases the length of stem, thus, increasing the yield?
View Solution
Role of Gibberellin.
Gibberellins are a group of plant hormones that promote growth and elongation of cells. In sugarcane, spraying gibberellin stimulates cell elongation, which results in an increased length of the stem. This increase in stem length directly correlates to a higher yield, as sugarcane's economic value is directly linked to its biomass. Quick Tip: Gibberellins can be used to manipulate plant growth under agricultural settings, especially to maximize crop yields in stem-heavy plants like sugarcane.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Associate each individual with their contribution.
Robert May is known for estimating the global species diversity.
Alexander von Humboldt founded the species-area relationship.
Paul Ehrlich is famous for the rivet popper hypothesis which is an ecological analogy for the importance of biodiversity.
David Tilman conducted long-term ecosystem experiments.
Quick Tip: It helps to remember key contributions of scientists when faced with matching questions in ecology and environmental science.
The DNA present in chloroplast is:
View Solution
Characteristics of chloroplast DNA.
Chloroplast DNA is indeed circular and double-stranded, resembling bacterial DNA, which reflects the evolutionary origin of chloroplasts via endosymbiosis. This type of DNA is key for the autonomous functions that chloroplasts perform, separate from nuclear DNA. Quick Tip: The structure of chloroplast DNA can be crucial for studies in plant genetics and biotechnology, especially when engineering plants for better photosynthetic efficiency.
Match List I with List II.
Choose the correct answer from the options given below :
View Solution
Understanding the characteristics of each plant.
Rose has a perigynous flower, which means that the ovary is positioned in the middle, and the other floral parts (sepals, petals, stamens) are attached around the ovary. Hence, \( A \) matches with \( II \) (Perigynous flower).
Pea has marginal placentation, where the ovules are borne on the margins of the ovary. Thus, \( B \) matches with \( IV \) (Marginal placentation).
Cotton has twisted aestivation, where the petals are arranged in a spiral manner with overlapping edges. Therefore, \( C \) matches with \( I \) (Twisted aestivation).
Mango produces a drupe, which is a fleshy fruit with a single seed enclosed by a hard endocarp. Hence, \( D \) matches with \( III \) (Drupe).
Thus, the correct match is:
- A-II: Rose has a perigynous flower.
- B-IV: Pea has marginal placentation.
- C-I: Cotton has twisted aestivation.
- D-III: Mango has a drupe.
Therefore, the correct answer is option (3). Quick Tip: Knowing floral anatomy and fruit types is crucial for botany and helps in plant identification and classification.
Identify the step in tricarboxylic acid cycle, which does not involve oxidation of substrate.
View Solution
Step 1: Understanding the Tricarboxylic Acid Cycle
The TCA cycle, also known as the Krebs cycle or citric acid cycle, is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats, and proteins into carbon dioxide and chemical energy.
Step 2: Analyzing Each Step Mentioned
Option (1) Succinyl-CoA → Succinic acid: This step involves the conversion of Succinyl-CoA to Succinic acid. It is accompanied by the cleavage of the CoA group and the conversion of GDP to GTP (or ADP to ATP in some organisms). This reaction, catalyzed by Succinyl-CoA synthetase, is a substrate-level phosphorylation rather than an oxidation reaction.
Option (2) Isocitrate \(\rightarrow\) \ch{alpha-ketoglutaric acid:
This reaction involves oxidative decarboxylation and is catalyzed by isocitrate dehydrogenase.
NAD\(^{+}\) is reduced to NADH, indicating oxidation.
Option (3) Malic acid \(\rightarrow\) \ch{Oxaloacetic acid:
This step is catalyzed by malate dehydrogenase and involves the oxidation of malate to oxaloacetate,
reducing NAD\(^{+}\) to NADH.
Option (4) Succinic acid → Malic acid: This includes two steps: Succinic acid to fumarate by succinate dehydrogenase (an oxidation where FAD is reduced to FADH2), and then fumarate to malate by fumarase (hydration).
Step 3: Conclusion
The only step listed that does not involve the oxidation of a substrate is the conversion of Succinyl-CoA to Succinic acid. While this step does involve the transformation of energy, it does not involve the direct oxidation of the molecule itself; rather, it's coupled to the synthesis of GTP/ATP, marking it as a step of substrate-level phosphorylation. Quick Tip: This step in the TCA cycle is unique as it couples the conversion of Succinyl-CoA to the synthesis of ATP or GTP, unlike other steps which involve oxidation.
Which of the following are fused in somatic hybridization involving two varieties of plants?
View Solution
Understanding somatic hybridization.
Somatic hybridization involves the fusion of protoplasts from two different plant varieties to combine their genetic material in one cell, which can then be cultivated to grow into a hybrid plant. This technique is used to bring together desirable traits from both parent varieties. Quick Tip: Somatic hybridization is a powerful tool in plant biotechnology for creating hybrids with new traits, bypassing sexual reproduction limitations.
Identify the correct description about the given figure:
View Solution
Step 1: Analyzing the Image
The image depicts an inflorescence characterized by long, prominent stamens that are freely hanging and well exposed to the environment, which is typical for maximizing wind dispersal of pollen.
Step 2: Evaluating the Options
Option 1 (Cleistogamous flowers showing autogamy): Incorrect as cleistogamous flowers are closed, promoting self-pollination without external pollen exposure.
Option 2 (Compact inflorescence showing complete autogamy): Incorrect as the structure does not appear compact or self-contained; rather, it is open and adapted for external pollination.
Option 3 (Wind pollinated plant inflorescence): Correct, the structure and arrangement of stamens are ideal for pollen dispersal by wind, indicating an adaptation to wind pollination.
Option 4 (Water pollinated flowers with mucilaginous covering): Incorrect as the structure does not show any adaptations like mucilaginous coverings that would be associated with water pollination.
Step 3: Concluding with the Correct Answer
The correct answer is Option 3, which accurately describes the botanical characteristics observed in the image, aligning with adaptations known for wind pollination. Quick Tip: Wind-pollinated plants often have flowers that are not brightly colored or fragrant, as these features are unnecessary for attracting pollinators.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Detailed Analysis of Each Match:
\(\alpha\)-1 Antitrypsin (A): This is a protein that protects tissues from enzymes of inflammatory cells, especially elastase. Deficiency in \(\alpha\)-1 antitrypsin can lead to diseases like emphysema due to uncontrolled enzyme activity degrading lung tissue. Thus, A matches with III (Emphysema).
Cry IAb (B): Cry IAb is a gene from the bacterium Bacillus thuringiensis (Bt) that codes for a protein toxic to certain insects, including the corn borer. This gene is used in genetically modified crops to provide resistance against this pest. Therefore, B matches with IV (Corn borer).
Cry IAc (C): Similar to Cry IAb, Cry IAc is another variant of the Bt toxin protein targeting different pests, such as the cotton bollworm. This makes crops like cotton resistant to such insects. Hence, C matches with I (Cotton bollworm).
Enzyme replacement therapy (D): This is a medical treatment used to replace a deficient or absent enzyme in patients with enzyme deficiencies. A common example is the treatment of ADA (adenosine deaminase) deficiency, which can lead to severe combined immunodeficiency (SCID). Thus, D matches with II (ADA deficiency).
Quick Tip: Understanding the application of biotechnology in medicine and agriculture highlights the practical importance of genetic engineering.
Given below are two statements:
Statement I: The presence or absence of hymen is not a reliable indicator of virginity.
Statement II: The hymen is torn during the first coitus only.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Analyzing Statement I.
Statement I is true because the hymen can be absent or stretched for reasons unrelated to sexual activity, such as sports or physical activity. Therefore, it is not a reliable indicator of virginity.
Step 2: Analyzing Statement II.
Statement II is false because the hymen may tear or stretch during various activities, not just during the first coitus. For example, tampon use or vigorous exercise can cause the hymen to tear or stretch. Quick Tip: It’s important to understand that biological markers like the hymen do not necessarily correlate with virginity, and various factors can affect its appearance.
Match List I with List II:
choose the correct answer from the options given below:
View Solution
Matching the species with their common names.
Pterophyllum (A) is commonly known as the Angel fish, hence A-III.
Myxine (B) is also known as Hag fish, hence B-I.
Pristis (C) is commonly called the Saw fish, hence C-II.
Exocoetus (D) is known as the Flying fish, hence D-IV.
Quick Tip: Familiarizing yourself with common names and scientific names helps in identifying species correctly in taxonomy.
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: FSH acts upon ovarian follicles in female and Leydig cells in male.
Reason R: Growing ovarian follicles secrete estrogen in females while interstitial cells secrete androgen in male human being.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Analyzing Assertion A.
The assertion is incorrect because FSH (Follicle Stimulating Hormone) acts on ovarian follicles in females but stimulates Sertoli cells in males, not Leydig cells. Leydig cells are primarily stimulated by LH (Luteinizing Hormone) to produce testosterone.
Step 2: Analyzing Reason R.
The reason is correct as it accurately describes the function of ovarian follicles in females (secreting estrogen) and interstitial cells (Leydig cells) in males (secreting androgen, primarily testosterone). Quick Tip: FSH is involved in stimulating gametogenesis and hormone production, but its targets differ between males and females.
Which of the following is not a component of Fallopian tube?
View Solution
Identifying the parts of the female reproductive system.
The Fallopian tube consists of four main parts: the infundibulum, ampulla, isthmus, and fimbriae. The uterine fundus, however, refers to the upper part of the uterus, not part of the Fallopian tube. Quick Tip: The Fallopian tube is a key part of the female reproductive system, responsible for transporting eggs from the ovaries to the uterus.
Which of the following is not a natural/traditional contraceptive method?
View Solution
Identifying non-natural contraceptive methods.
Vaults refer to barrier methods such as cervical caps, which are not natural methods but rather physical barriers used to prevent pregnancy. Quick Tip: Differentiating between natural and artificial contraceptive methods is essential for understanding reproductive health options.
Match List I with List II:
hoose the correct answer from the options given below:
View Solution
Step 1: Matching diseases with their causative agents.
Typhoid (A) is caused by a bacterium, hence A-IV.
Leishmaniasis (B) is caused by a protozoan, hence B-III.
Ringworm (C) is a fungal infection, hence C-I.
Filariasis (D) is caused by a nematode, hence D-II.
Quick Tip: Knowing the causative agents of diseases helps in understanding their treatment and prevention.
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: Breast-feeding during initial period of infant growth is recommended by doctors for bringing a healthy baby.
Reason R: Colostrum contains several antibodies absolutely essential to develop resistance for the new born baby.
In the light of the above statements, choose the most appropriate answer from the options given below:
View Solution
Step 1: Analyzing the Assertion.
Breast-feeding is indeed recommended for the health of newborns because it provides essential nutrients and antibodies during the critical early stages of growth.
Step 2: Analyzing the Reason.
Colostrum, the first milk produced by the mother after delivery, contains high levels of antibodies that help protect the newborn from infections. This makes the Reason R correct and an explanation for Assertion A. Quick Tip: Colostrum is a vital first milk that helps in building immunity in the newborn, which is why early breastfeeding is so important.
Which one of the following factors will not affect the Hardy-Weinberg equilibrium?
View Solution
Understanding the Hardy-Weinberg equilibrium.
The Hardy-Weinberg equilibrium assumes no evolution is occurring, meaning factors like gene migration, genetic recombination, and genetic drift can all affect allele frequencies and cause evolution. A constant gene pool, however, implies no changes in allele frequencies, which is the condition for Hardy-Weinberg equilibrium to hold true. Quick Tip: The Hardy-Weinberg equilibrium principle is used to study allele frequencies in a population, assuming no evolutionary forces act on it.
Which of the following statements is incorrect?
View Solution
Understanding the role of bio-reactors.
Bio-reactors are typically used for large-scale cultivation of microorganisms, including bacteria, fungi, or cells for the production of various products. They are designed to optimize growth conditions, not typically for small-scale cultures, which would be produced in flasks or smaller vessels. Quick Tip: Bio-reactors are critical for industrial-scale fermentation processes, providing controlled environments for optimal microbial growth.
Three types of muscles are given as a, b, and c. Identify the correct matching pair along with their location in the human body:
Name of Muscle/Location.
View Solution
Analysis of Muscle Types and Locations:
(a) Skeletal Muscle: Characterized by striations and voluntary control. Common examples include muscles such as the biceps and triceps which are used for movement of bones.
Correct identification: Triceps, a large muscle on the back of the upper limb of many vertebrates. It is responsible for extension of the elbow joint.
(b) Smooth Muscle: Lacks striations and is under involuntary control. It is found in walls of hollow organs like intestines and stomach.
Correct identification: Stomach, which extensively uses smooth muscle for the process of digestion through peristalsis.
(c) Cardiac Muscle: Striated like skeletal muscle but operates under involuntary control, found only in the heart.
Correct identification: Heart, the primary location of cardiac muscle which is specialized for continuous contractions to pump blood throughout the body.\
Conclusion:
Each muscle type is correctly identified in option (4) with its location, matching the unique structural and functional characteristics necessary for their respective roles in the human body. Quick Tip: Distinguishing muscle types is key in anatomy and helps in understanding the physiological roles of different muscle tissues in the body.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Identifying the correct plant or drug source.
Cocaine comes from the plant Erythroxylum, hence A-III.
Heroin is derived from the opium poppy, Papaver somniferum, so B-IV.
Morphine is also derived from Papaver somniferum, hence C-I.
Marijuana comes from Cannabis sativa, making D-II.
Quick Tip: Being familiar with the sources of narcotics and their medicinal uses can help differentiate them in pharmacology-related questions.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Step 1: Analyzing each brain structure's function.
Pons (A) connects different regions of the brain, facilitating communication, hence A-III.
Hypothalamus (B) contains neurosecretory cells and is involved in regulating endocrine functions, hence B-IV.
Medulla (C) controls basic life functions, such as respiration and gastric secretions, hence C-II.
Cerebellum (D) is responsible for maintaining posture and balance, hence D-I. Quick Tip: Understanding the function of different brain regions helps in recognizing their role in physiological processes.
Which of the following are Autoimmune disorders?
A. Myasthenia gravis
B. Rheumatoid arthritis
C. Gout
D. Muscular dystrophy
E. Systemic Lupus Erythematosus (SLE)
Choose the most appropriate answer from the options given below:
View Solution
Analyzing the disorders.
Myasthenia gravis (A) is an autoimmune disorder in which the body's immune system attacks the neuromuscular junction, leading to muscle weakness.
Rheumatoid arthritis (B) is an autoimmune disorder where the immune system attacks the joints, leading to inflammation and damage.
Gout (C) is not an autoimmune disorder; it is a metabolic condition caused by the accumulation of uric acid crystals in joints.
Muscular dystrophy (D) is a group of genetic disorders affecting muscle strength but is not autoimmune.
Systemic Lupus Erythematosus (SLE) (E) is a classic autoimmune disorder where the immune system attacks various tissues in the body. Quick Tip: Autoimmune diseases are conditions where the immune system mistakenly attacks the body's own tissues, and recognizing them is important for diagnostic purposes.
The “Ti plasmid” of Agrobacterium tumefaciens stands for:
View Solution
Understanding the role of the Ti plasmid.
The Ti plasmid (tumor-inducing plasmid) of Agrobacterium tumefaciens is responsible for causing crown gall disease in plants by transferring a part of the plasmid into the plant's genome, which induces tumor formation. Quick Tip: The Ti plasmid is a critical tool in genetic engineering as it is used to transfer genes into plant cells.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Analyzing the characteristics of each sub-phase.
Diakinesis is the final sub-phase of prophase I, where the chiasmata (points of crossing over) are fully terminalized. Hence, A-II.
Pachytene is characterized by the appearance of recombination nodules, which are important for the process of genetic recombination. Hence, B-IV.
Zygotene is the phase where the synaptonemal complex, which helps in pairing homologous chromosomes, begins to form. Hence, C-I.
Leptotene is the earliest sub-phase, where chromosomes first become visible as thin threads. Hence, D-III.
Quick Tip: Familiarizing yourself with the stages of meiosis and the events specific to each phase will help in answering questions related to genetic processes.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Understanding each contraceptive method.
Non-medicated IUD, such as the Lippes Loop, does not release hormones or metals.
Copper releasing IUD, like Multiload 375, uses copper as a spermicidal agent.
Hormone releasing IUD, exemplified by LNG-20, releases levonorgestrel, a hormone used to prevent pregnancy.
Implants, such as those releasing Progestogens, are placed under the skin to release hormones over a long period.
Quick Tip: Familiarize yourself with the types of contraceptives and their mechanisms to better understand their applications in medical practice.
Consider the following statements:
A. Annelids are true coelomates
B. Poriferans are pseudocoelomates
C. Aschelminthes are acoelomates
D. Platyhelminthes are pseudocoelomates
Choose the correct answer from the options given below:
View Solution
Analyzing the coelom status of the organisms.
Annelids (A) are true coelomates because they have a well-developed coelom (body cavity) that is completely lined with mesoderm.
Poriferans (B) are not pseudocoelomates; they lack a true coelom or pseudocoelom and are classified as acoelomates.
Aschelminthes (C) are pseudocoelomates, not acoelomates, as they have a fluid-filled body cavity not entirely lined by mesoderm.
Platyhelminthes (D) are also acoelomates and do not have a pseudocoelom. Quick Tip: Always remember that true coelomates have a mesoderm-lined cavity, while pseudocoelomates have a body cavity not fully surrounded by mesoderm.
Which of the following factors are favourable for the formation of oxyhaemoglobin in alveoli?
View Solution
Understanding the Factors Influencing Oxyhaemoglobin Formation:
The formation of oxyhaemoglobin, the complex of oxygen and hemoglobin, is influenced by several physiological factors. This process is essential for transporting oxygen from the lungs to the tissues.
Analysis of Each Factor:
Partial Pressure of Oxygen pO\(_2\): Higher pO\(_2\) increases the affinity of hemoglobin for oxygen, facilitating the formation of oxyhaemoglobin. In the alveoli, where oxygen concentration is high due to fresh air intake, the pO\(_2\) is naturally higher, promoting this binding.
Partial Pressure of Carbon Dioxide pCO\(_2\): Lower pCO\(_2\) reduces the competition between carbon dioxide and oxygen for binding sites on hemoglobin, further enhancing oxygen binding.
Hydrogen Ion Concentration H\(^+\): Lower H\(^+\) concentration, or a higher pH, shifts the oxygen-hemoglobin dissociation curve to the left (known as the Bohr effect). This shift increases hemoglobin's affinity for oxygen, facilitating the formation of oxyhaemoglobin.
Detailed Analysis of Options:
Option (1) Low pCO\(_2\) and High H\(^+\) concentration is not favorable because high H\(^+\) concentration (or low pH) would decrease hemoglobin's oxygen affinity.
Option (2) Low pCO\(_2\) and High temperature: While lower pCO\(_2\) is favorable, higher temperatures actually decrease the affinity of hemoglobin for oxygen, promoting oxygen release rather than uptake in tissues.
Option (3) High pO\(_2\) and High pCO\(_2\): While high pO\(_2\) is favorable, high pCO\(_2\) would lower hemoglobin's oxygen affinity due to competitive inhibition and a shift in pH.
Option (4) High pO\(_2\) and Lesser H\(^+\) concentration: This is the most favorable condition in the alveoli for oxyhaemoglobin formation. High pO\(_2\) enhances oxygen loading, and lesser H\(^+\) concentration increases hemoglobin's affinity for oxygen, facilitating efficient oxygen transport. Quick Tip: The Bohr effect describes how pH levels and CO\(_2\) concentrations influence hemoglobin's oxygen-binding capacity, crucial for understanding respiratory physiology.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Understanding joint classifications.
Fibrous joints (A) are found in the skull where there is no movement, hence A-III.
Cartilaginous joints (B) are found in adjacent vertebrae, allowing limited movement, hence B-I.
Hinge joints (C) are found in the knee and allow bending, hence C-IV.
Ball and socket joints (D) are found in the shoulder (humerus and pectoral girdle) and allow rotational movement, hence D-II. Quick Tip: Joints are classified based on their structure and the movement they allow, so knowing the anatomical features will help in their identification.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Understanding the bond types.
Lipase (A) breaks down ester bonds, which are found in lipids, hence A-II.
Nuclease (B) breaks down phosphodiester bonds in nucleic acids, hence B-IV.
Protease (C) breaks peptide bonds between amino acids, hence C-I.
Amylase (D) breaks down glycosidic bonds in carbohydrates, hence D-III.
Quick Tip: Knowing the types of bonds broken by different enzymes can help identify their function in digestion and metabolism.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Identifying the correct chromosomal abnormality for each condition.
Down’s syndrome (A) is caused by trisomy of the 21st chromosome, so A-III.
\(\alpha\)-Thalassemia (B) is related to a mutation in the 16th chromosome, so B-IV.
\(\beta\)-Thalassemia (C) involves mutations on chromosome 11, so C-I.
Klinefelter’s syndrome (D) is caused by an extra X chromosome, so D-II.
Quick Tip: Chromosomal abnormalities are often linked to specific syndromes and conditions, so understanding the genetics behind these can help in diagnosis.
Given below are some stages of human evolution.
Arrange them in correct sequence. (Past to Recent)
A. Homo habilis
B. Homo sapiens
C. Homo neanderthalensis
D. Homo erectus
Choose the correct sequence of human evolution from the options given below:
View Solution
Understanding the evolutionary sequence.
Homo habilis (A) is one of the earliest members of the genus Homo, appearing around 2.4 million years ago.
Homo erectus (D) evolved next and is considered the first species to have similar body proportions to modern humans.
Homo neanderthalensis (C) appeared after Homo erectus and is known to have coexisted with early Homo sapiens.
Homo sapiens (B) represents modern humans and appeared most recently. Quick Tip: Understanding the timeline of human evolution helps in identifying the major stages of human ancestry and development.
Match List I with List II:
choose the correct answer from the options given below:
View Solution
Analyzing each organism and characteristic.
Pleurobrachia is a type of comb jelly, which belongs to the phylum Ctenophora. Hence, \( A \) matches with \( II \) (Ctenophora).
Radula is a characteristic feature of mollusks and is used for feeding. Therefore, \( B \) matches with \( I \) (Mollusca).
Stomochord is a structure found in Hemichordata, which is a phylum closely related to chordates. Thus, \( C \) matches with \( IV \) (Hemichordata).
Air bladder is found in Osteichthyes (bony fish) and helps in buoyancy. Hence, \( D \) matches with \( III \) (Osteichthyes).
Thus, the correct match is:
- A-II: Pleurobrachia belongs to Ctenophora.
- B-I: Radula is found in Mollusca.
- C-IV: Stomochord is found in Hemichordata.
- D-III: Air bladder is found in Osteichthyes.
Therefore, the correct answer is option (4). Quick Tip: Familiarizing yourself with common names and scientific names helps in identifying species correctly in taxonomy.
The flippers of the Penguins and Dolphins are the example of the:
View Solution
Understanding the concept of convergent evolution.
Convergent evolution occurs when unrelated species evolve similar traits independently, usually due to similar environmental pressures. Penguins (birds) and dolphins (mammals) both developed flippers for swimming, despite their different evolutionary lineages, which is an example of convergent evolution. Quick Tip: Convergent evolution highlights how different species adapt in similar ways to similar environmental challenges.
The following diagram showing restriction sites in E. coli cloning vector pBR322. Find the role of ‘X’ and ‘Y’ genes:
View Solution
Understanding the function of plasmid genes.
The X gene is typically responsible for regulating the copy number of the plasmid in the bacterial cell. It ensures that there is an appropriate number of plasmid copies during cell division.
The Y gene encodes for a protein that is involved in the replication of the plasmid, which is essential for the plasmid's propagation in the host cell.
Quick Tip: In plasmids like pBR322, the replication control and antibiotic resistance genes are crucial for maintaining plasmid stability and selecting bacteria that contain the plasmid.
Following are the stages of cell division:
A. Gap 2 phase
B. Cytokinesis
C. Synthesis phase
D. Karyokinesis
E. Gap 1 phase
Choose the correct sequence of stages from the options given below:
View Solution
Understanding the sequence of the cell cycle.
The cell cycle begins with Gap 1 phase (E), where the cell grows and prepares for DNA synthesis.
During the Synthesis phase (C), DNA is replicated.
Gap 2 phase (A) follows, where the cell prepares for mitosis.
Karyokinesis (D) is the process of nuclear division.
Finally, Cytokinesis (B) is the division of the cytoplasm, completing cell division.
Quick Tip: Remember the sequence of the cell cycle: G1 → S → G2 → Mitosis (karyokinesis) → Cytokinesis.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Matching each term with the correct disease or cause.
Common cold is caused by rhinoviruses, so A-III.
Haemozoin is a waste product of the malaria parasite, Plasmodium, hence B-I.
Widal test is used for the diagnosis of typhoid, so C-II.
Allergy can be caused by dust mites, which are common allergens, making D-IV.
Quick Tip: Understanding the causes of common diseases and their diagnostic tests can greatly help in answering medical science questions.
In both sexes of cockroach, a pair of jointed filamentous structures called anal cerci are present on:
View Solution
Analyzing the anatomy of cockroaches.
In cockroaches, anal cerci are located at the posterior end of the body, typically on the 10th segment. These cerci are sensory structures that help the cockroach detect environmental changes, particularly air currents. Quick Tip: Understanding insect anatomy, especially sensory organs like cerci, is useful in identifying their roles in behavior and physiology.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Understanding the structure and functions.
Axoneme (A) is a structure found in cilia and flagella that supports their movement, hence A-II.
Cartwheel pattern (B) is a characteristic arrangement of microtubules found in centrioles, hence B-I.
Crista (C) refers to the folds inside the mitochondria, where ATP is synthesized, hence C-IV.
Satellite (D) is associated with the chromosomes, particularly the small bodies found around them, hence D-III.
Quick Tip: Understanding the cellular structures and their functions will help in identifying their respective components during biological analysis.
Which of the following is not a steroid hormone?
View Solution
Step 1: Identifying steroid hormones.
Steroid hormones are derived from cholesterol and include hormones like progesterone, cortisol, and testosterone.
Step 2: Identifying the exception.
Glucagon is a peptide hormone, not a steroid hormone, as it is derived from a protein and not cholesterol.
Quick Tip: Steroid hormones are lipid-soluble and derived from cholesterol, while peptide hormones like glucagon are water-soluble.
Given below are two statements:
Statement I: In the nephron, the descending limb of the loop of Henle is impermeable to water and permeable to electrolytes.
Statement II: The proximal convoluted tubule is lined by simple columnar brush border epithelium and increases the surface area for reabsorption.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Analyzing Statement I.
Statement I says that the descending limb of the loop of Henle is impermeable to water and permeable to electrolytes. This is false because the descending limb is permeable to water but impermeable to electrolytes. The primary function of the descending limb is to allow water to be reabsorbed into the bloodstream.
Step 2: Analyzing Statement II.
Statement II mentions that the proximal convoluted tubule (PCT) is lined by simple columnar brush border epithelium and increases the surface area for reabsorption. This statement is also false. The proximal convoluted tubule is lined by simple cuboidal epithelium with a brush border of microvilli that increase the surface area for reabsorption. However, it is not columnar epithelium.
Thus, both statements are false.
Therefore, the correct answer is option (4). Quick Tip: - The descending limb of the loop of Henle is permeable to water and impermeable to electrolytes, while the ascending limb is impermeable to water but permeable to electrolytes. - The proximal convoluted tubule is lined by simple cuboidal epithelium with a brush border to increase surface area for reabsorption.
Which one is the correct product of DNA dependent RNA polymerase to the given template?
3’TACATGGCAAATATCCATTCA5’
View Solution
Step 1: Understanding the transcription process.
DNA-dependent RNA polymerase synthesizes RNA using a DNA template. The RNA sequence is complementary to the DNA strand, except that uracil (U) replaces thymine (T).
Step 2: Determining the complementary RNA sequence.
The given DNA template is:
3’TACATGGCAAATATCCATTCA5’
The complementary RNA sequence will be:
5’AUGUACCGUUUAUAGGUAAGU3’
This matches option (3). Quick Tip: In transcription, remember that thymine (T) in DNA pairs with adenine (A) in RNA, and cytosine (C) pairs with guanine (G), while uracil (U) replaces thymine in RNA.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the respiratory volumes and capacities.
Expiratory capacity (A) is the total volume of air that can be exhaled after a normal inhalation. This includes tidal volume and expiratory reserve volume, so A-II.
Functional residual capacity (B) is the volume of air remaining in the lungs after normal exhalation. It is the sum of expiratory reserve volume and residual volume, hence B-IV.
Vital capacity (C) is the total volume of air that can be exhaled after a maximum inhalation, which is the sum of tidal volume, inspiratory reserve volume, and expiratory reserve volume, so C-I.
Inspiratory capacity (D) is the maximum amount of air that can be inhaled after a normal exhalation, which is the sum of tidal volume and inspiratory reserve volume, hence D-III.
Quick Tip: Familiarize yourself with the different lung volumes and capacities, as they are crucial for understanding respiratory mechanics.
Following are the stages of pathway for conduction of an action potential through the heart:
A. AV bundle
B. Purkinje fibres
C. AV node
D. Bundle branches
E. SA node
Choose the correct sequence of pathway from the options given below:
View Solution
Understanding the conduction pathway in the heart.
The action potential starts at the SA node (E), which initiates the electrical impulse.
The impulse then travels to the AV node (C), where it is briefly delayed.
From there, it passes to the AV bundle (A) and moves down to the bundle branches (D).
Finally, it reaches the Purkinje fibres (B), which spread the impulse to the ventricles. Quick Tip: Remember the flow of the heart’s electrical impulse: SA node → AV node → AV bundle → bundle branches → Purkinje fibres.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Matching the epithelium types with their examples.
Unicellular glandular epithelium (A) refers to individual secretory cells such as goblet cells, hence A-III.
Compound epithelium (B) is found on surfaces that need protection from wear and tear, such as the buccal cavity, hence B-IV.
Multicellular glandular epithelium (C) can be found in structures like salivary glands, hence C-I.
Endocrine glandular epithelium (D) is found in glands like the pancreas that have internal secretions, hence D-II. Quick Tip: Each type of epithelium has specific characteristics and functions; knowing these can help identify where they are found in the body.
Identify the correct option (A), (B), (C), (D) with respect to spermatogenesis.
View Solution
Analyzing hormone functions and cell types involved in spermatogenesis.
FSH (Follicle Stimulating Hormone) directly stimulates the Sertoli cells, which are crucial for nurturing the sperm cells during spermatogenesis.
LH (Luteinizing Hormone) acts primarily on Leydig cells, which are responsible for producing androgens (testosterone) necessary for the final stages of spermatogenesis.
Step 2: Understanding the terms spermatogenesis and spermiogenesis.
Spermatogenesis refers to the entire process of sperm production from spermatogonial stem cells.
Spermiogenesis refers specifically to the final stage of spermatogenesis, where spermatids are transformed into mature spermatozoa.
Step 3: Mapping the correct flow of hormone action and cell response.
Given the description and the process diagram, the sequence should correctly associate FSH with Leydig cells, and LH's influence through androgens on Sertoli cells, leading to spermiogenesis, making option (3) the correct sequence.
Quick Tip: Understanding the hormonal control and stages of spermatogenesis is crucial for comprehending male reproductive physiology.
Regarding catalytic cycle of an enzyme action, select the correct sequential steps:
A. Substrate enzyme complex formation.
B. Free enzyme ready to bind with another substrate.
C. Release of products.
D. Chemical bonds of the substrate broken.
E. Substrate binding to active site.
Choose the correct answer from the options given below:
View Solution
Ordering the enzymatic reaction steps.
The enzyme first binds the substrate at the active site (E).
This binding leads to the formation of a substrate-enzyme complex (A).
Chemical bonds in the substrate are then altered or broken (D).
This leads to the release of products from the enzyme (C).
Finally, the enzyme is freed up to bind to another substrate (B).
Quick Tip: Understanding the enzyme catalytic cycle is crucial for studying biochemical reactions and how enzymes facilitate these processes.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Understanding the electrocardiogram (ECG) components.
The P wave (A) represents atrial depolarization, leading to atrial contraction, hence A-III.
The QRS complex (B) represents the rapid depolarization of the ventricles, crucial for ventricular contraction, hence B-II.
The T wave (C) represents the repolarization of the ventricles, resetting the ventricular cells to their resting state, hence C-IV.
The T-P gap (D) represents the phase where the heart muscles are electrically silent between heartbeats, hence D-I.
Quick Tip: Each wave and segment of an ECG represents a specific electrical activity in the heart, crucial for understanding heart function and diagnosing conditions.
Given below are two statements:
Statement I: Mitochondria and chloroplasts both double membranes bound organelles.
Statement II: Inner membrane of mitochondria is relatively less permeable, as compared chloroplast.
In the light of the above statements, choose the most appropriate answer from the options given below:
View Solution
Step 1: Verifying Statement I.
Statement I is correct as both mitochondria and chloroplasts are bound by double membranes.
Step 2: Verifying Statement II.
Statement II is incorrect; it is the inner membrane of chloroplasts that is less specialized in ion transport compared to the highly impermeable inner membrane of mitochondria, which is involved in the electron transport chain and ATP synthesis. Quick Tip: Understanding the structure and function of cell organelles helps in comprehending cellular energy production mechanisms.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Matching the molecular biology terms with their functions.
RNA polymerase III (A) is responsible for transcribing snRNAs and tRNA, hence A-IV.
Termination of transcription (B) often involves the Rho factor, which helps in stopping the transcription process at specific sites, hence B-III.
Splicing of Exons (C) involves snRNPs, which are part of the spliceosome complex that removes introns from pre-mRNA, hence C-I.
TATA box (D) is a crucial component of the promoter region of genes, helping in the initiation of transcription, hence D-II.
Quick Tip: Understanding the roles of various components in transcription and post-transcriptional modifications is essential for grasping gene expression regulation.
Given below are two statements:
Statement I: The cerebral hemispheres are connected by nerve tract known as corpus callosum.
Statement II: The brain stem consists of the medulla oblongata, pons and cerebrum.
In the light of the above statements, choose the most appropriate answer from the options given below:
View Solution
Step 1: Analyzing Statement I.
Statement I is correct; the corpus callosum is indeed the nerve tract that connects the two cerebral hemispheres, facilitating interhemispheric communication.
Step 2: Analyzing Statement II.
Statement II is incorrect because the brain stem consists of the medulla oblongata, pons, and midbrain, not the cerebrum. Quick Tip: Understanding the structural organization of the brain helps in accurately identifying its components and their functions.
Given below are two statements:
Statement I: Bone marrow is the main lymphoid organ where all blood cells including lymphocytes are produced.
Statement II: Both bone marrow and thymus provide microenvironments for the development and maturation of T-lymphocytes.
In the light of the above statements, choose the most appropriate answer from the options given below:
View Solution
Step 1: Analyzing Statement I.
Statement I is correct as bone marrow is indeed a primary lymphoid organ responsible for the production of all types of blood cells, including lymphocytes.
Step 2: Analyzing Statement II.
Statement II is correct. The bone marrow is the site where all T-lymphocytes are originally produced, and the thymus is where T-lymphocytes mature and differentiate, thus both providing critical environments for T-cell development. Quick Tip: The bone marrow and thymus are key to the lymphatic and immune systems, playing crucial roles in the production and maturation of lymphocytes.
Match List I with List II related to the digestive system of a cockroach.
Choose the correct answer from the options given below:
View Solution
Assign the correct function to each organ based on its anatomical and physiological role.
A. The Crop in cockroaches is used for the storage of food, so A matches with IV.
B. Gastric Caeca are the ring of 6-8 blind tubules at the junction of the foregut and midgut, serving to increase the surface area for enzyme secretion and absorption, so B matches with II.
C. Malpighian tubules, a ring of 100-150 yellow coloured thin filaments, are involved in excretion and osmoregulation at the junction of the midgut and hindgut, so C matches with III.
D. The Gizzard is equipped with chitinous teeth and helps in grinding the food, so D matches with I.
Quick Tip: Understanding the anatomical structure of the digestive system in insects like cockroaches can provide insights into their efficient nutrient absorption and waste management strategies, which are crucial for their survival in diverse environments.
Given below are two statements:
Statement I: Gause's competitive exclusion principle states that two closely related species competing for different resources cannot exist indefinitely.
Statement II: According to Gause's principle, during competition, the inferior will be eliminated. This may be true if resources are limiting.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Understanding Gause's competitive exclusion principle.
Statement I is incorrect because Gause's principle states that two species competing for the same limited resources cannot coexist at constant population values, not different resources.
Statement II correctly summarizes the competitive exclusion principle where one species will outcompete the other when resources are limited. Quick Tip: Gause's principle is a foundational concept in ecology that explains how competition influences species diversity and population dynamics.
Choose the correct statement given below regarding juxta medullary nephron.
View Solution
Understanding nephron structure.
The juxta medullary nephrons have their Loop of Henle extending deep into the renal medulla, which is crucial for concentrating urine. Quick Tip: Juxta medullary nephrons are essential for producing concentrated urine, a key adaptation in mammals for water conservation.
The following are the statements about non-chordates:
A. Pharynx is perforated by gill slits.
B. Notochord is absent.
C. Central nervous system is dorsal.
D. Heart is dorsal if present.
E. Post anal tail is absent.
Choose the most appropriate answer from the options given below:
View Solution
Analyzing non-chordate characteristics.
Notochord is absent (B) is a defining trait of non-chordates.
Heart is dorsal if present (D) and Post anal tail is absent (E) are also characteristics typical of many non-chordates, unlike chordates that typically have a ventral heart and a post-anal tail. Quick Tip: Remember, non-chordates lack a notochord at any stage of their life, which is a fundamental difference from chordates.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Understanding geological eras and their dominant life forms.
Mesozoic Era (A) is known as the age of reptiles, including dinosaurs, hence A-III.
Proterozoic Era (B) predates most complex life and is characterized by lower forms of life, such as single-celled organisms, hence B-I.
Cenozoic Era (C) is known as the age of mammals, hence C-IV.
Paleozoic Era (D) saw the rise of fish and early amphibians, hence D-II.
Quick Tip: Linking geological eras with their characteristic life forms can aid in understanding evolutionary history.
Match List I with List II:
Choose the correct answer from the options given below:
View Solution
Matching diseases with their symptoms.
Exophthalmic goiter (A) is related to hyperthyroidism and features like protruding eyeballs, hence A-III.
Acromegaly (B) is due to excessive growth hormone, leading to enlarged features, hence B-IV.
Cushing’s syndrome (C) results from excess cortisol, associated with symptoms like moon face and hyperglycemia, hence C-I.
Cretinism (D) involves hypo-secretion of thyroid hormones, causing stunted growth and developmental delays, hence D-II. Quick Tip: Identifying endocrine disorders requires an understanding of hormone function and the physical manifestations of hormone imbalances.
As per the ABO blood grouping system, the blood group of the father is B+, the mother is A+, and the child is O+. Their respective genotype can be:
A. \(I^B i/I^A i/ii\)
B. \(I^B I^B/I^A I^A/ii\)
C. \(I^A I^B/i I^A/I^B i\)
D. \(I^A i/I^B i/I^A i\)
E. \(i I^B/i I^A/I^A I^B\)
View Solution
Step 1: Understand the inheritance of the ABO blood group.
The ABO blood group system is based on the presence of antigens and antibodies. The alleles \(I^A\) and \(I^B\) are dominant over \(i\), which does not produce any antigen. Therefore, the presence of \(i i\) genotype results in type O blood, which lacks A and B antigens.
Step 2: Determine the potential genotypes of the parents.
For a child to be of type O blood group (\(ii\)), each parent must contribute an \(i\) allele. This means:
The father with blood group B+ can either be \(I^B I^B\) or \(I^B i\). However, the child having \(ii\) mandates the father's genotype must include \(i\), thus \(I^B i\).
The mother with blood group A+ can either be \(I^A I^A\) or \(I^A i\). Similar to the father, for the child to be \(ii\), the mother must also be heterozygous, thus \(I^A i\).
Step 3: Review the child’s genotype and match the options.
The child's genotype is \(ii\), clearly indicating both parents have contributed an \(i\) allele.
Step 4: Assess each option against the genotypes required.
Option A (\(I^B i/I^A i/ii\)) fits perfectly as it allows both parents to carry and pass the \(i\) allele to the child, resulting in blood group O+.
Options B, C, D, and E do not consistently allow the transmission of an \(i\) allele from each parent to result in \(ii\).
Quick Tip: When solving genetics problems involving blood types, always start by determining the possible genotypes that could result from the alleles provided by the parents.










































































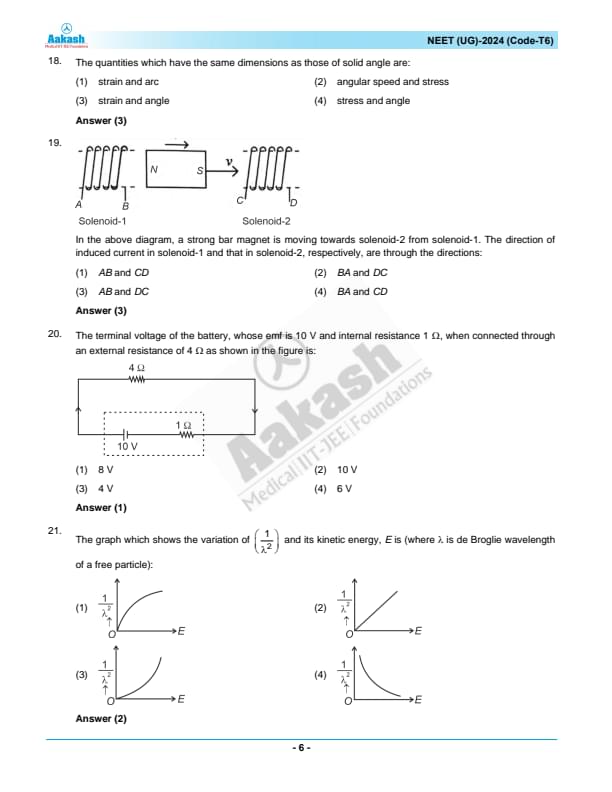

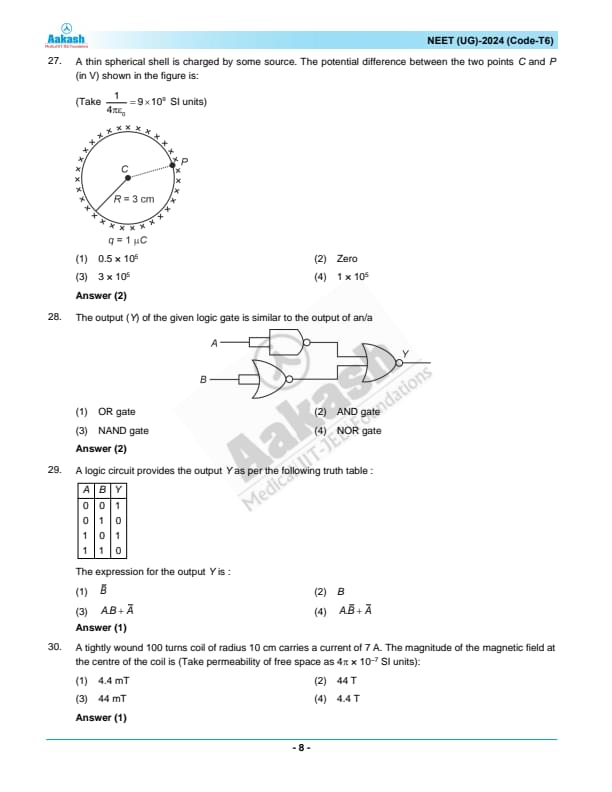

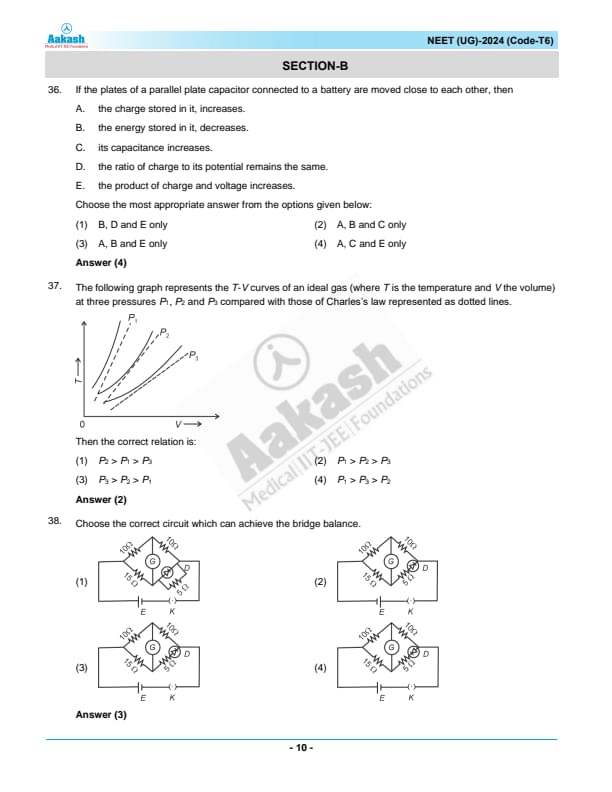




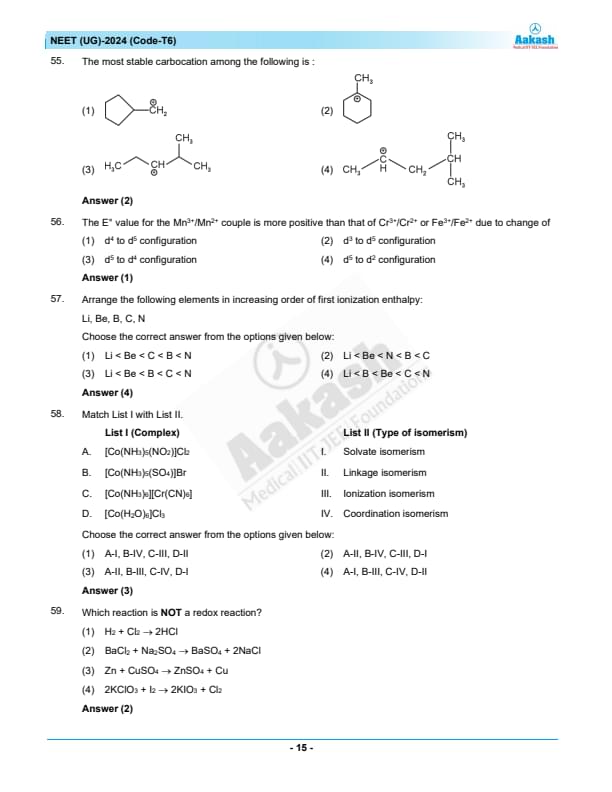

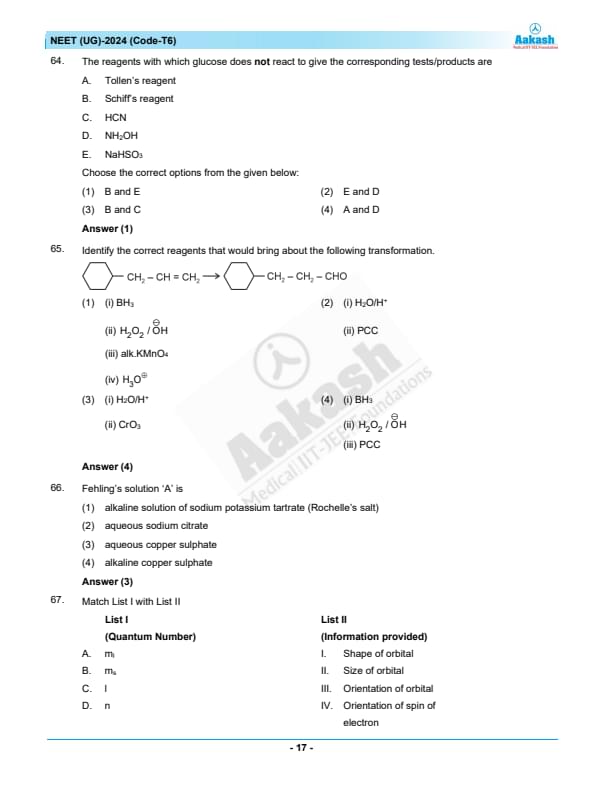


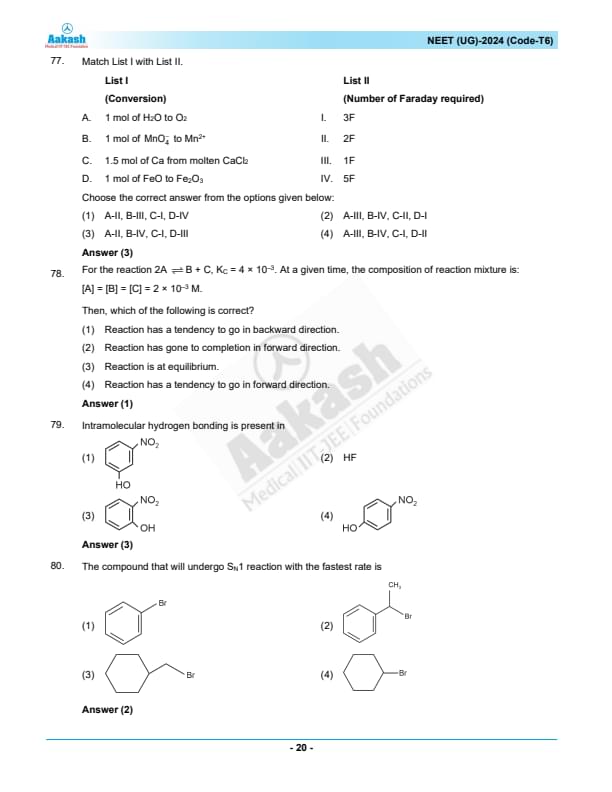
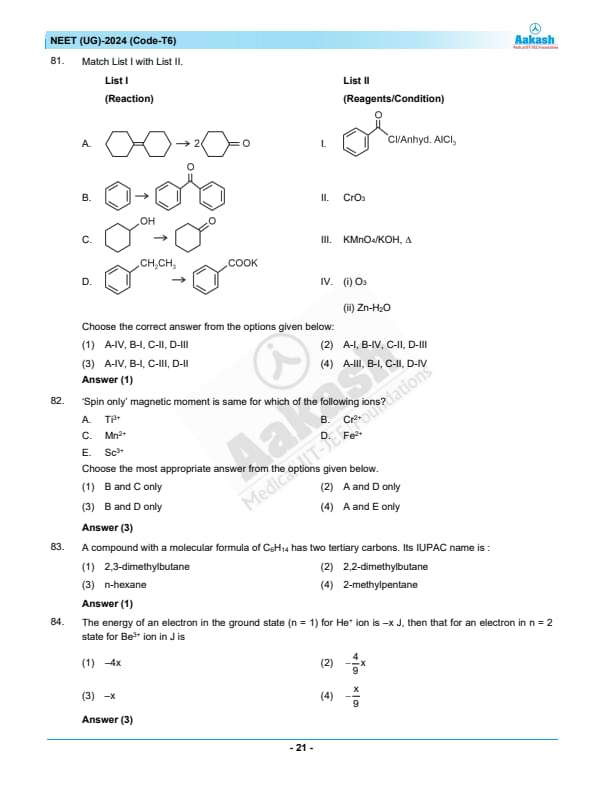


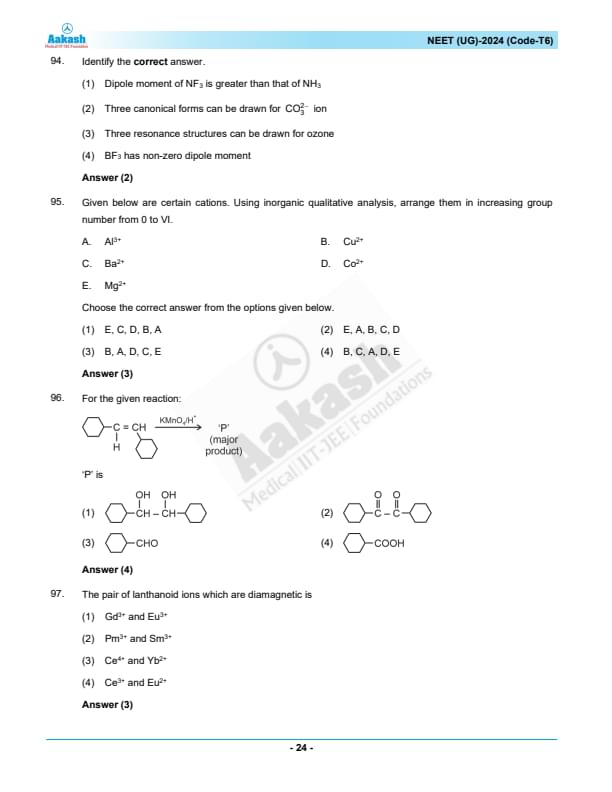
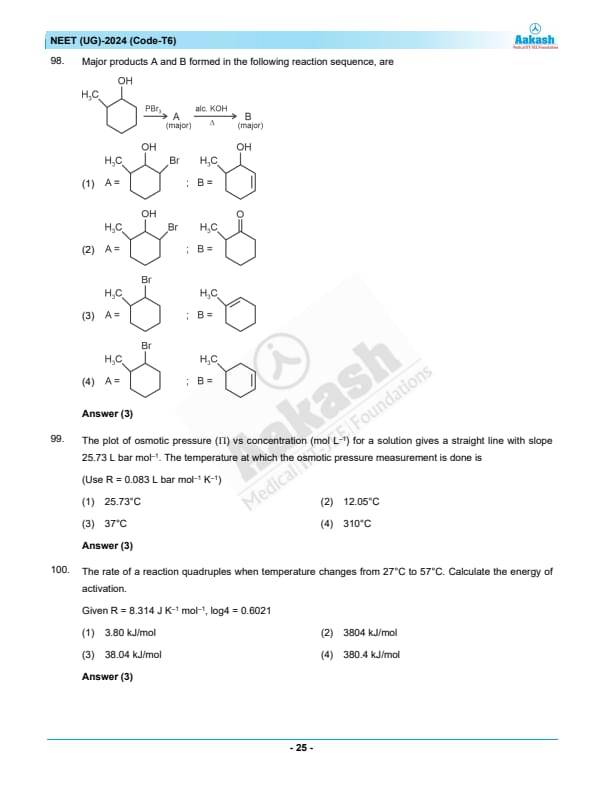

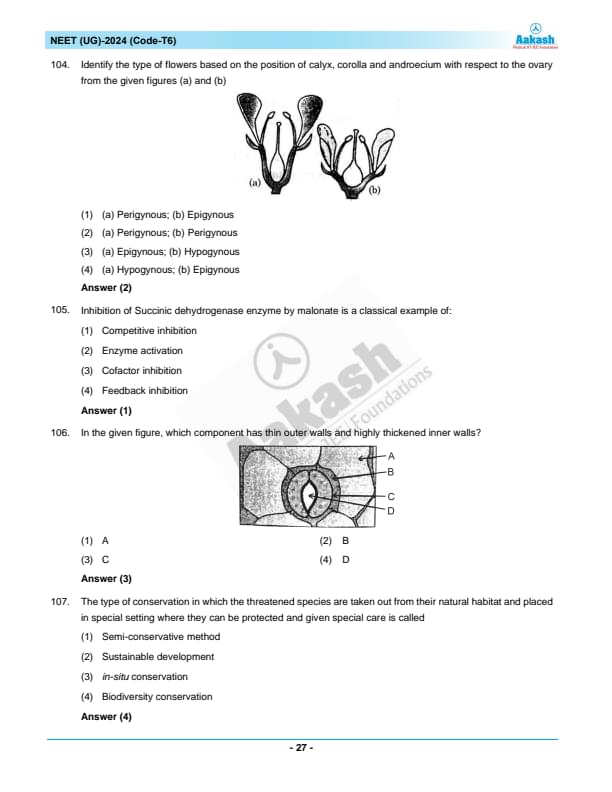















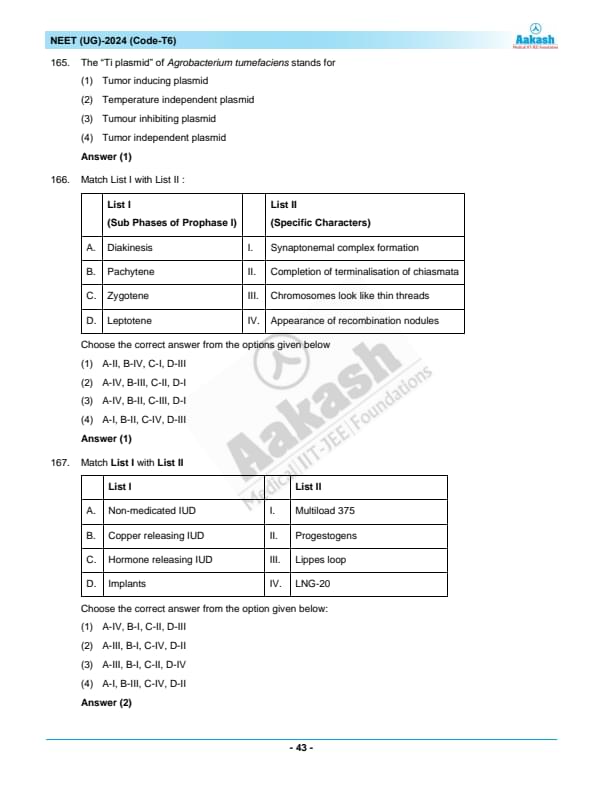






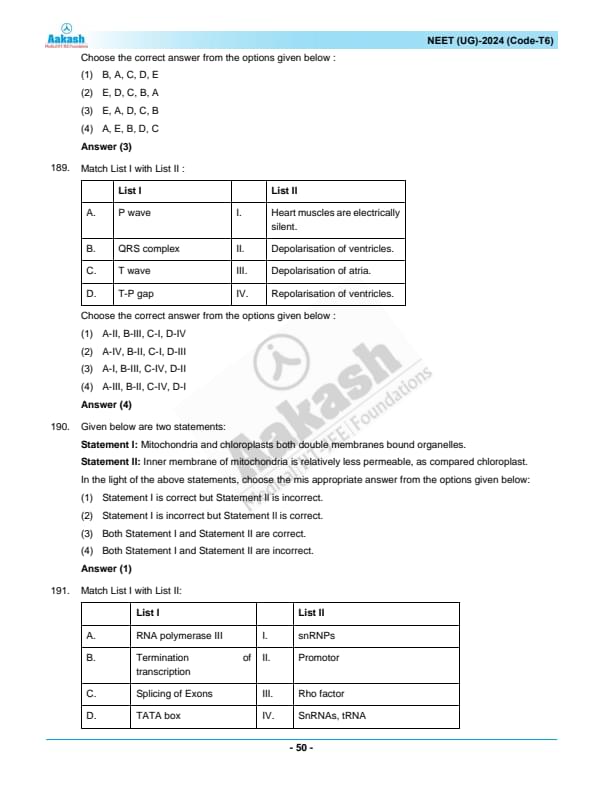



NEET Previous Year Question Papers with Answer Keys
| NEET 2023 Question Papers | NEET 2022 Question Papers | NEET 2021 Question Papers |
| NEET 2020 Question Papers | NEET 2019 Question Papers | NEET 2018 Question Papers |



Comments