NEET 2023 Question paper with answer key pdf G4 is available for download. NEET 2023 G4 question paper has been conducted by the NTA on May 7, 2023, in pen-paper mode. NEET 2023 question paper code G4 consists of 200 MCQs- 180 to be attempted in 200 minutes. Each of the 4 subjects (Zoology, Botany, Chemistry, Physics) in NEET G4 question paper 2023 have 50 MCQs (45 to be attempted).
You can download NEET 2023 question paper with answer key with solutions PDF for G4 using the links given below.
NEET 2023 Question Paper with Answer Key PDF G4 in English
| NEET 2023 G4 Question Paper with Answer Key PDF | Download PDF | Check Solutions |

For Young's double slit experiment, two statements are given below:
Statement I : If screen is moved away from the plane of slits, angular separation of the fringes remains constant.
Statement II : If the monochromatic source is replaced by another monochromatic source of larger wavelength, the angular separation of fringes decreases.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
We need to evaluate two statements about the angular separation of fringes in a Young's double-slit experiment (YDSE) and determine their validity.
Step 2: Key Formula or Approach:
In YDSE, two important quantities describe the fringe pattern:
1. Fringe Width (\(\beta\)): This is the linear distance between two consecutive bright or dark fringes. It is given by \(\beta = \frac{\lambda D}{d}\), where \(\lambda\) is the wavelength of light, \(D\) is the distance between the slits and the screen, and \(d\) is the distance between the two slits.
2. Angular Separation or Angular Fringe Width (\(\theta\)): This is the angle subtended by one fringe width at the slits. For small angles, it is given by \(\theta \approx \frac{\beta}{D} = \frac{(\lambda D/d)}{D}\). This simplifies to:
\[ \theta = \frac{\lambda}{d} \]
Step 3: Detailed Explanation:
Analysis of Statement I:
"If screen is moved away from the plane of slits, angular separation of the fringes remains constant."
The formula for angular separation is \(\theta = \frac{\lambda}{d}\). This formula depends only on the wavelength (\(\lambda\)) and the slit separation (\(d\)). It does not depend on the screen distance (\(D\)). Therefore, moving the screen away (increasing D) does not change the angular separation.
Conclusion: Statement I is true.
Analysis of Statement II:
"If the monochromatic source is replaced by another monochromatic source of larger wavelength, the angular separation of fringes decreases."
Again, the formula is \(\theta = \frac{\lambda}{d}\). This shows that the angular separation \(\theta\) is directly proportional to the wavelength \(\lambda\). If the wavelength is increased (using a source of "larger wavelength"), the angular separation \(\theta\) must also increase. The statement claims it decreases.
Conclusion: Statement II is false.
Step 4: Final Answer:
Based on the analysis, Statement I is true and Statement II is false.
Quick Tip: A common point of confusion is between linear fringe width (\(\beta\)) and angular fringe width (\(\theta\)). Remember: \(\beta\) depends on the screen distance D (\(\beta \propto D\)), but \(\theta\) is independent of D. Both are directly proportional to the wavelength \(\lambda\).
The ratio of frequencies of fundamental harmonic produced by an open pipe to that of closed pipe having the same length is :
View Solution
Step 1: Understanding the Question:
The question asks for the ratio of the fundamental frequency of an open organ pipe to that of a closed organ pipe, given that they both have the same length.
Step 2: Key Formula or Approach:
The fundamental frequency (\(f_1\)) is the lowest frequency at which a system can resonate.
1. Open Organ Pipe: An open pipe has antinodes at both ends. The fundamental mode of vibration has a wavelength \(\lambda_{open} = 2L\). The fundamental frequency is given by:
\[ f_{open} = \frac{v}{\lambda_{open = \frac{v}{2L} \]
where \(v\) is the speed of sound and L is the length of the pipe.
2. Closed Organ Pipe: A closed pipe has a node at the closed end and an antinode at the open end. The fundamental mode of vibration has a wavelength \(\lambda_{closed} = 4L\). The fundamental frequency is given by:
\[ f_{closed} = \frac{v}{\lambda_{closed = \frac{v}{4L} \]
Step 3: Detailed Explanation:
We are asked to find the ratio \(\frac{f_{open{f_{closed\).
Using the formulas from Step 2:
\[ \frac{f_{open{f_{closed = \frac{v/2L}{v/4L} \]
The terms \(v\) and \(L\) are the same for both pipes, so they cancel out.
\[ \frac{f_{open{f_{closed = \frac{1/2}{1/4} = \frac{1}{2} \times \frac{4}{1} = \frac{4}{2} = 2 \]
So, the ratio of the frequencies is 2:1.
Step 4: Final Answer:
The ratio of the fundamental frequency of the open pipe to the closed pipe is 2:1.
Quick Tip: A simple way to remember the harmonics: - Open pipe: All harmonics are present (\(f_n = nf_1\), where n=1, 2, 3,...). Frequencies are in the ratio 1:2:3... - Closed pipe: Only odd harmonics are present (\(f_n = (2n-1)f_1\), where n=1, 2, 3,...). Frequencies are in the ratio 1:3:5...
The ratio of radius of gyration of a solid sphere of mass M and radius R about its own axis to the radius of gyration of the thin hollow sphere of same mass and radius about its axis is :
View Solution
Step 1: Understanding the Question:
We need to find the ratio of the radius of gyration of a solid sphere to that of a thin hollow sphere, both having the same mass M and radius R, rotating about an axis passing through their centers.
Step 2: Key Formula or Approach:
1. The moment of inertia (\(I\)) is related to the radius of gyration (\(k\)) by \(I = Mk^2\), which means \(k = \sqrt{\frac{I}{M\).
2. The moment of inertia of a solid sphere about its central axis is \(I_{solid} = \frac{2}{5}MR^2\).
3. The moment of inertia of a thin hollow sphere (spherical shell) about its central axis is \(I_{hollow} = \frac{2}{3}MR^2\).
Step 3: Detailed Explanation:
First, find the radius of gyration for the solid sphere (\(k_{solid}\)):
\[ k_{solid} = \sqrt{\frac{I_{solid{M = \sqrt{\frac{\frac{2}{5}MR^2}{M = \sqrt{\frac{2}{5R \]
Next, find the radius of gyration for the hollow sphere (\(k_{hollow}\)):
\[ k_{hollow} = \sqrt{\frac{I_{hollow{M = \sqrt{\frac{\frac{2}{3}MR^2}{M = \sqrt{\frac{2}{3R \]
Now, find the required ratio:
\[ \frac{k_{solid{k_{hollow = \frac{\sqrt{\frac{2}{5R}{\sqrt{\frac{2}{3R} = \sqrt{\frac{2/5}{2/3 = \sqrt{\frac{2}{5} \times \frac{3}{2 = \sqrt{\frac{3}{5 \]
The exact ratio is \(\sqrt{3} : \sqrt{5}\). Quick Tip: Be aware that exam questions can sometimes be flawed. If your correct derivation leads to an answer not in the options, re-read the question carefully. If the issue persists, check if a simpler, related quantity (like the ratio of moments of inertia instead of radii of gyration) matches an option. This often reveals the intended, albeit imprecisely stated, question.
An ac source is connected to a capacitor C. Due to decrease in its operating frequency :
View Solution
Step 1: Understanding the Question:
We are examining how the capacitive reactance and displacement current in a capacitor change when the frequency of the connected AC source decreases.
Step 2: Key Formula or Approach:
1. Capacitive Reactance (\(X_C\)): The opposition offered by a capacitor to the flow of alternating current. It is given by:
\[ X_C = \frac{1}{\omega C} = \frac{1}{2\pi f C} \]
where \(\omega\) is the angular frequency, f is the operating frequency, and C is the capacitance.
2. Current in a Capacitive Circuit (I): According to Ohm's law for AC circuits, the current is given by \(I = \frac{V}{X_C}\), where V is the RMS voltage of the source.
3. Displacement Current (\(I_D\)): In a capacitor, the displacement current is equal to the conduction current in the connecting wires. Thus, \(I_D = I\).
Step 3: Detailed Explanation:
The problem states that the operating frequency (f) decreases.
Let's analyze the effect on capacitive reactance (\(X_C\)).
From the formula \(X_C = \frac{1}{2\pi f C}\), we can see that \(X_C\) is inversely proportional to f (\(X_C \propto \frac{1}{f}\)).
Therefore, as frequency (f) decreases, the capacitive reactance (\(X_C\)) will increase.
This eliminates options (A) and (B).
Now, let's analyze the effect on the current. The current in the circuit is \(I = \frac{V}{X_C}\).
Since \(X_C\) increases and the source voltage V is assumed to be constant, the current (I) will decrease.
The displacement current (\(I_D\)) inside the capacitor is equal to the conduction current (I) in the circuit.
Since the conduction current (I) decreases, the displacement current (\(I_D\)) also decreases.
This confirms that option (D) is correct and (C) is incorrect.
Step 4: Final Answer:
Due to a decrease in operating frequency, the capacitive reactance increases, which causes the current (and thus the displacement current) to decrease.
Quick Tip: Remember the frequency dependence for capacitors and inductors: - Capacitor: \(X_C \propto 1/f\). At low frequencies (like DC), \(X_C\) is very high (infinite), blocking the current. - Inductor: \(X_L = \omega L = 2\pi f L\), so \(X_L \propto f\). At low frequencies, \(X_L\) is low, offering little opposition.
In hydrogen spectrum, the shortest wavelength in the Balmer series is \(\lambda\). The shortest wavelength in the Bracket series is :
View Solution
Step 1: Understanding the Question:
We are given the shortest wavelength (\(\lambda\)) for the Balmer series of the hydrogen spectrum and asked to find the shortest wavelength for the Brackett series in terms of \(\lambda\).
Step 2: Key Formula or Approach:
The Rydberg formula for the wavelength of spectral lines in the hydrogen spectrum is:
\[ \frac{1}{\lambda} = R_H \left( \frac{1}{n_f^2} - \frac{1}{n_i^2} \right) \]
where \(R_H\) is the Rydberg constant, \(n_f\) is the principal quantum number of the final state, and \(n_i\) is the principal quantum number of the initial state.
The shortest wavelength in any series corresponds to the maximum energy transition, which occurs when the electron transitions from \(n_i = \infty\) to the final state \(n_f\). This is also known as the series limit.
Step 3: Detailed Explanation:
For the Balmer series, the final state is \(n_f = 2\). The shortest wavelength (\(\lambda\)) occurs for the transition from \(n_i = \infty\) to \(n_f = 2\).
Using the Rydberg formula:
\[ \frac{1}{\lambda} = R_H \left( \frac{1}{2^2} - \frac{1}{\infty^2} \right) = R_H \left( \frac{1}{4} - 0 \right) \] \[ \frac{1}{\lambda} = \frac{R_H}{4} \quad \Rightarrow \quad R_H = \frac{4}{\lambda} \quad (Equation 1) \]
For the Brackett series, the final state is \(n_f = 4\). Let the shortest wavelength be \(\lambda_{Br}\). This occurs for the transition from \(n_i = \infty\) to \(n_f = 4\).
Using the Rydberg formula:
\[ \frac{1}{\lambda_{Br = R_H \left( \frac{1}{4^2} - \frac{1}{\infty^2} \right) = R_H \left( \frac{1}{16} - 0 \right) \] \[ \frac{1}{\lambda_{Br = \frac{R_H}{16} \quad (Equation 2) \]
Now, substitute the value of \(R_H\) from Equation 1 into Equation 2:
\[ \frac{1}{\lambda_{Br = \frac{1}{16} \left( \frac{4}{\lambda} \right) \] \[ \frac{1}{\lambda_{Br = \frac{1}{4\lambda} \] \[ \lambda_{Br} = 4\lambda \]
Step 4: Final Answer:
The shortest wavelength in the Brackett series is \(4\lambda\).
Quick Tip: Remember the final states for the first few series in the hydrogen spectrum: Lyman (\(n_f=1\)), Balmer (\(n_f=2\)), Paschen (\(n_f=3\)), Brackett (\(n_f=4\)), Pfund (\(n_f=5\)). "Shortest wavelength" means "highest energy," which corresponds to the transition from \(n_i = \infty\) (the series limit).
Resistance of a carbon resistor determined from colour codes is (22000 \(\pm\) 5%) \(\Omega\). The colour of third band must be :
View Solution
Step 1: Understanding the Question:
We are given the value of a carbon resistor and need to determine the color of the third band based on the standard resistor color code.
Step 2: Key Formula or Approach:
The value of a four-band carbon resistor is given by the formula: \(R = (AB \times 10^C) \pm D%\), where:
- A is the digit corresponding to the first color band.
- B is the digit corresponding to the second color band.
- C is the multiplier corresponding to the third color band.
- D is the tolerance corresponding to the fourth color band.
The color code mnemonic is "BB ROY of Great Britain has a Very Good Wife" for Black(0), Brown(1), Red(2), Orange(3), Yellow(4), Green(5), Blue(6), Violet(7), Grey(8), White(9).
Step 3: Detailed Explanation:
The given resistance is \(22000 \, \Omega\). We can write this in scientific notation to match the formula:
\[ R = 22 \times 1000 \, \Omega = 22 \times 10^3 \, \Omega \]
Comparing this with \(R = AB \times 10^C\):
- The first significant digit, A, is 2. The color for digit 2 is Red.
- The second significant digit, B, is 2. The color for digit 2 is Red.
- The multiplier is \(10^C = 10^3\). The value of C is 3. The color for multiplier \(10^3\) is Orange.
- The tolerance is \(\pm 5%\). The color for this tolerance is Gold.
The question asks for the color of the third band, which corresponds to the multiplier C. Since C=3, the color is Orange.
Step 4: Final Answer:
The colour of the third band must be Orange.
Quick Tip: To quickly find the multiplier band, write the resistance value in standard engineering notation (\(XY \times 10^Z\)). The power 'Z' directly gives you the color number for the third band (e.g., \(10^3 \rightarrow 3 \rightarrow\) Orange).
A vehicle travels half the distance with speed \(v\) and the remaining distance with speed 2\(v\). Its average speed is:
View Solution
Step 1: Understanding the Question:
We need to calculate the average speed of a vehicle that covers two equal halves of its total journey at two different constant speeds.
Step 2: Key Formula or Approach:
The definition of average speed is:
\[ Average Speed = \frac{Total Distance Travelled}{Total Time Taken} \]
It is important not to simply average the speeds, as the time spent at each speed is different.
Step 3: Detailed Explanation:
Let the total distance of the journey be \(2D\).
The first half of the distance is \(D\), and the second half is also \(D\).
For the first half of the journey:
Distance = \(D\).
Speed = \(v\).
Time taken, \(t_1 = \frac{Distance}{Speed} = \frac{D}{v}\).
For the second half of the journey:
Distance = \(D\).
Speed = \(2v\).
Time taken, \(t_2 = \frac{Distance}{Speed} = \frac{D}{2v}\).
Now, we can calculate the average speed for the entire journey.
Total Distance = \(D + D = 2D\).
Total Time = \(t_1 + t_2 = \frac{D}{v} + \frac{D}{2v}\).
To add the fractions, find a common denominator:
Total Time = \(\frac{2D}{2v} + \frac{D}{2v} = \frac{3D}{2v}\).
Average Speed = \(\frac{Total Distance}{Total Time} = \frac{2D}{3D / 2v}\).
Average Speed = \(2D \times \frac{2v}{3D}\).
The 'D' terms cancel out.
Average Speed = \(\frac{4v}{3}\).
Step 4: Final Answer:
The average speed of the vehicle is \(\frac{4v}{3}\).
Quick Tip: When an object travels two equal distances at speeds \(v_1\) and \(v_2\), the average speed is the harmonic mean of the two speeds: \(v_{avg} = \frac{2v_1v_2}{v_1+v_2}\). In this case, \(v_1=v\) and \(v_2=2v\), so \(v_{avg} = \frac{2(v)(2v)}{v+2v} = \frac{4v^2}{3v} = \frac{4v}{3}\). This formula is a useful shortcut.
The potential energy of a long spring when stretched by 2 cm is U. If the spring is stretched by 8 cm, potential energy stored in it will be :
View Solution
Step 1: Understanding the Question:
The question asks to find the new potential energy of a spring when its stretch is increased, given its initial potential energy at a smaller stretch.
Step 2: Key Formula or Approach:
The potential energy (\(U_{sp}\)) stored in a spring is given by:
\[ U_{sp} = \frac{1}{2} k x^2 \]
where \(k\) is the spring constant and \(x\) is the extension or compression from the equilibrium position. This shows that the potential energy is directly proportional to the square of the extension (\(U_{sp} \propto x^2\)).
Step 3: Detailed Explanation:
Let the initial state be State 1 and the final state be State 2.
In State 1:
Extension, \(x_1 = 2 \, cm\).
Potential energy, \(U_1 = U\).
So, \(U = \frac{1}{2} k (2)^2 = \frac{1}{2} k \times 4 = 2k\).
In State 2:
Extension, \(x_2 = 8 \, cm\).
Potential energy, \(U_2\).
So, \(U_2 = \frac{1}{2} k (8)^2 = \frac{1}{2} k \times 64 = 32k\).
To find \(U_2\) in terms of U, we can use a ratio method.
\[ \frac{U_2}{U_1} = \frac{\frac{1}{2} k x_2^2}{\frac{1}{2} k x_1^2} = \left(\frac{x_2}{x_1}\right)^2 \]
Substituting the values:
\[ \frac{U_2}{U} = \left(\frac{8 \, cm}{2 \, cm}\right)^2 = (4)^2 = 16 \] \[ U_2 = 16U \]
Step 4: Final Answer:
The potential energy stored in the spring when stretched by 8 cm will be 16U.
Quick Tip: For problems involving changes in spring extension, using the proportionality \(U \propto x^2\) is much faster than calculating the spring constant explicitly. If the stretch is increased by a factor of 'n', the potential energy increases by a factor of 'n\(^2\)'. Here, the stretch increases by a factor of 4 (from 2 cm to 8 cm), so the energy increases by a factor of \(4^2 = 16\).
A bullet is fired from a gun at the speed of 280 m s\(^{-1}\) in the direction 30\(^\circ\) above the horizontal. The maximum height attained by the bullet is (g=9.8 m s\(^{-2}\), sin 30\(^\circ\) = 0.5) :
View Solution
Step 1: Understanding the Question:
We are asked to calculate the maximum vertical height reached by a projectile, given its initial speed and launch angle.
Step 2: Key Formula or Approach:
The motion can be analyzed by separating it into horizontal and vertical components. The maximum height is reached when the vertical component of the velocity becomes zero.
The initial vertical velocity is \(u_y = u \sin\theta\).
Using the kinematic equation \(v_y^2 = u_y^2 + 2a_y s_y\):
At maximum height (\(H\)), the final vertical velocity \(v_y = 0\). The acceleration is \(a_y = -g\). The vertical displacement is \(s_y = H\).
\[ 0^2 = (u\sin\theta)^2 + 2(-g)H \] \[ 2gH = u^2 \sin^2\theta \] \[ H = \frac{u^2 \sin^2\theta}{2g} \]
Step 3: Detailed Explanation:
Given values:
Initial speed, \(u = 280\) m/s.
Launch angle, \(\theta = 30^\circ\).
Acceleration due to gravity, \(g = 9.8\) m/s\(^2\).
We are also given \(\sin 30^\circ = 0.5\).
Substitute these values into the formula for maximum height:
\[ H = \frac{(280)^2 \times (\sin 30^\circ)^2}{2 \times 9.8} \] \[ H = \frac{(280)^2 \times (0.5)^2}{19.6} \] \[ H = \frac{78400 \times 0.25}{19.6} \] \[ H = \frac{19600}{19.6} \] \[ H = 1000 \, m \]
Step 4: Final Answer:
The maximum height attained by the bullet is 1000 m.
Quick Tip: Memorize the standard formulas for projectile motion: - Maximum Height: \(H = \frac{u^2 \sin^2\theta}{2g}\) - Time of Flight: \(T = \frac{2u \sin\theta}{g}\) - Range: \(R = \frac{u^2 \sin(2\theta)}{g}\) Knowing these formulas saves valuable time during exams.
The half life of a radioactive substance is 20 minutes. In how much time, the activity of substance drops to \( \left(\frac{1}{16}\right)^{th} \) of its initial value?
View Solution
Step 1: Understanding the Question:
We are given the half-life of a radioactive substance and asked to find the total time it takes for its activity to decrease to 1/16th of its original activity.
Step 2: Key Formula or Approach:
The activity (A) of a radioactive substance after a certain time is related to its initial activity (\(A_0\)) by the formula:
\[ A = A_0 \left(\frac{1}{2}\right)^n \]
where \(n\) is the number of half-lives that have passed. The total time elapsed (\(t\)) is related to the number of half-lives (\(n\)) and the half-life period (\(T_{1/2}\)) by:
\[ t = n \times T_{1/2} \]
Step 3: Detailed Explanation:
Given values:
Half-life, \(T_{1/2} = 20\) minutes.
The final activity is \(\frac{1}{16}\) of the initial activity, so \(\frac{A}{A_0} = \frac{1}{16}\).
First, let's find the number of half-lives, \(n\).
\[ \frac{A}{A_0} = \left(\frac{1}{2}\right)^n \] \[ \frac{1}{16} = \left(\frac{1}{2}\right)^n \]
We need to express 16 as a power of 2. Since \(2^4 = 16\), we have:
\[ \left(\frac{1}{2}\right)^4 = \left(\frac{1}{2}\right)^n \]
Therefore, the number of half-lives is \(n = 4\).
Now, calculate the total time elapsed:
\[ t = n \times T_{1/2} \] \[ t = 4 \times 20 \, minutes = 80 \, minutes \]
Step 4: Final Answer:
It will take 80 minutes for the activity to drop to 1/16th of its initial value.
Quick Tip: For fractions that are integer powers of 1/2 (like 1/2, 1/4, 1/8, 1/16, etc.), you can quickly count the number of half-lives. 1 half-life \(\rightarrow\) 1/2, 2 half-lives \(\rightarrow\) 1/4, 3 half-lives \(\rightarrow\) 1/8, 4 half-lives \(\rightarrow\) 1/16. This avoids formal calculation for simple cases.
The net magnetic flux through any closed surface is :
View Solution
Step 1: Understanding the Question:
The question asks for the value of the net magnetic flux passing through any arbitrary closed surface.
Step 2: Key Formula or Approach:
This question relates to one of Maxwell's equations, specifically Gauss's Law for Magnetism. The law is stated mathematically as:
\[ \Phi_B = \oint \vec{B} \cdot d\vec{A} = 0 \]
where \(\Phi_B\) is the magnetic flux, \(\vec{B}\) is the magnetic field, and the integral is taken over a closed surface A.
Step 3: Detailed Explanation:
Gauss's Law for Magnetism states that the net magnetic flux out of any closed surface is zero.
This is a fundamental law of physics with a deep physical meaning:
1. Magnetic monopoles do not exist: Unlike electric charges (which can be positive or negative), there are no isolated "magnetic charges" (north or south poles).
2. Magnetic field lines are continuous loops: Every magnetic field line that enters a closed surface must also exit that surface. Therefore, the total incoming flux (which can be considered negative) is always perfectly balanced by the total outgoing flux (positive), making the net flux zero.
This holds true for any closed surface, regardless of its shape or size, or the magnetic fields present.
Step 4: Final Answer:
The net magnetic flux through any closed surface is always zero.
Quick Tip: Remember the contrast with Gauss's Law for electricity: \(\oint \vec{E} \cdot d\vec{A} = \frac{Q_{enc{\epsilon_0}\). The net electric flux is proportional to the enclosed charge because isolated electric charges (monopoles) exist. For magnetism, the equivalent of enclosed charge is zero.
A metal wire has mass \((0.4 \pm 0.002)\) g, radius \((0.3 \pm 0.001)\) mm and length \((5 \pm 0.02)\) cm. The maximum possible percentage error in the measurement of density will nearly be:
View Solution
Step 1: Understanding the Question:
We are given the measured values and absolute errors for the mass, radius, and length of a wire. We need to calculate the maximum percentage error in the calculated density.
Step 2: Key Formula or Approach:
1. The density (\(\rho\)) is mass (\(m\)) per unit volume (\(V\)): \(\rho = \frac{m}{V}\).
2. The wire is a cylinder, so its volume is \(V = \pi r^2 l\), where \(r\) is the radius and \(l\) is the length.
3. The formula for density is \(\rho = \frac{m}{\pi r^2 l}\).
4. The rule for propagation of errors for a quantity \(X = \frac{A^a B^b}{C^c}\) is:
\[ \frac{\Delta X}{X} = a\frac{\Delta A}{A} + b\frac{\Delta B}{B} + c\frac{\Delta C}{C} \]
For density, the maximum relative error is \(\frac{\Delta \rho}{\rho} = \frac{\Delta m}{m} + 2\frac{\Delta r}{r} + \frac{\Delta l}{l}\). The percentage error is this value multiplied by 100.
Step 3: Detailed Explanation:
First, calculate the relative error for each measurement:
- Mass (m): \(\frac{\Delta m}{m} = \frac{0.002 \, g}{0.4 \, g} = \frac{2}{400} = 0.005\).
- Radius (r): \(\frac{\Delta r}{r} = \frac{0.001 \, mm}{0.3 \, mm} = \frac{1}{300} \approx 0.00333\).
- Length (l): \(\frac{\Delta l}{l} = \frac{0.02 \, cm}{5 \, cm} = \frac{2}{500} = 0.004\).
Now, use the error propagation formula for density. Note the power of 2 for the radius term.
\[ \frac{\Delta \rho}{\rho} = \frac{\Delta m}{m} + 2\left(\frac{\Delta r}{r}\right) + \frac{\Delta l}{l} \] \[ \frac{\Delta \rho}{\rho} \approx 0.005 + 2(0.00333) + 0.004 \] \[ \frac{\Delta \rho}{\rho} \approx 0.005 + 0.00666 + 0.004 = 0.01566 \]
To find the percentage error, multiply by 100:
\[ Percentage Error = 0.01566 \times 100% \approx 1.566% \]
This value is nearly 1.6%.
Step 4: Final Answer:
The maximum possible percentage error in the measurement of density will be nearly 1.6%.
Quick Tip: When calculating percentage error, remember to multiply the relative error of each variable by the magnitude of its power in the formula. For density of a wire (\(\rho \propto m r^{-2} l^{-1}\)), the errors are added, and the error for radius is multiplied by 2.
The angular acceleration of a body, moving along the circumference of a circle, is :
View Solution
Step 1: Understanding the Question:
We need to identify the direction of the angular acceleration vector (\(\vec{\alpha}\)) for an object undergoing circular motion.
Step 2: Detailed Explanation:
Let's distinguish between linear and angular quantities in circular motion.
- Linear quantities describe the motion of the particle itself and lie in the plane of rotation:
- Tangential velocity (\(\vec{v}_t\)): Always tangent to the circular path.
- Tangential acceleration (\(\vec{a}_t\)): Also tangent to the path, responsible for changing the speed of the particle.
- Centripetal (or radial) acceleration (\(\vec{a}_c\)): Always points along the radius towards the center of the circle, responsible for changing the direction of the velocity.
- Angular quantities describe the rotation of the body as a whole. These are axial vectors, meaning their direction is along the axis of rotation, perpendicular to the plane of motion. The direction is determined by the right-hand thumb rule.
- Angular velocity (\(\vec{\omega}\)): Points along the axis of rotation.
- Angular acceleration (\(\vec{\alpha}\)): Defined as the rate of change of angular velocity, \(\vec{\alpha} = \frac{d\vec{\omega{dt}\). Since \(\vec{\omega}\) is an axial vector, its change (\(d\vec{\omega}\)) and hence \(\vec{\alpha}\) must also be directed along the axis of rotation. If the body speeds up, \(\vec{\alpha}\) is in the same direction as \(\vec{\omega}\). If it slows down, \(\vec{\alpha}\) is in the opposite direction to \(\vec{\omega}\). In both cases, it is along the axis of rotation.
Therefore, options (B), (C), and (D) describe linear acceleration components, not angular acceleration.
Step 3: Final Answer:
The angular acceleration of a body in circular motion is directed along the axis of rotation.
Quick Tip: Remember the rule of thumb: If the quantity's name starts with "angular" (angular velocity, angular acceleration, angular momentum) or is a "moment" (torque, moment of inertia), it's related to rotation, and its vector representation (if it's a vector) is typically along the axis of rotation.
The magnetic energy stored in an inductor of inductance 4 \(\mu\)H carrying a current of 2 A is :
View Solution
Step 1: Understanding the Question:
The question asks for the calculation of the magnetic potential energy stored in an inductor with given inductance and current.
Step 2: Key Formula or Approach:
The energy (U) stored in an inductor is given by the formula:
\[ U = \frac{1}{2} L I^2 \]
where L is the inductance and I is the current flowing through it.
Step 3: Detailed Explanation:
We are given the following values:
Inductance, \(L = 4 \, \mu H = 4 \times 10^{-6} \, H\).
Current, \(I = 2 \, A\).
Substitute these values into the formula:
\[ U = \frac{1}{2} \times (4 \times 10^{-6} \, H) \times (2 \, A)^2 \] \[ U = \frac{1}{2} \times 4 \times 10^{-6} \times 4 \] \[ U = 2 \times 10^{-6} \times 4 \] \[ U = 8 \times 10^{-6} \, J \]
Since \(1 \, \mu J = 10^{-6} \, J\), the energy is:
\[ U = 8 \, \mu J \]
Step 4: Final Answer:
The magnetic energy stored in the inductor is 8 \(\mu\)J.
Quick Tip: Pay close attention to the units and prefixes (\(\mu\) for micro = \(10^{-6}\), m for milli = \(10^{-3}\)). This is a common source of errors. Also, remember the analogous formula for energy stored in a capacitor: \(U_C = \frac{1}{2} C V^2 = \frac{Q^2}{2C}\).
A full wave rectifier circuit consists of two p-n junction diodes, a centre-tapped transformer, capacitor and a load resistance. Which of these components remove the ac ripple from the rectified output?
View Solution
Step 1: Understanding the Question:
We need to identify the component in a full-wave rectifier circuit that is responsible for smoothing the output voltage, i.e., removing the AC ripple.
Step 2: Detailed Explanation:
Let's analyze the role of each component mentioned:
- Centre-tapped transformer and p-n junction diodes: These components together perform the rectification. The transformer steps down the AC voltage and provides two out-of-phase inputs. The diodes allow current to flow in only one direction, converting the AC input into a pulsating DC output. This output, however, still has significant voltage variations, known as AC ripple.
- Load resistance: This is the component across which the output voltage is delivered. It does not perform any filtering.
- Capacitor: A capacitor placed in parallel with the load resistance acts as a filter. It works by storing charge when the rectified voltage is increasing and releasing that charge to the load when the rectified voltage is decreasing. This process smooths out the peaks and valleys of the pulsating DC, significantly reducing the AC ripple and providing a more stable DC output. This is often called a smoothing capacitor or filter capacitor.
Step 3: Final Answer:
The capacitor is the component used to remove the AC ripple from the rectified output.
Quick Tip: In rectifier circuits, the process of converting AC to DC has two main stages: 1. \textbf{Rectification:} Using diodes to convert AC to pulsating DC. 2. \textbf{Filtering:} Using components like capacitors (or inductors) to smooth the pulsating DC into a more constant DC. The capacitor is the key element for filtering.
A football player is moving southward and suddenly turns eastward with the same speed to avoid an opponent. The force that acts on the player while turning is :
View Solution
Step 1: Understanding the Question:
We need to determine the direction of the net force acting on a player who changes their direction of motion from south to east, while keeping their speed constant.
Step 2: Key Formula or Approach:
According to Newton's Second Law of Motion, the net force \(\vec{F}\) acting on an object is proportional to its acceleration \(\vec{a}\), and hence to the change in its velocity \(\Delta \vec{v}\).
\[ \vec{F} = m\vec{a} = m \frac{\Delta \vec{v{\Delta t} \]
The direction of the force is the same as the direction of the change in velocity, \(\Delta \vec{v}\). The change in velocity is calculated as \(\Delta \vec{v} = \vec{v}_f - \vec{v}_i\), where \(\vec{v}_f\) is the final velocity and \(\vec{v}_i\) is the initial velocity.
Step 3: Detailed Explanation:
Let's set up a coordinate system. Let the \(+\hat{j}\) direction be North and the \(+\hat{i}\) direction be East. Consequently, South is \(-\hat{j}\) and West is \(-\hat{i}\).
Let the speed of the player be \(v\).
Initial Velocity (\(\vec{v}_i\)):
The player is moving southward. So, \(\vec{v}_i = -v\hat{j}\).
Final Velocity (\(\vec{v}_f\)):
The player turns and moves eastward with the same speed. So, \(\vec{v}_f = v\hat{i}\).
Change in Velocity (\(\Delta \vec{v}\)):
\[ \Delta \vec{v} = \vec{v}_f - \vec{v}_i \] \[ \Delta \vec{v} = (v\hat{i}) - (-v\hat{j}) \] \[ \Delta \vec{v} = v\hat{i} + v\hat{j} \]
The direction of the force is the direction of \(\Delta \vec{v}\). The vector \(\Delta \vec{v} = v(\hat{i} + \hat{j})\) has a positive component in the East direction (\(\hat{i}\)) and a positive component in the North direction (\(\hat{j}\)). A vector with equal positive components along East and North points in the north-east direction.
Step 4: Final Answer:
The force that acts on the player is along the north-east direction.
Quick Tip: You can visualize vector subtraction \(\vec{v}_f - \vec{v}_i\) as vector addition \(\vec{v}_f + (-\vec{v}_i)\). Here, \(-\vec{v}_i\) is a vector of magnitude \(v\) pointing North. Adding a North vector to an East vector gives a resultant vector pointing North-East.
The work functions of Caesium (Cs), Potassium (K) and Sodium (Na) are 2.14 eV, 2.30 eV and 2.75 eV respectively. If incident electromagnetic radiation has an incident energy of 2.20 eV, which of these photosensitive surfaces may emit photoelectrons?
View Solution
Step 1: Understanding the Question:
We are given the work functions for three different metals and the energy of incident photons. We need to determine which of these metals will exhibit the photoelectric effect.
Step 2: Key Formula or Approach:
The condition for the photoelectric effect to occur is that the energy of the incident photon (E) must be greater than or equal to the work function (\(\phi\)) of the metal.
\[ E \geq \phi \]
The work function is the minimum energy required to remove an electron from the surface of the material.
Step 3: Detailed Explanation:
Given values:
Incident photon energy, \(E = 2.20\) eV.
Work function of Caesium, \(\phi_{Cs} = 2.14\) eV.
Work function of Potassium, \(\phi_{K} = 2.30\) eV.
Work function of Sodium, \(\phi_{Na} = 2.75\) eV.
Let's check the condition \(E \geq \phi\) for each metal:
1. Caesium (Cs): Is \(2.20 \, eV \geq 2.14 \, eV\)? Yes. Therefore, photoemission will occur from Caesium.
2. Potassium (K): Is \(2.20 \, eV \geq 2.30 \, eV\)? No. Therefore, photoemission will not occur from Potassium.
3. Sodium (Na): Is \(2.20 \, eV \geq 2.75 \, eV\)? No. Therefore, photoemission will not occur from Sodium.
Only Caesium satisfies the condition for photoelectron emission.
Step 4: Final Answer:
Of the given surfaces, only Cs may emit photoelectrons.
Quick Tip: Remember that the work function is a "threshold" energy. If the incoming photon's energy is below this threshold, no electrons will be emitted, no matter how intense the light is. If the energy is above the threshold, the excess energy (\(E - \phi\)) becomes the maximum kinetic energy of the emitted photoelectron.
If \( \oint_S \vec{E} \cdot d\vec{S} = 0 \) over a surface, then :
View Solution
Step 1: Understanding the Question:
The question asks for the physical implication of the net electric flux through a closed surface being zero.
Step 2: Key Formula or Approach:
The given equation, \( \oint_S \vec{E} \cdot d\vec{S} = 0 \), is a mathematical statement about the net electric flux through a closed surface S. According to Gauss's Law for electrostatics, the net electric flux through any closed surface is proportional to the net electric charge enclosed by that surface:
\[ \oint_S \vec{E} \cdot d\vec{S} = \frac{Q_{enc{\epsilon_0} \]
where \(Q_{enc}\) is the net charge inside the surface and \(\epsilon_0\) is the permittivity of free space.
Step 3: Detailed Explanation:
If \( \oint_S \vec{E} \cdot d\vec{S} = 0 \), then from Gauss's Law, it implies that \( \frac{Q_{enc{\epsilon_0} = 0 \), which means the net charge enclosed within the surface is zero (\(Q_{enc} = 0\)).
Let's analyze this in terms of electric field lines (flux lines):
- Electric flux is a measure of the number of electric field lines passing through a surface.
- By convention, flux lines leaving a closed surface contribute positively to the total flux, while lines entering contribute negatively.
- A net flux of zero means that the total positive contribution (flux out) is exactly cancelled by the total negative contribution (flux in).
- Therefore, the number of electric field lines entering the surface must be equal to the number of electric field lines leaving it.
Let's evaluate the given options:
- (A) is incorrect. The field inside could be zero or non-uniform (e.g., from an electric dipole placed inside the surface, where \(Q_{enc} = +q -q = 0\)).
- (B) is correct. This is the direct physical interpretation of zero net flux
- (C) is incorrect. The field strength can vary across the surface.
- (D) is incorrect and poorly phrased. The statement implies that there cannot be any charge outside, which is false. Also, it's about the net charge inside being zero, not that all charges are inside.
Step 4: Final Answer:
The condition of zero net electric flux means that the number of flux lines entering the surface must be equal to the number of flux lines leaving it.
Quick Tip: Gauss's Law is a powerful tool. Remember its core implications: - Net flux depends ONLY on the enclosed charge. - Charges outside the surface contribute to the electric field at the surface but do not contribute to the net flux through the surface. - Zero net flux implies zero net enclosed charge.
Given below are two statements:
Statement I : Photovoltaic devices can convert optical radiation into electricity.
Statement II : Zener diode is designed to operate under reverse bias in breakdown region.
In the light of the above statements, choose the most appropriate answer from the options given below:
View Solution
Step 1: Understanding the Question:
We need to assess the correctness of two separate statements related to semiconductor devices.
Step 2: Detailed Explanation:
Analysis of Statement I:
"Photovoltaic devices can convert optical radiation into electricity."
This statement describes the fundamental principle of the photovoltaic effect. Devices like solar cells and photodiodes are designed to absorb photons (optical radiation) and generate a potential difference (voltage) or a current. This is a direct conversion of light energy into electrical energy.
Conclusion: Statement I is correct.
Analysis of Statement II:
"Zener diode is designed to operate under reverse bias in breakdown region."
A Zener diode is a special type of diode that is engineered to have a precise and sharp reverse breakdown voltage, known as the Zener voltage. Its primary application is as a voltage regulator. In these circuits, it is intentionally operated in the reverse breakdown region, where it maintains a nearly constant voltage across its terminals despite changes in current.
Conclusion: Statement II is correct.
Step 3: Final Answer:
Since both statements are factually correct descriptions of their respective devices, the correct option is that both Statement I and Statement II are correct.
Quick Tip: Remember the specific operating regions for different diodes: - Rectifier diode: Forward bias (conduction), Reverse bias (blocking). - Zener diode: Reverse bias in the breakdown region (voltage regulation). - LED: Forward bias (light emission). - Photodiode/Solar cell: Reverse bias or unbiased (light detection/power generation).
In a plane electromagnetic wave travelling in free space, the electric field component oscillates sinusoidally at a frequency of \(2.0 \times 10^{10}\) Hz and amplitude 48 Vm\(^{-1}\). Then the amplitude of oscillating magnetic field is : (Speed of light in free space = \(3 \times 10^8\) m s\(^{-1}\))
View Solution
Step 1: Understanding the Question:
We are given the amplitude of the electric field component of an electromagnetic wave and asked to find the amplitude of the magnetic field component.
Step 2: Key Formula or Approach:
In an electromagnetic wave traveling in a vacuum (free space), the magnitudes of the electric field (E) and magnetic field (B) at any instant are related by the speed of light (c). The same relationship holds for their amplitudes (\(E_0\) and \(B_0\)):
\[ \frac{E_0}{B_0} = c \]
Step 3: Detailed Explanation:
Given values:
Amplitude of electric field, \(E_0 = 48\) V/m.
Speed of light, \(c = 3 \times 10^8\) m/s.
The frequency information is not needed to find the magnetic field amplitude.
Rearrange the formula to solve for the amplitude of the magnetic field, \(B_0\):
\[ B_0 = \frac{E_0}{c} \]
Substitute the given values:
\[ B_0 = \frac{48 \, V/m}{3 \times 10^8 \, m/s} \] \[ B_0 = 16 \times 10^{-8} \, T \]
To express this in standard scientific notation, we can write it as:
\[ B_0 = 1.6 \times 10^1 \times 10^{-8} \, T = 1.6 \times 10^{-7} \, T \]
Step 4: Final Answer:
The amplitude of the oscillating magnetic field is \(1.6 \times 10^{-7}\) T.
Quick Tip: A simple way to remember the E, B, and c relationship is \(E = cB\). Note that the electric field value is much larger than the magnetic field value because the speed of light 'c' is a very large number. The frequency is irrelevant for this calculation, so watch out for extraneous information in problems.
The temperature of a gas is \(-50^\circ\) C. To what temperature the gas should be heated so that the rms speed is increased by 3 times?
View Solution
Step 1: Understanding the Question:
The question asks for the final temperature required to increase the root-mean-square (rms) speed of a gas. The phrase "increased by 3 times" is crucial to interpret correctly. It means the final speed is the initial speed plus three times the initial speed.
Step 2: Key Formula or Approach:
The rms speed (\(v_{rms}\)) of gas molecules is related to the absolute temperature (T) by the formula:
\[ v_{rms} = \sqrt{\frac{3RT}{M \]
where R is the ideal gas constant and M is the molar mass. From this, we can see the proportionality:
\[ v_{rms} \propto \sqrt{T} \]
where T must be in Kelvin.
Step 3: Detailed Explanation:
Let the initial state be 1 and the final state be 2.
Initial State (1):
Initial temperature, \(T_1 = -50^\circ C\). First, convert to Kelvin:
\[ T_1 = -50 + 273 = 223 \, K \]
Let the initial rms speed be \(v_1\).
Final State (2):
The rms speed is "increased by 3 times". This means:
\[ v_2 = v_1 + 3v_1 = 4v_1 \]
So, the final speed is 4 times the initial speed.
Now, use the proportionality \(v_{rms} \propto \sqrt{T}\):
\[ \frac{v_2}{v_1} = \sqrt{\frac{T_2}{T_1 \]
Substitute the known values:
\[ \frac{4v_1}{v_1} = \sqrt{\frac{T_2}{223 \, K \] \[ 4 = \sqrt{\frac{T_2}{223 \]
Square both sides to solve for \(T_2\):
\[ 16 = \frac{T_2}{223} \] \[ T_2 = 16 \times 223 = 3568 \, K \]
The options are given in both K and \(^\circ\)C. Let's convert \(T_2\) to Celsius:
\[ T_{2(C)} = 3568 - 273 = 3295^\circ C \]
Step 4: Final Answer:
The gas should be heated to a temperature of 3295\(^\circ\) C.
Quick Tip: Be very careful with phrasing like "increased by X times" versus "increased to X times". "Increased by X times" means \(v_{final} = v_{initial} + X \cdot v_{initial} = (1+X)v_{initial}\). "Increased to X times" means \(v_{final} = X \cdot v_{initial}\). Here, X=3, so the factor is (1+3)=4.
The magnitude and direction of the current in the following circuit is
View Solution
Step 1: Understanding the Question:
The question asks to find the magnitude and direction of the current in the given circuit. The circuit appears to be a single loop containing resistors and voltage sources (batteries).
Step 2: Key Formula or Approach:
We will use Kirchhoff's Voltage Law (KVL), which states that the algebraic sum of the potential differences (voltages) around any closed loop is zero. The procedure is:
1. Identify the components in the single loop.
2. Sum the resistances to find the total resistance \(R_{total}\).
3. Sum the electromotive forces (EMFs) to find the net EMF \(E_{net}\), paying attention to their polarities.
4. Calculate the current using Ohm's law for the entire circuit: \( I = \frac{E_{net{R_{total \).
5. Determine the direction of the current based on the polarity of the net EMF.
Step 3: Detailed Explanation:
1. Total Resistance: The resistors are all in series in the single loop. \[ R_{total} = 2 \, \Omega + 1 \, \Omega + 7 \, \Omega = 10 \, \Omega \]
2. Net EMF: There are two voltage sources, 10 V and 5 V. Tracing the loop, we see that their terminals are connected in opposition (positive to positive or negative to negative). The 10 V source tries to drive the current clockwise (from A to B in the top part), while the 5 V source tries to drive it counter-clockwise. The net EMF is the difference between them, and its direction is determined by the larger source. \[ E_{net} = 10 \, V - 5 \, V = 5 \, V \]
The direction of the net EMF is the same as the 10 V source.
3. Calculate Current: \[ I = \frac{E_{net{R_{total = \frac{5 \, V}{10 \, \Omega} = 0.5 \, A \]
4. Determine Direction: Since the 10 V source is stronger, it determines the direction of the current. The current will flow out of its positive terminal and into its negative terminal, meaning it flows in a clockwise direction around the loop. In the top part of the circuit (where 'E' is mentioned), this corresponds to a direction from left to right, which is from node A to node B.
Step 4: Final Answer:
The magnitude of the current is 0.5 A, and its direction is from A to B. This corresponds to option (C).
Quick Tip: When applying KVL to a loop with multiple batteries, first determine if they are aiding or opposing each other. If they are connected in series aiding (positive to negative), add their EMFs. If they are in series opposing (positive to positive), subtract the smaller EMF from the larger one. The overall current direction is set by the resulting net EMF.
An electric dipole is placed at an angle of 30\(^\circ\) with an electric field of intensity \(2 \times 10^5\) N C\(^{-1}\). It experiences a torque equal to 4 Nm. Calculate the magnitude of charge on the dipole, if the dipole length is 2 cm.
View Solution
Step 1: Understanding the Question:
We are given the torque experienced by an electric dipole in a uniform electric field, along with the field strength, the angle, and the dipole length. We need to calculate the magnitude of the charge on the dipole.
Step 2: Key Formula or Approach:
The torque (\(\tau\)) on an electric dipole in an electric field (\(E\)) is given by:
\[ \tau = pE\sin\theta \]
where \(p\) is the magnitude of the electric dipole moment and \(\theta\) is the angle between the dipole moment vector and the electric field vector.
The electric dipole moment \(p\) is defined as the product of the magnitude of one of the charges (\(q\)) and the separation between the charges (\(d\), or dipole length):
\[ p = qd \]
Step 3: Detailed Explanation:
Given values:
Angle, \(\theta = 30^\circ\).
Electric field, \(E = 2 \times 10^5\) N/C.
Torque, \(\tau = 4\) Nm.
Dipole length, \(d = 2\) cm = 0.02 m.
First, combine the two formulas:
\[ \tau = (qd)E\sin\theta \]
Now, rearrange the formula to solve for the charge \(q\):
\[ q = \frac{\tau}{dE\sin\theta} \]
Substitute the given values:
\[ q = \frac{4}{(0.02) \times (2 \times 10^5) \times \sin(30^\circ)} \]
We know that \(\sin(30^\circ) = 0.5\).
\[ q = \frac{4}{(0.02) \times (2 \times 10^5) \times 0.5} \] \[ q = \frac{4}{(0.04 \times 10^5) \times 0.5} \] \[ q = \frac{4}{0.02 \times 10^5} = \frac{4}{2 \times 10^3} \] \[ q = 2 \times 10^{-3} \, C \]
Since \(1 \, mC = 10^{-3} \, C\), the charge is:
\[ q = 2 \, mC \]
Step 4: Final Answer:
The magnitude of the charge on the dipole is 2 mC.
Quick Tip: Always ensure your units are consistent before calculation. In this problem, the dipole length was given in cm and needed to be converted to meters to match the SI units of other quantities (Nm, N/C).
Let a wire be suspended from the ceiling (rigid support) and stretched by a weight W attached at its free end. The longitudinal stress at any point of cross-sectional area A of the wire is :
View Solution
Step 1: Understanding the Question:
We need to find the formula for longitudinal stress in a wire that is supporting a weight W.
Step 2: Key Formula or Approach:
Stress is defined as the internal restoring force (\(F_{restoring}\)) acting per unit of cross-sectional area (A).
\[ Stress = \frac{F_{restoring{A} \]
In equilibrium, the internal restoring force is equal in magnitude to the external deforming force.
Longitudinal stress (or tensile stress) occurs when the force is applied perpendicular to the cross-section, causing a change in length.
Step 3: Detailed Explanation:
- A wire of cross-sectional area A is suspended vertically.
- A weight W is attached to its free end. This weight W is the external deforming force that stretches the wire.
- The wire is in static equilibrium. This means that at any cross-section of the wire, the upward internal restoring force (tension, T) must balance the downward external force.
- If we neglect the weight of the wire itself, the only downward force is the attached weight W.
- Therefore, the tension at any point in the wire is \(T = W\).
- This tension T is the internal restoring force.
- Now, we can apply the formula for stress: \[ Longitudinal Stress = \frac{Internal Restoring Force}{Cross-sectional Area} = \frac{T}{A} \]
Substituting \(T=W\): \[ Longitudinal Stress = \frac{W}{A} \]
Step 4: Final Answer:
The longitudinal stress at any point in the wire is W/A.
Quick Tip: In problems like this, unless the mass or density of the wire is mentioned and you are asked to account for it, you should assume the wire is massless. The stress is then uniform throughout the wire and depends only on the attached weight.
The equivalent capacitance of the system shown in the following circuit is :
View Solution
Step 1: Understanding the Question:
We need to find the equivalent capacitance between points A and B for the given arrangement of four capacitors, each with a capacitance of 3 \(\mu\)F.
Step 2: Key Formula or Approach:
1. Capacitors in Series: The equivalent capacitance \(C_{eq}\) for two capacitors \(C_1\) and \(C_2\) in series is given by \(\frac{1}{C_{eq = \frac{1}{C_1} + \frac{1}{C_2}\) or \(C_{eq} = \frac{C_1C_2}{C_1+C_2}\).
2. Capacitors in Parallel: The equivalent capacitance for capacitors in parallel is the sum of their individual capacitances: \(C_{eq} = C_1 + C_2 + \dots\).
3. Circuit Analysis: We need to identify which parts of the circuit are in series and which are in parallel. Let's label the junctions to analyze the circuit. Let the junction after the first capacitor be P, the top junction be Q, and the bottom junction be R. From the diagram, we can see that point B is connected to both Q and R.
Step 3: Detailed Explanation:
Let's analyze the circuit structure.
- There is a 3 \(\mu\)F capacitor between A and a central node P.
- From node P, the circuit splits. There is a 3 \(\mu\)F capacitor between P and Q (top path), and another 3 \(\mu\)F capacitor between P and R (bottom path).
- There is a 3 \(\mu\)F capacitor connected between nodes Q and R.
- Both nodes Q and R are connected to the terminal B.
Since both Q and R are connected to the same point B, they are at the same potential. This means the potential difference across the capacitor between Q and R is zero. A capacitor with zero potential difference across it carries no charge and can be removed from the circuit for analysis. It is effectively short-circuited.
The simplified circuit becomes:
- A capacitor \(C_1 = 3 \, \mu F\) from A to P.
- Two capacitors, \(C_{top} = 3 \, \mu F\) (from P to Q/B) and \(C_{bottom} = 3 \, \mu F\) (from P to R/B), are now in parallel with each other between node P and node B.
The equivalent capacitance of the parallel combination (\(C_p\)) is: \[ C_p = C_{top} + C_{bottom} = 3 \, \mu F + 3 \, \mu F = 6 \, \mu F \]
Now, this equivalent capacitance \(C_p\) is in series with the first capacitor \(C_1\).
The total equivalent capacitance of the system (\(C_{eq}\)) is: \[ \frac{1}{C_{eq = \frac{1}{C_1} + \frac{1}{C_p} = \frac{1}{3} + \frac{1}{6} \] \[ \frac{1}{C_{eq = \frac{2+1}{6} = \frac{3}{6} = \frac{1}{2} \] \[ C_{eq} = 2 \, \mu F \]
Step 4: Final Answer:
The equivalent capacitance of the system is 2 \(\mu\)F.
Quick Tip: When analyzing complex capacitor networks, always look for points that are at the same potential. Capacitors connected between such points can be removed from the circuit as they are shorted out, which often simplifies the problem significantly.
The venturi-meter works on :
View Solution
Step 1: Understanding the Question:
The question asks for the underlying physical principle behind the operation of a venturi-meter.
Step 2: Detailed Explanation:
A venturi-meter is a device used for measuring the rate of flow of a fluid through a pipe. It consists of a converging section, a throat (the narrowest part), and a diverging section.
1. As the fluid flows from the wider section into the narrow throat, the cross-sectional area decreases. According to the principle of continuity (\(A_1 v_1 = A_2 v_2\)), the speed of the fluid (\(v\)) must increase.
2. Bernoulli's principle states that for a horizontal flow, an increase in the speed of a fluid occurs simultaneously with a decrease in pressure. The principle is expressed as \(P + \frac{1}{2}\rho v^2 = constant\) for a horizontal pipe.
3. In the venturi-meter, since the speed is highest in the throat, the pressure is lowest there. By measuring the pressure difference between the main pipe and the throat using a manometer, one can calculate the fluid's velocity and thus the flow rate.
Therefore, the operation of the venturi-meter is a direct application of Bernoulli's principle, combined with the principle of continuity.
The other options are irrelevant:
- Principles of perpendicular and parallel axes are theorems related to the moment of inertia in rotational mechanics.
- Huygen's principle is a concept in wave optics that describes how wavefronts propagate.
Step 3: Final Answer:
The venturi-meter works on Bernoulli's principle.
Quick Tip: Many practical applications in fluid dynamics, such as the lift of an airplane wing, the curve of a spinning ball (Magnus effect), and atomizers/sprayers, are explained by Bernoulli's principle: where speed is high, pressure is low.
A 12 V, 60 W lamp is connected to the secondary of a step down transformer, whose primary is connected to ac mains of 220 V. Assuming the transformer to be ideal, what is the current in the primary winding?
View Solution
Step 1: Understanding the Question:
We have an ideal transformer with given primary voltage, and secondary voltage and power. We need to find the current in the primary coil.
Step 2: Key Formula or Approach:
For an ideal transformer, there is no power loss. This means the power in the primary coil (\(P_p\)) is equal to the power in the secondary coil (\(P_s\)).
\[ P_p = P_s \]
The power in a coil is given by \(P = V \times I\), where V is the voltage and I is the current.
Therefore, for an ideal transformer:
\[ V_p I_p = V_s I_s = P_s \]
Step 3: Detailed Explanation:
Given values:
Secondary Voltage, \(V_s = 12\) V.
Secondary Power (power of the lamp), \(P_s = 60\) W.
Primary Voltage, \(V_p = 220\) V.
Since the transformer is ideal, the power drawn by the primary winding from the mains is equal to the power delivered by the secondary winding to the lamp.
\[ P_p = P_s = 60 \, W \]
Now we can use the power formula for the primary coil to find the primary current (\(I_p\)):
\[ P_p = V_p \times I_p \] \[ 60 \, W = 220 \, V \times I_p \]
Solve for \(I_p\):
\[ I_p = \frac{60}{220} \, A = \frac{6}{22} \, A = \frac{3}{11} \, A \]
Now, convert the fraction to a decimal:
\[ I_p \approx 0.2727... \, A \]
This is approximately 0.27 A.
Step 4: Final Answer:
The current in the primary winding is approximately 0.27 A.
Quick Tip: For ideal transformers, the core principle is \(Power_{in} = Power_{out}\). This directly relates the primary voltage and current to the output power. You don't always need to calculate the secondary current or the turns ratio if the power is known.
In a series LCR circuit, the inductance L is 10 mH, capacitance C is 1 \(\mu\)F and resistance R is 100 \(\Omega\). The frequency at which resonance occurs is :
View Solution
Step 1: Understanding the Question:
We are given the values of L, C, and R for a series LCR circuit and asked to find the resonant frequency.
Step 2: Key Formula or Approach:
Resonance in a series LCR circuit occurs when the inductive reactance (\(X_L\)) equals the capacitive reactance (\(X_C\)). The frequency at which this happens is the resonant frequency (\(f_0\)). The formula for resonant frequency is:
\[ f_0 = \frac{1}{2\pi\sqrt{LC \]
The angular resonant frequency (\(\omega_0\)) is \(\omega_0 = \frac{1}{\sqrt{LC\). Note that the resistance R does not affect the resonant frequency itself.
Step 3: Detailed Explanation:
Given values:
L = 10 mH = \(10 \times 10^{-3}\) H = \(10^{-2}\) H.
C = 1 \(\mu\)F = \(1 \times 10^{-6}\) F.
R = 100 \(\Omega\).
First, calculate the product LC:
\[ LC = (10^{-2} \, H) \times (10^{-6} \, F) = 10^{-8} \, s^2 \]
Next, calculate the square root of LC:
\[ \sqrt{LC} = \sqrt{10^{-8} \, s^2} = 10^{-4} \, s \]
Now, substitute this into the formula for the resonant frequency \(f_0\):
\[ f_0 = \frac{1}{2\pi (10^{-4})} = \frac{10^4}{2\pi} \, Hz \] \[ f_0 = \frac{10000}{2\pi} \approx \frac{10000}{2 \times 3.14159} \approx \frac{10000}{6.283} \approx 1591.5 \, Hz \]
To express this in kHz, we divide by 1000:
\[ f_0 \approx 1.59 \, kHz \]
Step 4: Final Answer:
The frequency at which resonance occurs is approximately 1.59 kHz.
Quick Tip: Remember that the resistance R in a series LCR circuit affects the "sharpness" or quality factor (Q-factor) of the resonance, but not the resonant frequency itself. The resonant frequency depends only on L and C.
Two bodies of mass m and 9m are placed at a distance R. The gravitational potential on the line joining the bodies where the gravitational field equals zero, will be (G = gravitational constant) :
View Solution
Step 1: Understanding the Question:
We are asked to find the gravitational potential at a specific point on the line connecting two masses, m and 9m. This point is where the net gravitational field due to the two masses is zero.
Step 2: Key Formula or Approach:
1. Gravitational Field (E): The magnitude of the gravitational field due to a mass M at a distance r is \(E = \frac{GM}{r^2}\). It is a vector quantity.
2. Gravitational Potential (V): The gravitational potential due to a mass M at a distance r is \(V = -\frac{GM}{r}\). It is a scalar quantity.
The approach is to first find the point where the net gravitational field is zero and then calculate the net potential at that point.
Step 3: Detailed Explanation:
Let the two masses, m and 9m, be placed along the x-axis at x=0 and x=R, respectively. Let P be the point on the line joining them where the gravitational field is zero. Let the distance of P from mass m be 'x'. Then, the distance of P from mass 9m will be (R-x).
For the net gravitational field at P to be zero, the magnitudes of the fields due to both masses must be equal.
\[ E_m = E_{9m} \] \[ \frac{Gm}{x^2} = \frac{G(9m)}{(R-x)^2} \]
Taking the square root on both sides:
\[ \frac{1}{x} = \frac{3}{R-x} \] \[ R-x = 3x \] \[ R = 4x \] \[ x = \frac{R}{4} \]
So, the point where the field is zero is at a distance of R/4 from mass m. The distance from mass 9m is \(R-x = R - \frac{R}{4} = \frac{3R}{4}\).
Now, we calculate the net gravitational potential at this point P. Since potential is a scalar, we simply add the potentials due to each mass.
\[ V_{net} = V_m + V_{9m} \] \[ V_{net} = \left(-\frac{Gm}{x}\right) + \left(-\frac{G(9m)}{R-x}\right) \]
Substitute the values of x and (R-x):
\[ V_{net} = \left(-\frac{Gm}{R/4}\right) - \left(\frac{G(9m)}{3R/4}\right) \] \[ V_{net} = -\frac{4Gm}{R} - \frac{36Gm}{3R} \] \[ V_{net} = -\frac{4Gm}{R} - \frac{12Gm}{R} \] \[ V_{net} = -\frac{16Gm}{R} \]
Step 4: Final Answer:
The gravitational potential at the point where the gravitational field is zero is \(-\frac{16Gm}{R}\).
Quick Tip: The point where the gravitational field is zero (the null point) between two masses always lies closer to the smaller mass. Remember that gravitational potential is a scalar quantity, so you add the potentials algebraically (including the negative sign), whereas the gravitational field is a vector, requiring vector addition.
Light travels a distance x in time \(t_1\) in air and 10x in time \(t_2\) in another denser medium. What is the critical angle for this medium?
View Solution
Step 1: Understanding the Question:
We need to find the critical angle for a denser medium, given the time it takes for light to travel certain distances in air and in that medium.
Step 2: Key Formula or Approach:
1. Velocity of light: \(v = \frac{distance}{time}\).
2. Refractive index (n): \(n = \frac{speed of light in vacuum (c)}{speed of light in medium (v)}\). For air, we can approximate \(n_{air} \approx 1\).
3. Critical angle (\(i_c\)): When light travels from a denser medium (\(n_d\)) to a rarer medium (\(n_r\)), the critical angle is given by Snell's law: \(\sin(i_c) = \frac{n_r}{n_d}\).
Step 3: Detailed Explanation:
First, let's find the speed of light in air (\(v_{air}\)) and in the denser medium (\(v_{medium}\)).
Speed in air: \(v_{air} = \frac{x}{t_1}\). We'll consider air as the rarer medium, so \(n_r = n_{air} = 1\).
Speed in the denser medium: \(v_{medium} = \frac{10x}{t_2}\).
Next, let's find the refractive index of the denser medium (\(n_d\)). The refractive index is the ratio of the speed of light in air to the speed of light in the medium.
\[ n_d = \frac{v_{air{v_{medium = \frac{x/t_1}{10x/t_2} \] \[ n_d = \frac{x}{t_1} \times \frac{t_2}{10x} = \frac{t_2}{10t_1} \]
Now, we can find the critical angle (\(i_c\)) for light going from the denser medium to air.
\[ \sin(i_c) = \frac{n_{rarer{n_{denser = \frac{n_{air{n_d} \] \[ \sin(i_c) = \frac{1}{t_2 / (10t_1)} \] \[ \sin(i_c) = \frac{10t_1}{t_2} \]
Therefore, the critical angle is:
\[ i_c = \sin^{-1}\left(\frac{10t_1}{t_2}\right) \]
Step 4: Final Answer:
The critical angle for the medium is \(\sin^{-1}\left(\frac{10t_1}{t_2}\right)\).
Quick Tip: The critical angle only exists when light travels from a denser medium to a rarer medium. The value of \(\sin(i_c)\) must be less than or equal to 1. In this problem, \(\frac{n_r}{n_d} = \frac{v_d}{v_r}\), which can be a useful shortcut if you calculate the velocities first.
The amount of energy required to form a soap bubble of radius 2 cm from a soap solution is nearly : (surface tension of soap solution = 0.03 N m\(^{-1}\))
View Solution
Step 1: Understanding the Question:
We need to calculate the work done (or energy required) to create a soap bubble of a given radius. The surface tension of the soap solution is provided.
Step 2: Key Formula or Approach:
The energy required to form a liquid surface is equal to the product of the surface tension (S) and the increase in the surface area (\(\Delta A\)).
\[ W = S \times \Delta A \]
A crucial point for a soap bubble is that it has two surfaces: an inner surface and an outer surface. Therefore, the total surface area is twice the area of a single sphere.
Step 3: Detailed Explanation:
Given values:
Radius, \(r = 2 \, cm = 0.02 \, m\).
Surface tension, \(S = 0.03 \, N \, m^{-1}\).
First, calculate the total surface area of the soap bubble. Since it has two surfaces, the area is:
\[ A = 2 \times (4 \pi r^2) = 8 \pi r^2 \]
The bubble is formed from a solution, so the initial area is zero. Thus, the increase in area is \(\Delta A = A\).
\[ \Delta A = 8 \pi (0.02 \, m)^2 = 8 \pi (0.0004 \, m^2) = 0.0032 \pi \, m^2 \]
Now, calculate the energy required (work done):
\[ W = S \times \Delta A \] \[ W = 0.03 \, N \, m^{-1} \times 0.0032 \pi \, m^2 \] \[ W = 0.000096 \pi \, J \]
Using the approximation \(\pi \approx 3.14\):
\[ W \approx 0.000096 \times 3.14 \, J \approx 0.00030144 \, J \]
Expressing this in scientific notation:
\[ W \approx 3.0144 \times 10^{-4} \, J \]
This is approximately \(3.01 \times 10^{-4} \, J\).
Step 4: Final Answer:
The amount of energy required is nearly \(3.01 \times 10^{-4} \, J\).
Quick Tip: A common mistake is forgetting that a soap bubble has two surfaces. A liquid drop in air has only one surface. Always check if the object is a bubble (two surfaces) or a drop (one surface) when dealing with surface tension energy problems.
The minimum wavelength of X-rays produced by an electron accelerated through a potential difference of V volts is proportional to:
View Solution
Step 1: Understanding the Question:
We need to find the relationship between the minimum wavelength of produced X-rays and the accelerating potential difference applied to the electrons.
Step 2: Key Formula or Approach:
1. Energy of an accelerated electron: When an electron (charge e) is accelerated through a potential difference V, it gains kinetic energy \(E_k = eV\).
2. Energy of a photon: The energy of a photon (like an X-ray) is given by \(E_{photon} = hf = \frac{hc}{\lambda}\), where h is Planck's constant, c is the speed of light, f is the frequency, and \(\lambda\) is the wavelength.
The minimum wavelength (\(\lambda_{min}\)) of the X-ray is produced when the electron loses all of its kinetic energy in a single interaction to create one photon. This photon will have the maximum possible energy.
Step 3: Detailed Explanation:
Equating the maximum kinetic energy of the electron to the maximum energy of the X-ray photon:
\[ E_{k, max} = E_{photon, max} \] \[ eV = \frac{hc}{\lambda_{min \]
We want to find the proportionality of \(\lambda_{min}\) with respect to V. Rearranging the equation for \(\lambda_{min}\):
\[ \lambda_{min} = \frac{hc}{e} \frac{1}{V} \]
Since h (Planck's constant), c (speed of light), and e (electron charge) are all constants, we can see the relationship between \(\lambda_{min}\) and V:
\[ \lambda_{min} \propto \frac{1}{V} \]
The minimum wavelength is inversely proportional to the accelerating voltage.
Step 4: Final Answer:
The minimum wavelength of the produced X-rays is proportional to \(\frac{1}{V}\).
Quick Tip: This minimum wavelength is also known as the "cutoff wavelength." A higher accelerating voltage means electrons have more energy, which allows them to produce higher-energy (and thus shorter-wavelength) X-ray photons.
If the galvanometer G does not show any deflection in the circuit shown, the value of R is given by :
View Solution
Step 1: Understanding the Question:
We are given a circuit and told that the galvanometer shows no deflection. This is a key condition that simplifies the circuit, and we need to find the value of the unknown resistance R.
Step 2: Key Formula or Approach:
The condition of "no deflection in the galvanometer" means that no current is flowing through it. This implies that the potential difference across the galvanometer is zero. In this circuit, this means the potential difference across the resistor R must be equal and opposite to the EMF of the 2V battery. This is the principle of a potentiometer.
Step 3: Detailed Explanation:
Since no current flows through the galvanometer, we can analyze the main circuit loop (the one with the 10V battery and resistors 400 \(\Omega\) and R) as if the galvanometer and the 2V battery branch were not there.
The resistors 400 \(\Omega\) and R are in series with the 10V battery.
The total resistance in this primary loop is \(R_{total} = 400\Omega + R\).
The current flowing through the primary loop is given by Ohm's law:
\[ I = \frac{V_{total{R_{total = \frac{10}{400 + R} \]
This current 'I' flows through the resistor R. The potential difference (voltage drop) across R is:
\[ V_R = I \times R = \left(\frac{10}{400 + R}\right)R \]
For the galvanometer to show no deflection, the potential at the points across it must be equal. This means the potential drop across resistor R must exactly balance the EMF of the 2V battery in the secondary loop.
\[ V_R = 2V \]
Now we can set up the equation:
\[ \left(\frac{10}{400 + R}\right)R = 2 \]
Now, solve for R:
\[ 10R = 2(400 + R) \] \[ 10R = 800 + 2R \] \[ 10R - 2R = 800 \] \[ 8R = 800 \] \[ R = \frac{800}{8} = 100 \, \Omega \]
Step 4: Final Answer:
The value of R for which the galvanometer shows no deflection is 100 \(\Omega\).
Quick Tip: This circuit is an application of the potentiometer principle. The main loop with the 10V battery acts as the potentiometer wire, creating a potential gradient. The galvanometer shows a null point when the potential drop across a portion of the main circuit (resistor R here) equals the EMF of the cell in the secondary circuit (the 2V battery).
A Carnot engine has an efficiency of 50% when its source is at a temperature 327\(^\circ\) C. The temperature of the sink is :
View Solution
Step 1: Understanding the Question:
We are given the efficiency of a Carnot engine and the temperature of its source. We need to find the temperature of the sink.
Step 2: Key Formula or Approach:
The efficiency (\(\eta\)) of a Carnot engine is given by:
\[ \eta = 1 - \frac{T_L}{T_H} \]
where \(T_L\) is the temperature of the sink and \(T_H\) is the temperature of the source. It is crucial that both temperatures are in an absolute scale (Kelvin).
The conversion from Celsius (\(T_C\)) to Kelvin (\(T_K\)) is \(T_K = T_C + 273\).
Step 3: Detailed Explanation:
Given values:
Efficiency, \(\eta = 50% = 0.5\).
Source temperature, \(T_{H(C)} = 327^\circ C\).
First, convert the source temperature to Kelvin:
\[ T_H = 327 + 273 = 600 \, K \]
Now, substitute the values into the efficiency formula:
\[ 0.5 = 1 - \frac{T_L}{600} \]
Rearrange the formula to solve for \(T_L\):
\[ \frac{T_L}{600} = 1 - 0.5 = 0.5 \] \[ T_L = 0.5 \times 600 = 300 \, K \]
The question asks for the temperature of the sink in degrees Celsius. Convert \(T_L\) back to Celsius:
\[ T_{L(C)} = T_L - 273 = 300 - 273 = 27^\circ C \]
Step 4: Final Answer:
The temperature of the sink is 27\(^\circ\) C.
Quick Tip: Always convert temperatures to Kelvin before using them in any thermodynamics formula involving ratios or absolute temperatures, such as the ideal gas law, Carnot efficiency, or Stefan-Boltzmann law. Forgetting this conversion is a very common mistake.
The errors in the measurement which arise due to unpredictable fluctuations in temperature and voltage supply are :
View Solution
Step 1: Understanding the Question:
The question asks to classify the type of measurement error that results from unpredictable changes in environmental or experimental conditions.
Step 2: Detailed Explanation:
Let's define the different types of errors:
- Random errors: These are errors that occur irregularly and are unpredictable. They are random in both magnitude and direction. They are caused by uncontrolled variables, such as fluctuations in temperature, voltage, pressure, or mechanical vibrations. Because they are random, their effects can be minimized by taking multiple measurements and calculating the average.
- Systematic errors: These errors have a consistent direction or magnitude. They are typically caused by a flaw in the experimental setup or the instrument itself.
- Instrumental errors: Arise from imperfections in the measuring instrument, such as incorrect calibration (a zero error) or faulty design.
- Personal errors: Occur due to the observer's bias, carelessness in taking readings, or incorrect experimental procedure (e.g., parallax error).
- Least count errors: This error is associated with the resolution of the instrument. The least count is the smallest value that can be measured by the instrument, and any measurement is only accurate up to this value.
The question specifically mentions "unpredictable fluctuations," which is the defining characteristic of random errors.
Step 3: Final Answer:
Errors arising from unpredictable fluctuations in temperature and voltage supply are classified as random errors.
Quick Tip: A key distinction: Systematic errors can, in principle, be identified and corrected for, as they are consistent. Random errors cannot be eliminated but can be reduced by repeated measurements and statistical analysis.
A wire carrying a current I along the positive x-axis has length L. It is kept in a magnetic field \(\vec{B}=(2\hat{i} + 3\hat{j} - 4\hat{k})\) T. The magnitude of the magnetic force acting on the wire is :
View Solution
Step 1: Understanding the Question:
We need to find the magnitude of the magnetic force on a straight current-carrying wire placed in a uniform magnetic field.
Step 2: Key Formula or Approach:
The magnetic force \(\vec{F}\) on a straight wire of length vector \(\vec{L}\) carrying a current \(I\) in a uniform magnetic field \(\vec{B}\) is given by the Lorentz force law:
\[ \vec{F} = I (\vec{L} \times \vec{B}) \]
The magnitude of the force is \(|\vec{F}| = I |\vec{L} \times \vec{B}|\).
Step 3: Detailed Explanation:
First, we need to define the length vector \(\vec{L}\).
The wire has length L and carries current along the positive x-axis. Therefore, the length vector is:
\[ \vec{L} = L\hat{i} \]
The magnetic field vector is given as:
\[ \vec{B} = (2\hat{i} + 3\hat{j} - 4\hat{k}) \, T \]
Next, we calculate the cross product \(\vec{L} \times \vec{B}\):
\[ \vec{L} \times \vec{B} = (L\hat{i}) \times (2\hat{i} + 3\hat{j} - 4\hat{k}) \]
Using the distributive property of the cross product:
\[ \vec{L} \times \vec{B} = L [(\hat{i} \times 2\hat{i}) + (\hat{i} \times 3\hat{j}) + (\hat{i} \times -4\hat{k})] \]
Recall the properties of unit vector cross products: \(\hat{i} \times \hat{i} = 0\), \(\hat{i} \times \hat{j} = \hat{k}\), and \(\hat{i} \times \hat{k} = -\hat{j}\).
\[ \vec{L} \times \vec{B} = L [0 + 3(\hat{i} \times \hat{j}) - 4(\hat{i} \times \hat{k})] \] \[ \vec{L} \times \vec{B} = L [3\hat{k} - 4(-\hat{j})] = L(4\hat{j} + 3\hat{k}) \]
Now, calculate the force vector \(\vec{F}\):
\[ \vec{F} = I (\vec{L} \times \vec{B}) = I [L(4\hat{j} + 3\hat{k})] = IL(4\hat{j} + 3\hat{k}) \]
Finally, find the magnitude of the force vector:
\[ |\vec{F}| = |IL(4\hat{j} + 3\hat{k})| = IL |4\hat{j} + 3\hat{k}| \] \[ |\vec{F}| = IL \sqrt{(4)^2 + (3)^2} = IL \sqrt{16 + 9} = IL \sqrt{25} \] \[ |\vec{F}| = 5IL \]
Step 4: Final Answer:
The magnitude of the magnetic force acting on the wire is 5 IL.
Quick Tip: Remember that the component of the magnetic field that is parallel to the current (\(2\hat{i}\) in this case) does not contribute to the magnetic force, as the cross product of parallel vectors is zero. Only the components of \(\vec{B}\) perpendicular to \(\vec{L}\) contribute to the force.
A very long conducting wire is bent in a semi-circular shape from A to B as shown in figure. The magnetic field at point P for steady current configuration is given by :
View Solution
Step 1: Understanding the Question:
The diagram shows a current configuration consisting of a very long straight wire and a semi-circular arc. We need to find the net magnetic field at point P, which is the center of the semi-circle. The wording "A very long conducting wire is bent..." is ambiguous. A more plausible interpretation, matching the options, is that the total field is the superposition of the field from a long straight wire and the field from the semi-circular arc.
Step 2: Key Formula or Approach:
We use the principle of superposition. The total magnetic field \(\vec{B}_{net}\) is the vector sum of the magnetic field from the straight wire (\(\vec{B}_{straight}\)) and the semi-circular arc (\(\vec{B}_{arc}\)).
1. Field from a long straight wire: At a perpendicular distance \(R\), the magnetic field is \(B_{straight} = \frac{\mu_0 i}{2\pi R}\).
2. Field from a semi-circular arc: At its center, the magnetic field is \(B_{arc} = \frac{1}{2} \left( \frac{\mu_0 i}{2R} \right) = \frac{\mu_0 i}{4R}\).
The direction of each field is found using the right-hand rule.
Step 3: Detailed Explanation:
Let's analyze the contribution from each part based on the diagram:
1. The very long straight wire (top part):
- The current \(i\) flows to the left.
- Point P is at a perpendicular distance \(R\) from this wire.
- Using the right-hand grip rule (point your thumb in the direction of the current, i.e., to the left), your fingers curl such that the magnetic field at point P (which is below the wire in the plane of the page) points into the page.
- Magnitude: \(B_{straight} = \frac{\mu_0 i}{2\pi R}\).
2. The semi-circular arc (A to B):
- The current flows from A to B, which is a counter-clockwise direction.
- Using the right-hand curl rule (curl the fingers of your right hand in the direction of the current flow around the arc), your thumb points away from the page (outward).
- Magnitude: \(B_{arc} = \frac{\mu_0 i}{4R}\).
3. Net Field:
The two fields are in opposite directions. Let's take the direction "away from the page" (outward) as positive.
\[ B_{net} = B_{arc} - B_{straight} \] \[ B_{net} = \frac{\mu_0 i}{4R} - \frac{\mu_0 i}{2\pi R} \]
Factor out the common term \(\frac{\mu_0 i}{4R}\):
\[ B_{net} = \frac{\mu_0 i}{4R} \left( 1 - \frac{4R}{2\pi R} \right) = \frac{\mu_0 i}{4R} \left( 1 - \frac{2}{\pi} \right) \]
To determine the final direction, we compare the magnitudes. \(1 > 2/\pi\) (since \(\pi \approx 3.14\), \(2/\pi \approx 0.637\)). So the net result is positive, which means the direction is away from the page.
Step 4: Final Answer:
The net magnetic field is \( \frac{\mu_0 i}{4R} \left[ 1 - \frac{2}{\pi} \right] \) pointed away from the page.
Quick Tip: When a problem presents a complex shape, always break it down into simpler, standard shapes (like straight lines, arcs, loops) for which you know the magnetic field formulas. Then, apply the principle of superposition, paying close attention to the direction of the field from each part.
A bullet from a gun is fired on a rectangular wooden block with velocity u. When bullet travels 24 cm through the block along its length horizontally, velocity of bullet becomes \( \frac{u}{3} \). Then it further penetrates into the block in the same direction before coming to rest exactly at the other end of the block. The total length of the block is :
View Solution
Step 1: Understanding the Question:
A bullet enters a wooden block and slows down. We are given its initial velocity, its velocity after traveling a certain distance, and that it stops at the end of the block. We need to find the total length of the block, assuming constant resistive force (and thus constant deceleration).
Step 2: Key Formula or Approach:
We can use the third equation of motion, which relates initial velocity (u), final velocity (v), acceleration (a), and displacement (s):
\[ v^2 = u^2 + 2as \]
Alternatively, we can use the work-energy theorem, which states that the work done by the net force is equal to the change in kinetic energy: \(W = \Delta K\). Here, the work is done by the resistive force of the wood.
Step 3: Detailed Explanation (Using Work-Energy Theorem):
Let \(F\) be the constant resistive force exerted by the block on the bullet. Let \(m\) be the mass of the bullet.
Part 1: Bullet travels the first 24 cm.
Initial velocity = \(u\).
Final velocity = \(u/3\).
Distance, \(s_1 = 24\) cm.
Work done by resistive force, \(W_1 = -F \times s_1 = -F \times 24\).
Change in kinetic energy, \(\Delta K_1 = \frac{1}{2}m(\frac{u}{3})^2 - \frac{1}{2}mu^2 = \frac{1}{2}m(\frac{u^2}{9} - u^2) = -\frac{1}{2}m(\frac{8u^2}{9})\).
According to the work-energy theorem, \(W_1 = \Delta K_1\):
\[ -F \times 24 = -\frac{4mu^2}{9} \quad \Rightarrow \quad F \times 24 = \frac{4mu^2}{9} \quad (Equation 1) \]
Part 2: Bullet travels the remaining distance \(s_2\) and stops.
Initial velocity = \(u/3\).
Final velocity = 0.
Distance = \(s_2\).
Work done by resistive force, \(W_2 = -F \times s_2\).
Change in kinetic energy, \(\Delta K_2 = 0 - \frac{1}{2}m(\frac{u}{3})^2 = -\frac{mu^2}{18}\).
According to the work-energy theorem, \(W_2 = \Delta K_2\):
\[ -F \times s_2 = -\frac{mu^2}{18} \quad \Rightarrow \quad F \times s_2 = \frac{mu^2}{18} \quad (Equation 2) \]
Now, divide Equation 2 by Equation 1:
\[ \frac{F \times s_2}{F \times 24} = \frac{mu^2/18}{4mu^2/9} \] \[ \frac{s_2}{24} = \frac{1}{18} \times \frac{9}{4} = \frac{1}{8} \] \[ s_2 = \frac{24}{8} = 3 \, cm \]
The total length of the block is the sum of the two distances:
Total Length = \(s_1 + s_2 = 24 \, cm + 3 \, cm = 27 \, cm\).
Step 4: Final Answer:
The total length of the block is 27 cm.
Quick Tip: The work-energy theorem is often simpler than kinematics when forces and distances are involved, as it bypasses the need to calculate acceleration and time. For constant force, Work \(\propto\) distance and \(\Delta K \propto (v^2 - u^2)\).
An electric dipole is placed as shown in the figure. The electric potential (in 10\(^2\) V) at point P due to the dipole is (\(\epsilon_0\)=permittivity of free space and \( \frac{1}{4\pi\epsilon_0} = K \)):
View Solution
Step 1: Understanding the Question:
We need to calculate the net electric potential at point P due to an electric dipole. Point P lies on the axial line of the dipole.
Step 2: Key Formula or Approach:
Electric potential is a scalar quantity. The total potential at a point due to multiple charges is the algebraic sum of the potentials due to individual charges. The potential V at a distance r from a point charge Q is given by:
\[ V = \frac{KQ}{r} \]
where \(K = \frac{1}{4\pi\epsilon_0}\).
Step 3: Detailed Explanation:
From the figure, we have:
- A negative charge \(-q\) at \(x = -3\) cm.
- A positive charge \(+q\) at \(x = +3\) cm.
- Point P is on the x-axis at \(x = +5\) cm.
1. Calculate the distance of P from each charge:
- Distance of P from the positive charge \(+q\):
\(r_+ = (5 \, cm) - (3 \, cm) = 2 \, cm\).
- Distance of P from the negative charge \(-q\):
\(r_- = (5 \, cm) - (-3 \, cm) = 5 \, cm + 3 \, cm = 8 \, cm\).
2. Calculate the potential at P:
The total potential \(V_P\) is the sum of the potential from \(+q\) (\(V_+\)) and the potential from \(-q\) (\(V_-\)).
\[ V_P = V_+ + V_- = \frac{K(+q)}{r_+} + \frac{K(-q)}{r_-} \] \[ V_P = Kq \left( \frac{1}{r_+} - \frac{1}{r_-} \right) \]
Substitute the distances (we can keep them in cm as we are looking for a ratio which makes the units cancel):
\[ V_P = Kq \left( \frac{1}{2} - \frac{1}{8} \right) \]
Find a common denominator:
\[ V_P = Kq \left( \frac{4}{8} - \frac{1}{8} \right) = Kq \left( \frac{3}{8} \right) \] \[ V_P = \left(\frac{3}{8}\right)qK \]
The phrase "in 10\(^2\) V" seems to be extraneous information, as the options are symbolic expressions.
Step 4: Final Answer:
The electric potential at point P due to the dipole is \( \left(\frac{3}{8}\right)qK \).
Quick Tip: Remember that electric potential is a scalar, so you perform algebraic addition (including signs of the charges). The distances used are the absolute distances from the charge to the point of interest. For points on the axis of a dipole, be careful to add or subtract distances correctly from the center.
In the figure shown here, what is the equivalent focal length of the combination of lenses (Assume that all layers are thin)?
View Solution
Step 1: Understanding the Question:
The given setup consists of a biconvex lens made of material \(n_1=1.5\) enclosed within a container filled with a medium of refractive index \(n_2=1.6\). This combination forms a system of three thin lenses in contact. We need to find the equivalent focal length of this system, assuming it is placed in air (\(n_{air}=1\)).
Step 2: Key Formula or Approach:
We can treat the system as three thin lenses placed in contact:
1. A plano-concave lens of material \(n_2\). (\(L_1\))
2. A biconvex lens of material \(n_1\). (\(L_2\))
3. Another plano-concave lens of material \(n_2\). (\(L_3\))
The equivalent focal length \(F\) is given by: \( \frac{1}{F} = \frac{1}{f_1} + \frac{1}{f_2} + \frac{1}{f_3} \).
The focal length of each lens is calculated using the Lens Maker's Formula:
\[ \frac{1}{f} = (n_{lens} - n_{medium}) \left( \frac{1}{R_1} - \frac{1}{R_2} \right) \]
Here, the surrounding medium is air, so \(n_{medium}=1\).
Step 3: Detailed Explanation:
Given: \(n_1 = 1.5\), \(n_2 = 1.6\), and \(|R_1| = |R_2| = 20\) cm.
Using the standard sign convention (light travels from left to right):
For lens \(L_1\) (plano-concave, \(n_2 = 1.6\)):
The first surface is plane (\(R_{1,1} = \infty\)). The second surface is concave with radius 20 cm (\(R_{1,2} = +20\) cm).
\[ \frac{1}{f_1} = (1.6 - 1) \left(\frac{1}{\infty} - \frac{1}{20}\right) = 0.6 \times \left(-\frac{1}{20}\right) = -\frac{0.6}{20} = -\frac{3}{100} \]
For lens \(L_2\) (biconvex, \(n_1 = 1.5\)):
The first surface is convex (\(R_{2,1} = +20\) cm). The second surface is also convex from the right side, so its radius of curvature is (\(R_{2,2} = -20\) cm).
\[ \frac{1}{f_2} = (1.5 - 1) \left(\frac{1}{20} - \frac{1}{-20}\right) = 0.5 \times \left(\frac{2}{20}\right) = 0.5 \times \frac{1}{10} = \frac{5}{100} \]
For lens \(L_3\) (plano-concave, \(n_2 = 1.6\)):
The first surface is concave (\(R_{3,1} = -20\) cm). The second surface is plane (\(R_{3,2} = \infty\)).
\[ \frac{1}{f_3} = (1.6 - 1) \left(\frac{1}{-20} - \frac{1}{\infty}\right) = 0.6 \times \left(-\frac{1}{20}\right) = -\frac{0.6}{20} = -\frac{3}{100} \]
For the combination:
\[ \frac{1}{F} = \frac{1}{f_1} + \frac{1}{f_2} + \frac{1}{f_3} = \left(-\frac{3}{100}\right) + \left(\frac{5}{100}\right) + \left(-\frac{3}{100}\right) \] \[ \frac{1}{F} = \frac{-3 + 5 - 3}{100} = \frac{-1}{100} \] \[ F = -100 \, cm \]
Step 4: Final Answer:
The equivalent focal length of the combination is \(-100\) cm.
Quick Tip: Complex lens arrangements can often be simplified by treating them as multiple thin lenses in contact. Remember to apply the sign convention for radii of curvature carefully for each lens component. A convex surface has a positive radius if light hits it from the left, while a concave surface has a negative radius.
A satellite is orbiting just above the surface of the earth with period T. If d is the density of the earth and G is the universal constant of gravitation, the quantity \( \frac{3\pi}{Gd} \) represents :
View Solution
Step 1: Understanding the Question:
We need to find what the expression \(\frac{3\pi}{Gd}\) represents in terms of the orbital period T of a satellite orbiting just above the Earth's surface.
Step 2: Key Formula or Approach:
1. The orbital period \(T\) of a satellite is given by Kepler's third law applied to circular orbits: \( T^2 = \frac{4\pi^2 r^3}{GM} \), where \(r\) is the orbital radius and \(M\) is the mass of the central body (Earth).
2. For a satellite orbiting "just above the surface," the orbital radius \(r\) is approximately equal to the Earth's radius \(R\).
3. The mass of the Earth \(M\) can be expressed in terms of its density \(d\) and radius \(R\): \(M = Volume \times Density = \frac{4}{3}\pi R^3 d\).
Step 3: Detailed Explanation:
Start with the formula for the orbital period squared:
\[ T^2 = \frac{4\pi^2 R^3}{GM} \]
Now, substitute the expression for the mass of the Earth \(M\):
\[ T^2 = \frac{4\pi^2 R^3}{G \left(\frac{4}{3}\pi R^3 d\right)} \]
We can cancel out several terms: \(4\), \(\pi\), and \(R^3\).
\[ T^2 = \frac{\pi}{G \left(\frac{1}{3} d\right)} \] \[ T^2 = \frac{3\pi}{Gd} \]
This shows that the given quantity \(\frac{3\pi}{Gd}\) is exactly equal to the square of the orbital period, \(T^2\).
Step 4: Final Answer:
The quantity \( \frac{3\pi}{Gd} \) represents \(T^2\).
Quick Tip: This is a classic derivation. It shows that for a satellite orbiting at the surface of any spherical body, the square of the orbital period is inversely proportional to the body's density (\(T^2 \propto 1/d\)), regardless of its size.
10 resistors, each of resistance R are connected in series to a battery of emf E and negligible internal resistance. Then those are connected in parallel to the same battery, the current is increased n times. The value of n is:
View Solution
Step 1: Understanding the Question:
We have two scenarios with 10 identical resistors and the same battery. First, they are in series, and second, they are in parallel. We need to find the factor 'n' by which the current increases in the parallel case compared to the series case.
Step 2: Key Formula or Approach:
1. Series Combination: The equivalent resistance \(R_s\) of N resistors each of resistance R in series is \(R_s = NR\).
2. Parallel Combination: The equivalent resistance \(R_p\) of N resistors each of resistance R in parallel is \(R_p = R/N\).
3. Ohm's Law: The current \(I\) from a battery of emf E is \(I = E/R_{eq}\).
Step 3: Detailed Explanation:
Let N = 10 and each resistor has resistance R. The battery emf is E.
Case 1: Series Connection
The equivalent resistance is \(R_{series} = 10R\).
The current flowing from the battery is:
\[ I_{series} = \frac{E}{R_{series = \frac{E}{10R} \]
Case 2: Parallel Connection
The equivalent resistance is \(R_{parallel} = \frac{R}{10}\).
The current flowing from the battery is:
\[ I_{parallel} = \frac{E}{R_{parallel = \frac{E}{R/10} = \frac{10E}{R} \]
Finding the value of n
We are given that the current is increased n times, which means \(I_{parallel} = n \times I_{series}\).
\[ \frac{10E}{R} = n \times \left(\frac{E}{10R}\right) \]
We can cancel E and R from both sides of the equation:
\[ 10 = n \times \frac{1}{10} \] \[ n = 10 \times 10 = 100 \]
Step 4: Final Answer:
The value of n is 100.
Quick Tip: For N identical resistors, the ratio of series to parallel equivalent resistance is \(R_s/R_p = (NR)/(R/N) = N^2\). Since current is inversely proportional to resistance, the ratio of currents will be \(I_p/I_s = R_s/R_p = N^2\). In this case, \(n = 10^2 = 100\).
A horizontal bridge is built across a river. A student standing on the bridge throws a small ball vertically upwards with a velocity 4 m s\(^{-1}\). The ball strikes the water surface after 4 s. The height of bridge above water surface is (Take g = 10 m s\(^{-2}\)) :
View Solution
Step 1: Understanding the Question:
We are given the initial upward velocity of a ball thrown from a bridge and the total time until it hits the water below. We need to find the height of the bridge.
Step 2: Key Formula or Approach:
We will use the second equation of motion for displacement under constant acceleration (gravity). We must be careful with the sign convention. Let's choose the point of projection (on the bridge) as the origin (s=0) and the upward direction as positive.
The formula is:
\[ s = ut + \frac{1}{2}at^2 \]
where \(s\) is the displacement, \(u\) is the initial velocity, \(t\) is the time, and \(a\) is the acceleration.
Step 3: Detailed Explanation:
According to our sign convention:
- Initial velocity, \(u = +4\) m/s (since it's thrown upwards).
- Time, \(t = 4\) s.
- Acceleration, \(a = -g = -10\) m/s\(^2\) (since gravity acts downwards).
Now, we calculate the displacement (\(s\)) of the ball from the bridge to the water surface:
\[ s = (4)(4) + \frac{1}{2}(-10)(4)^2 \] \[ s = 16 - 5(16) \] \[ s = 16 - 80 \] \[ s = -64 \, m \]
The negative sign indicates that the final position (the water surface) is 64 meters below the initial position (the bridge). Therefore, the height of the bridge above the water is the magnitude of this displacement.
Height \(H = |s| = 64\) m.
Step 4: Final Answer:
The height of the bridge above the water surface is 64 m.
Quick Tip: In projectile motion problems, carefully establishing a coordinate system and consistently applying a sign convention (e.g., up is positive, down is negative) is crucial to avoid errors. The displacement 's' is a vector quantity, while height is a scalar distance.
Two thin lenses are of same focal lengths (f), but one is convex and the other one is concave. When they are placed in contact with each other, the equivalent focal length of the combination will be :
View Solution
Step 1: Understanding the Question:
We have a combination of two thin lenses in contact: a convex lens and a concave lens, both having the same magnitude of focal length, \(f\). We need to find the equivalent focal length of this combination.
Step 2: Key Formula or Approach:
The equivalent focal length, \(F_{eq}\), of two thin lenses with focal lengths \(f_1\) and \(f_2\) placed in contact is given by:
\[ \frac{1}{F_{eq = \frac{1}{f_1} + \frac{1}{f_2} \]
By convention, the focal length of a convex lens is positive, and that of a concave lens is negative.
Step 3: Detailed Explanation:
Let \(f_1\) be the focal length of the convex lens and \(f_2\) be the focal length of the concave lens.
According to the sign convention:
- \(f_1 = +f\) (for the convex lens)
- \(f_2 = -f\) (for the concave lens)
Now, substitute these into the combination formula:
\[ \frac{1}{F_{eq = \frac{1}{+f} + \frac{1}{-f} \] \[ \frac{1}{F_{eq = \frac{1}{f} - \frac{1}{f} = 0 \]
If the reciprocal of the equivalent focal length is zero, the focal length itself must be infinitely large.
\[ F_{eq} = \frac{1}{0} \rightarrow \infty \]
Step 4: Final Answer:
The equivalent focal length of the combination will be infinite. Such a combination acts like a plane glass plate and has zero power.
Quick Tip: The power of a lens is the reciprocal of its focal length (\(P = 1/f\)). For lenses in contact, the powers add up: \(P_{eq} = P_1 + P_2\). In this case, \(P_1 = +1/f\) and \(P_2 = -1/f\), so \(P_{eq} = 0\). A system with zero power has an infinite focal length.
The radius of inner most orbit of hydrogen atom is \(5.3 \times 10^{-11}\) m. What is the radius of third allowed orbit of hydrogen atom?
View Solution
Step 1: Understanding the Question:
We are given the radius of the first orbit (n=1) of a hydrogen atom and asked to find the radius of the third orbit (n=3).
Step 2: Key Formula or Approach:
According to the Bohr model for the hydrogen atom, the radius of the n-th allowed orbit is given by:
\[ r_n = r_1 \times n^2 \]
where \(r_1\) is the radius of the first orbit (also known as the Bohr radius, \(a_0\)) and \(n\) is the principal quantum number.
Step 3: Detailed Explanation:
Given values:
- Radius of the innermost orbit, \(r_1 = 5.3 \times 10^{-11}\) m.
- We need to find the radius of the third orbit, so \(n=3\).
Substitute the values into the formula:
\[ r_3 = r_1 \times (3)^2 = r_1 \times 9 \] \[ r_3 = (5.3 \times 10^{-11} \, m) \times 9 \] \[ r_3 = 47.7 \times 10^{-11} \, m \]
The options are in Angstroms (Å). We know that \(1 \, Å = 10^{-10}\) m. Let's convert our answer to Angstroms.
\[ r_3 = 4.77 \times 10^{-10} \, m = 4.77 \, Å \]
Step 4: Final Answer:
The radius of the third allowed orbit of the hydrogen atom is 4.77 Å.
Quick Tip: For hydrogen-like atoms, the key proportionalities from the Bohr model are very useful: - Radius: \(r_n \propto \frac{n^2}{Z}\) - Velocity: \(v_n \propto \frac{Z}{n}\) - Energy: \(E_n \propto -\frac{Z^2}{n^2}\) For hydrogen, Z=1, so the relationships simplify.
The resistance of platinum wire at 0°C is 2\(\Omega\) and 6.8\(\Omega\) at 80°C. The temperature coefficient of resistance of the wire is :
View Solution
Step 1: Understanding the Question:
We are given the resistance of a wire at two different temperatures and asked to find its temperature coefficient of resistance, \(\alpha\).
Step 2: Key Formula or Approach:
The relationship between resistance and temperature is given by the formula:
\[ R_T = R_0 (1 + \alpha \Delta T) \]
where \(R_T\) is the resistance at temperature T, \(R_0\) is the resistance at a reference temperature (here, 0°C), \(\alpha\) is the temperature coefficient of resistance, and \(\Delta T\) is the change in temperature.
Step 3: Detailed Explanation:
Given values:
- Resistance at 0°C, \(R_0 = 2 \, \Omega\).
- Resistance at 80°C, \(R_{80} = 6.8 \, \Omega\).
- The change in temperature is \(\Delta T = 80°C - 0°C = 80°C\).
Substitute these values into the formula:
\[ R_{80} = R_0 (1 + \alpha \times 80) \] \[ 6.8 = 2 (1 + 80\alpha) \]
Divide both sides by 2:
\[ 3.4 = 1 + 80\alpha \]
Subtract 1 from both sides:
\[ 2.4 = 80\alpha \]
Solve for \(\alpha\):
\[ \alpha = \frac{2.4}{80} = \frac{24}{800} = \frac{3}{100} = 0.03 \, °C^{-1} \]
Expressing this in scientific notation:
\[ \alpha = 3 \times 10^{-2} \, °C^{-1} \]
Step 4: Final Answer:
The temperature coefficient of resistance of the wire is \(3 \times 10^{-2}\) °C\(^{-1}\).
Quick Tip: The formula \(R_T = R_0(1+\alpha\Delta T)\) is an approximation that works well for metals over a limited temperature range. \(\alpha\) itself can vary slightly with temperature. For exam purposes, assume \(\alpha\) is constant unless stated otherwise.
The net impedance of circuit (as shown in figure) will be :
View Solution
Step 1: Understanding the Question:
We are asked to find the net impedance (Z) of a series LCR circuit with given values for the inductor (L), capacitor (C), resistor (R), and the AC source frequency (f).
Step 2: Key Formula or Approach:
The impedance Z of a series LCR circuit is given by:
\[ Z = \sqrt{R^2 + (X_L - X_C)^2} \]
where \(R\) is the resistance, \(X_L\) is the inductive reactance, and \(X_C\) is the capacitive reactance.
The reactances are calculated as:
\[ X_L = \omega L = 2\pi f L \] \[ X_C = \frac{1}{\omega C} = \frac{1}{2\pi f C} \]
Step 3: Detailed Explanation:
Given values:
- \(R = 10 \, \Omega\)
- \(L = \frac{50}{\pi} \, mH = \frac{50}{\pi} \times 10^{-3} \, H\)
- \(C = \frac{10^3}{\pi} \, \muF = \frac{10^3}{\pi} \times 10^{-6} \, F = \frac{10^{-3{\pi} \, F\)
- \(f = 50 \, Hz\)
First, calculate the inductive reactance \(X_L\):
\[ X_L = 2\pi f L = 2\pi (50) \left(\frac{50}{\pi} \times 10^{-3}\right) = 100\pi \left(\frac{50}{\pi} \times 10^{-3}\right) = 5000 \times 10^{-3} = 5 \, \Omega \]
Next, calculate the capacitive reactance \(X_C\):
\[ X_C = \frac{1}{2\pi f C} = \frac{1}{2\pi (50) \left(\frac{10^{-3{\pi}\right)} = \frac{1}{100\pi \left(\frac{10^{-3{\pi}\right)} = \frac{1}{100 \times 10^{-3 = \frac{1}{10^{-1 = 10 \, \Omega \]
Now, calculate the impedance Z:
\[ Z = \sqrt{R^2 + (X_L - X_C)^2} = \sqrt{(10)^2 + (5 - 10)^2} \] \[ Z = \sqrt{100 + (-5)^2} = \sqrt{100 + 25} = \sqrt{125} \] \[ Z = \sqrt{25 \times 5} = 5\sqrt{5} \, \Omega \]
Step 4: Final Answer:
The net impedance of the circuit is \(5\sqrt{5} \, \Omega\).
Quick Tip: In LCR circuit calculations, the values of L, C, and f are often chosen to give simple integer values for \(X_L\) and \(X_C\). Always calculate the reactances first before finding the impedance. The voltage of the source (220 V) is extra information not needed for calculating impedance.
Calculate the maximum acceleration of a moving car so that a body lying on the floor of the car remains stationary. The coefficient of static friction between the body and the floor is 0.15 (g = 10 m s\(^{-2}\)).
View Solution
Step 1: Understanding the Question:
We need to find the maximum acceleration a car can have without an object on its floor slipping. This is a problem involving static friction.
Step 2: Key Formula or Approach:
The object accelerates along with the car because of the force of static friction (\(f_s\)) between the object and the car floor. According to Newton's second law:
\[ F_{net} = ma \]
Here, the net horizontal force on the body is the static friction force, so \(f_s = ma\).
The static friction force has a maximum possible value, \(f_{s,max} = \mu_s N\), where \(\mu_s\) is the coefficient of static friction and \(N\) is the normal force.
For the object not to slip, \(f_s \leq f_{s,max}\). The maximum acceleration (\(a_{max}\)) occurs when the required force is equal to the maximum available friction force, i.e., \(ma_{max} = f_{s,max}\).
Step 3: Detailed Explanation:
On a horizontal floor, the normal force \(N\) balances the weight of the body \(mg\). So, \(N = mg\).
The maximum static friction force is:
\[ f_{s,max} = \mu_s N = \mu_s mg \]
The condition for maximum acceleration is:
\[ ma_{max} = f_{s,max} \] \[ ma_{max} = \mu_s mg \]
The mass \(m\) cancels out from both sides:
\[ a_{max} = \mu_s g \]
Given values:
- \(\mu_s = 0.15\)
- \(g = 10\) m/s\(^2\)
Substitute the values:
\[ a_{max} = 0.15 \times 10 = 1.5 \, m/s^2 \]
Step 4: Final Answer:
The maximum acceleration of the car is 1.5 m/s\(^{-2}\).
Quick Tip: This result, \(a_{max} = \mu_s g\), is a standard and useful one for problems involving an object on an accelerating horizontal surface. Notice that the maximum acceleration is independent of the mass of the object.
For the following logic circuit, the truth table is:
View Solution
Step 1: Understanding the Question:
We are asked to determine the output Y for all possible combinations of inputs A and B for the given logic circuit.
Step 2: Key Formula or Approach:
1. Identify the logic gates in the circuit.
2. Write the Boolean expression for the output Y in terms of the inputs A and B.
3. Construct the truth table based on the Boolean expression.
Step 3: Detailed Explanation:
The circuit consists of two gates:
- A NOT gate with input A. Its output is \(\bar{A}\).
- A NOR gate with two inputs: one from the output of the NOT gate (\(\bar{A}\)) and the other from input B.
The Boolean expression for the output Y of the NOR gate is:
\[ Y = \overline{\bar{A} + B} \]
Using De Morgan's theorem, which states \(\overline{X+Y} = \bar{X} \cdot \bar{Y}\), we can simplify the expression:
\[ Y = \overline{(\bar{A})} \cdot \bar{B} = A \cdot \bar{B} \]
This expression represents the operation "A AND NOT B".
Let's construct the truth table for \(Y = A \cdot \bar{B}\):
When A=0, B=0: \(Y = 0 \cdot \overline{0} = 0 \cdot 1 = 0\)
When A=0, B=1: \(Y = 0 \cdot \overline{1} = 0 \cdot 0 = 0\)
When A=1, B=0: \(Y = 1 \cdot \overline{0} = 1 \cdot 1 = 1\)
When A=1, B=1: \(Y = 1 \cdot \overline{1} = 1 \cdot 0 = 0\)
The resulting truth table is:
\begin{tabular{|c|c|c|
A & B & Y
0 & 0 & 0
0 & 1 & 0
1 & 0 & 1
1 & 1 & 0
Step 4: Final Answer:
The logical operation of the circuit is \(Y = A \cdot \bar{B}\). The correct truth table for this circuit is option (C) .
Quick Tip: Always double-check the symbols for logic gates. A D-shape is for AND/NAND, while a curved input line is for OR/NOR. In case of discrepancies between your result and the given options in an exam, re-read the question and re-check your work. If the discrepancy persists, it might indicate an error in the question paper.
The x-t graph of a particle performing simple harmonic motion is shown in the figure. The acceleration of the particle at t=2 s is :
View Solution
Step 1: Understanding the Question:
We are given the position-time (x-t) graph for a particle in Simple Harmonic Motion (SHM) and asked to find its acceleration at a specific time, t = 2 s.
Step 2: Key Formula or Approach:
The acceleration \(a\) of a particle in SHM is related to its displacement \(x\) by the formula:
\[ a = -\omega^2 x \]
where \(\omega\) is the angular frequency. The angular frequency can be found from the time period \(T\) using \(\omega = \frac{2\pi}{T}\). We can determine \(A\) and \(T\) from the graph.
Step 3: Detailed Explanation:
1. Read parameters from the graph:
- Amplitude (A): The maximum displacement from the mean position. From the graph, the maximum value of x is 1 m. So, \(A = 1\) m.
- Time Period (T): The time taken for one complete oscillation. The graph shows one full cycle is completed at t = 8 s. So, \(T = 8\) s.
2. Calculate angular frequency (\(\omega\)):
\[ \omega = \frac{2\pi}{T} = \frac{2\pi}{8} = \frac{\pi}{4} \, rad/s \]
3. Find the displacement at t = 2 s:
From the graph, at \(t = 2\) s, the particle is at its maximum positive displacement.
\[ x(t=2s) = +1 \, m \]
4. Calculate the acceleration at t = 2 s:
Using the formula \(a = -\omega^2 x\):
\[ a(t=2s) = - \left(\frac{\pi}{4}\right)^2 \times (1) \] \[ a(t=2s) = -\frac{\pi^2}{16} \, m/s^2 \]
Step 4: Final Answer:
The acceleration of the particle at t=2 s is \(-\frac{\pi^2}{16}\) m s\(^{-2}\).
Quick Tip: In SHM, acceleration is maximum in magnitude at the extreme positions (where displacement is maximum) and is directed towards the mean position. At the positive extreme (\(x = +A\)), acceleration is maximum negative (\(a = -\omega^2 A\)). At the negative extreme (\(x = -A\)), acceleration is maximum positive (\(a = +\omega^2 A\)).
The right option for the mass of CO\(_2\) produced by heating 20 g of 20% pure limestone is (Atomic mass of Ca = 40)
[CaCO\(_3 \xrightarrow{1200 K} CaO + CO_2\)]
View Solution
Step 1: Understanding the Question:
This is a stoichiometry problem. We need to calculate the mass of carbon dioxide produced from the thermal decomposition of an impure sample of limestone (CaCO\(_3\)).
Step 2: Key Formula or Approach:
1. Calculate the mass of the pure reactant (CaCO\(_3\)) in the sample.
2. Use the balanced chemical equation to establish the molar relationship between the reactant and the product.
3. Convert the mass of the pure reactant to moles.
4. Use the mole ratio to find the moles of the product (CO\(_2\)).
5. Convert the moles of the product to mass.
Step 3: Detailed Explanation:
1. Mass of pure CaCO\(_3\):
Total mass of limestone sample = 20 g.
Purity = 20%.
Mass of pure CaCO\(_3\) = 20 g \(\times \frac{20}{100} = 4\) g.
2. Molar Masses:
Molar mass of CaCO\(_3\) = 40 (Ca) + 12 (C) + 3 \(\times\) 16 (O) = 100 g/mol.
Molar mass of CO\(_2\) = 12 (C) + 2 \(\times\) 16 (O) = 44 g/mol.
3. Stoichiometric Calculation:
The balanced equation is: \[ CaCO_3 (s) \rightarrow CaO (s) + CO_2 (g) \]
The mole ratio between CaCO\(_3\) and CO\(_2\) is 1:1.
This means that 1 mole of CaCO\(_3\) produces 1 mole of CO\(_2\).
In terms of mass, 100 g of CaCO\(_3\) produces 44 g of CO\(_2\).
4. Calculate the mass of CO\(_2\):
We can use a simple ratio to find the mass of CO\(_2\) produced from 4 g of pure CaCO\(_3\):
\[ Mass of CO_2 = (Mass of CaCO_3) \times \frac{Molar mass of CO_2}{Molar mass of CaCO_3} \] \[ Mass of CO_2 = 4 \, g \times \frac{44 \, g/mol}{100 \, g/mol} \] \[ Mass of CO_2 = \frac{4 \times 44}{100} = \frac{176}{100} = 1.76 \, g \]
Step 4: Final Answer:
The mass of CO\(_2\) produced is 1.76 g.
Quick Tip: In stoichiometry problems involving impure samples, always perform calculations based on the mass of the pure substance. The impurities are assumed to be inert and do not participate in the reaction.
Some tranquilizers are listed below. Which one from the following belongs to barbiturates?
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given tranquilizers is a member of the barbiturate class of drugs.
Step 2: Detailed Explanation:
Tranquilizers are neurological drugs used to treat anxiety, fear, tension, and disturbances of the mind. They are classified into different chemical groups. Let's analyze the given options:
(1) Veronal: Veronal is the trade name for barbital. It is a derivative of barbituric acid and was one of the first commercially available barbiturate hypnotics. Therefore, Veronal is a barbiturate.
(2) Chlordiazepoxide: This is sold under the trade name Librium. It belongs to the benzodiazepine class of drugs, not barbiturates.
(3) Meprobamate: This is sold under trade names like Miltown and Equanil. It is a carbamate derivative and is classified as a non-barbiturate anxiolytic.
(4) Valium: Valium is the well-known trade name for Diazepam. It is a classic example of a benzodiazepine, a different class of tranquilizers from barbiturates.
Step 3: Final Answer:
Among the given options, only Veronal is a barbiturate.
Quick Tip: In the chapter "Chemistry in Everyday Life", it's important to memorize the classification of common drugs and at least one or two examples from each class. For tranquilizers, remember the main classes: barbiturates (e.g., Veronal, Luminal) and benzodiazepines (e.g., Valium, Librium).
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : Helium is used to dilute oxygen in diving apparatus.
Reason R : Helium has high solubility in O\(_2\).
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
We need to evaluate an assertion and a reason related to the use of helium in deep-sea diving equipment.
Step 2: Detailed Explanation:
Analysis of Assertion A:
"Helium is used to dilute oxygen in diving apparatus."
This is a factually correct statement. For deep-sea diving, a mixture of helium and oxygen (called heliox) is used instead of compressed air (nitrogen and oxygen). This is done to prevent a condition called nitrogen narcosis, which occurs at high pressures when divers breathe nitrogen.
Conclusion: Assertion A is true.
Analysis of Reason R:
"Helium has high solubility in O\(_2\)."
This statement is irrelevant to the application. The important property is the solubility of the diluent gas (helium or nitrogen) in the diver's blood under pressure. The primary reason for using helium is its very low solubility in blood compared to nitrogen. When a diver ascends, the pressure decreases, and dissolved gases can come out of solution to form bubbles in the bloodstream, leading to a painful and dangerous condition called decompression sickness or "the bends". Because helium is much less soluble in blood, the risk of the bends is significantly reduced. Therefore, the statement that helium is used because it has *high solubility* is incorrect in context and factually wrong regarding its key property.
Conclusion: Reason R is false.
Step 3: Final Answer:
Since Assertion A is true and Reason R is false, the correct option is (4).
Quick Tip: The use of helium in deep-sea diving is a classic example demonstrating the application of Henry's Law, which states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. The key to avoiding "the bends" is using a breathing gas that has low solubility in blood.
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R : The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
This is an Assertion-Reason question about the properties of solutions of alkali metals in liquid ammonia. We must evaluate the truthfulness of both statements and the validity of the reason.
Step 2: Detailed Explanation:
Analysis of Assertion A:
When alkali metals, like sodium (Na), are dissolved in liquid ammonia (NH\(_3\)), they ionize to form metal cations and release electrons. The reaction is:
\[ Na(s) + (x+y)NH_3(l) \rightarrow [Na(NH_3)_x]^+ + [e(NH_3)_y]^- \]
The species \([e(NH_3)_y]^-\) is known as the ammoniated electron or solvated electron. This unpaired electron absorbs energy in the visible region of the spectrum, imparting a deep blue color to the solution. Since it is an unpaired electron, its presence also makes the solution paramagnetic. Therefore, Assertion A is true.
Analysis of Reason R:
The reason states that the deep blue color is due to the formation of amide. Sodium amide (NaNH\(_2\)) can be formed in these solutions, but it happens slowly, and this reaction causes the blue color to fade, not to appear. The formation of amide is represented by:
\[ 2Na(s) + 2NH_3(l) \xrightarrow{catalyst or time} 2NaNH_2(s) + H_2(g) \]
The primary cause of the blue color is the ammoniated electron, not the sodium amide. Therefore, Reason R is false.
Step 3: Final Answer:
Since Assertion A is true and Reason R is false, the correct option is (4).
Quick Tip: Remember the three key properties of alkali metal-liquid ammonia solutions and their common cause, the ammoniated electron: 1. \textbf{Deep blue color}: Due to electronic transitions of the ammoniated electron. 2. \textbf{Paramagnetism}: Due to the unpaired spin of the ammoniated electron. 3. \textbf{High electrical conductivity}: Due to both ammoniated cations and ammoniated electrons being mobile charge carriers.
The correct order of energies of molecular orbitals of N\(_2\) molecule, is :
View Solution
Step 1: Understanding the Question:
The question asks for the correct increasing order of energy for the molecular orbitals (MOs) of the nitrogen molecule (N\(_2\)).
Step 2: Key Formula or Approach:
According to Molecular Orbital Theory (MOT), the order of energy levels of MOs for diatomic molecules of the second period elements depends on s-p mixing.
For diatomic molecules up to N\(_2\) (i.e., Li\(_2\), Be\(_2\), B\(_2\), C\(_2\), N\(_2\)), significant s-p mixing occurs. This mixing raises the energy of the \(\sigma2p_z\) orbital above that of the \(\pi2p_x\) and \(\pi2p_y\) orbitals.
For diatomic molecules after N\(_2\) (i.e., O\(_2\), F\(_2\), Ne\(_2\)), the energy gap between 2s and 2p atomic orbitals is larger, so s-p mixing is less effective. The \(\sigma2p_z\) orbital remains below the \(\pi2p_x\) and \(\pi2p_y\) orbitals.
Step 3: Detailed Explanation:
Since the question is about the N\(_2\) molecule (total 14 electrons), we must use the energy order that accounts for s-p mixing.
The correct sequence of molecular orbitals in increasing order of energy is:
\[ \sigma1s < \sigma^*1s < \sigma2s < \sigma^*2s < (\pi2p_x = \pi2p_y) < \sigma2p_z < (\pi^*2p_x = \pi^*2p_y) < \sigma^*2p_z \]
The \(\pi2p_x\) and \(\pi2p_y\) orbitals are degenerate (have the same energy), as are the \(\pi^*2p_x\) and \(\pi^*2p_y\) orbitals.
Step 4: Final Answer:
Comparing this correct order with the given options:
Option (A) incorrectly places \( (\pi^*2p_x = \pi^*2p_y) \) before \( \sigma2p_z \).
Option (B) matches the correct energy order for N\(_2\).
Option (C) shows the order for O\(_2\) and F\(_2\), where \( \sigma2p_z \) is lower in energy than \( \pi2p_x \) and \( \pi2p_y \).
Option (D) shows a completely incorrect sequence.
Therefore, the correct order is given in option (2).
Quick Tip: A simple mnemonic to remember the MO energy order: For N\(_2\) and lighter diatomic molecules (14 or fewer electrons), the order is "pi-sigma" for the 2p bonding orbitals (\(\pi_{2p} < \sigma_{2p}\)). For O\(_2\) and heavier ones (more than 14 electrons), the order is "sigma-pi" (\(\sigma_{2p} < \pi_{2p}\)). This is a crucial distinction and a frequently tested concept.
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R:
Assertion A : A reaction can have zero activation energy.
Reason R : The minimum extra amount of energy absorbed by reactant molecules so that their energy becomes equal to threshold value, is called activation energy.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
This Assertion-Reason question tests the fundamental concepts of activation energy in chemical kinetics. We need to evaluate the validity of both statements and the causal link between them.
Step 2: Detailed Explanation:
Analysis of Reason R:
Reason R provides the standard definition of activation energy (\(E_a\)). It is the minimum amount of energy that must be provided to reactant molecules to overcome the energy barrier and form the transition state, which then proceeds to products. The total energy required is the threshold energy. This definition is perfectly correct.
Therefore, Reason R is true.
Analysis of Assertion A:
Assertion A claims that a reaction can have zero activation energy. According to the Arrhenius equation, \( k = Ae^{-E_a/RT} \), if \(E_a = 0\), then \( k = A \), meaning the reaction rate is independent of temperature and every collision is effective. While some reactions, particularly the combination of free radicals in the gas phase (e.g., \( CH_3\cdot + CH_3\cdot \rightarrow C_2H_6 \)), have very low or negligible activation energies, the concept of a truly zero activation energy is an idealization. For the purposes of general chemistry curriculum and examinations, it is generally considered that a reaction involves some form of bond rearrangement or formation, which necessitates overcoming an energy barrier, however small. Therefore, the statement that a reaction can have zero activation energy is considered to be false in this context.
Step 3: Final Answer:
Since Assertion A is considered false and Reason R is true, the correct option is (1).
Quick Tip: In chemical kinetics, activation energy is a fundamental concept representing an energy barrier. While some barrierless reactions exist, for exam purposes, assume that reactions generally have a non-zero activation energy unless dealing with specific exceptions like radical recombination. The definition of activation energy (Reason R) is a core concept you must know.
Weight (g) of two moles of the organic compound, which is obtained by heating sodium ethanoate with sodium hydroxide in presence of calcium oxide is :
View Solution
Step 1: Understanding the Question:
The question asks for the mass of two moles of the organic product formed from a specific chemical reaction: the decarboxylation of sodium ethanoate.
Step 2: Key Formula or Approach:
1. Identify the reaction. Heating a sodium salt of a carboxylic acid with soda-lime (a mixture of NaOH and CaO) is a standard method for preparing alkanes, known as decarboxylation.
2. Write the balanced chemical equation to identify the organic product.
3. Calculate the molar mass of the product.
4. Calculate the total mass for two moles of the product using the formula: Mass = moles \(\times\) Molar Mass.
Step 3: Detailed Explanation:
The reaction is the decarboxylation of sodium ethanoate (CH\(_3\)COONa) using soda-lime (NaOH + CaO). The CaO does not directly participate but helps to keep the NaOH dry and facilitates the reaction.
The reaction is: \[ \underset{Sodium ethanoate}{CH_3COONa} + NaOH \xrightarrow{CaO, \Delta} \underset{Methane}{CH_4} + Na_2CO_3 \]
The organic compound obtained is methane (CH\(_4\)).
Now, we need to find the weight of two moles of methane.
First, calculate the molar mass of methane (CH\(_4\)):
Molar Mass (CH\(_4\)) = (Atomic mass of C) + 4 \(\times\) (Atomic mass of H)
Molar Mass (CH\(_4\)) = 12.0 g/mol + 4 \(\times\) 1.0 g/mol = 16.0 g/mol.
Finally, calculate the weight of two moles:
Weight = 2 moles \(\times\) 16.0 g/mol = 32 g.
Step 4: Final Answer:
The weight of two moles of the organic product (methane) is 32 g.
Quick Tip: Soda-lime decarboxylation is a step-down reaction for alkanes. The alkane produced has one less carbon atom than the parent carboxylic acid salt. The -COONa group is effectively replaced by an -H atom.
Complete the following reaction :
View Solution
Step 1: Understanding the Question:
The question asks for the final product [C] of a two-step reaction sequence starting from cyclohexanone [A].
Step 2: Key Formula or Approach:
The reaction sequence involves two key transformations:
1. Cyanohydrin formation: A ketone reacts with HCN to form a cyanohydrin.
2. Acid hydrolysis and dehydration: The cyanohydrin is treated with concentrated acid (H\(_2\)SO\(_4\)) and heat. The nitrile group (-CN) hydrolyzes to a carboxylic acid (-COOH), and the tertiary alcohol group (-OH) undergoes dehydration to form an alkene.
Step 3: Detailed Explanation:
Step I: Formation of Cyanohydrin [B]
The carbonyl group of cyclohexanone [A] is attacked by the nucleophilic cyanide ion (from HCN) to form cyclohexanone cyanohydrin [B].
\[ [A: Cyclohexanone] \xrightarrow{HCN} [B: 1-hydroxycyclohexanecarbonitrile] \]
The structure of [B] has both a hydroxyl (-OH) group and a nitrile (-CN) group attached to the same carbon atom (C1) of the ring.
Step II: Formation of Product [C]
Product [B] is heated with concentrated sulfuric acid. Two reactions occur simultaneously:
Hydrolysis of Nitrile: The nitrile group (-C\(\equiv\)N) is completely hydrolyzed by the strong acid to a carboxylic acid group (-COOH).
Dehydration of Alcohol: The hydroxyl group (-OH) is on a tertiary carbon, making it susceptible to dehydration (elimination of a water molecule) in the presence of a strong acid like conc. H\(_2\)SO\(_4\) and heat. A double bond is formed between C1 and an adjacent carbon (C2 or C6) of the ring.
The combined result is the formation of cyclohex-1-ene-1-carboxylic acid.
\[ [B] \xrightarrow{conc. H_2SO_4, \Delta} [C: Cyclohex-1-ene-1-carboxylic acid] + NH_4HSO_4 + H_2O \]
Step 4: Final Answer:
The final product [C] is cyclohex-1-ene-1-carboxylic acid, which corresponds to the structure shown in option (1).
Quick Tip: Recognize that concentrated H\(_2\)SO\(_4\) is a powerful dehydrating agent. When you see it used with heat on a molecule containing an alcohol group (especially secondary or tertiary), always anticipate an elimination reaction to form an alkene.
Select the correct statements from the following:
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is \(9.10939 \times 10^{-31}\) kg.
C. All the isotopes of a given element show same chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks us to identify the correct statements among the five given options related to atomic structure and theory.
Step 2: Detailed Explanation:
Let's evaluate each statement for its correctness.
Statement A: "Atoms of all elements are composed of two fundamental particles." This is incorrect. Atoms are composed of three fundamental particles: protons, neutrons, and electrons. An exception is the protium isotope of hydrogen (\(^{1}H\)), which has one proton and one electron but no neutron, but the statement refers to "all elements".
Statement B: "The mass of the electron is \(9.10939 \times 10^{-31}\) kg." This is a factual statement and is correct. The accepted value for the rest mass of an electron is approximately \(9.1093837 \times 10^{-31}\) kg, so the given value is accurate for exam purposes.
Statement C: "All the isotopes of a given element show same chemical properties." This is correct. Isotopes of an element have the same number of protons and, in a neutral atom, the same number of electrons. Since chemical properties are primarily determined by the electron configuration, isotopes exhibit identical chemical behavior. They differ only in the number of neutrons, which affects their mass and nuclear properties.
Statement D: "Protons and electrons are collectively known as nucleons." This is incorrect. Nucleons are the particles that reside in the atomic nucleus. These are protons and neutrons. Electrons orbit the nucleus.
Statement E: "Dalton's atomic theory, regarded the atom as an ultimate particle of matter." This is correct. A fundamental postulate of John Dalton's atomic theory was that atoms are indivisible and indestructible fundamental particles of matter. Although we now know atoms are divisible, this was a cornerstone of his original theory.
Step 3: Final Answer:
The correct statements are B, C, and E. Therefore, the correct option is (1).
Quick Tip: For questions involving multiple statements, evaluate each one individually as true or false. This systematic approach helps eliminate incorrect options and pinpoint the correct combination. Pay close attention to absolute words like "all" or "always".
The stability of Cu\(^{2+}\) is more than Cu\(^+\) salts in aqueous solution due to -
View Solution
Step 1: Understanding the Question:
The question asks for the thermodynamic reason behind the greater stability of the copper(II) ion (Cu\(^{2+}\)) compared to the copper(I) ion (Cu\(^+\)) in an aqueous environment.
Step 2: Key Formula or Approach:
The stability of an ion in an aqueous solution is determined by the overall Gibbs free energy change for its formation from the elemental state. This involves several energy terms, primarily the enthalpy of atomization, ionization enthalpy (IE), and hydration enthalpy (\(\Delta_{hyd}H\)).
\[ M(s) \xrightarrow{\Delta_{at}H} M(g) \xrightarrow{IE} M^{n+}(g) \xrightarrow{\Delta_{hyd}H} M^{n+}(aq) \]
A more stable ion will have a more negative overall enthalpy change.
Step 3: Detailed Explanation:
Let's compare the formation of Cu\(^+\)(aq) and Cu\(^{2+}\)(aq).
Ionization Enthalpy: The second ionization enthalpy (IE\(_2\)) of copper (the energy required to remove an electron from Cu\(^+\) to form Cu\(^{2+}\)) is very high. Based on IE alone, Cu\(^+\) should be more stable than Cu\(^{2+}\). So, option (1) is incorrect as a reason for Cu\(^{2+}\) stability; it's actually a factor that opposes it.
Hydration Enthalpy: Hydration enthalpy is the energy released when one mole of gaseous ions is dissolved in water. It depends strongly on the charge density of the ion (charge/size ratio). The formula for hydration energy is roughly proportional to the square of the charge (\(q^2\)) and inversely proportional to the radius (\(r\)).
\[ \Delta_{hyd}H \propto -\frac{q^2}{r} \]
The Cu\(^{2+}\) ion has a greater charge (+2) and a smaller ionic radius compared to the Cu\(^+\) ion (+1). Consequently, the hydration enthalpy of Cu\(^{2+}\) is much more negative (i.e., much more energy is released) than that of Cu\(^+\).
Conclusion: This large release of hydration energy for Cu\(^{2+}\) more than compensates for the high energy input required for the second ionization. The overall energy change is more favorable for the formation of Cu\(^{2+}\) in an aqueous solution, making it more stable.
Step 4: Final Answer:
The primary factor responsible for the greater stability of Cu\(^{2+}\) in aqueous solution is its high hydration energy. This corresponds to option (4).
Quick Tip: When comparing the stability of different oxidation states of an ion *in aqueous solution*, always consider the hydration enthalpy. It's often the deciding factor, especially for ions with higher charges, as hydration energy increases significantly with charge.
The relation between n\(_m\), (n\(_m\) = the number of permissible values of magnetic quantum number (m)) for a given value of azimuthal quantum number (\(l\)), is
View Solution
Step 1: Understanding the Question:
The question asks for the mathematical relationship between the total number of possible values for the magnetic quantum number (\(m\)), denoted as n\(_m\), and the azimuthal quantum number (\(l\)).
Step 2: Key Formula or Approach:
The rules for quantum numbers state that for a given value of the azimuthal quantum number, \(l\), the magnetic quantum number, \(m_l\) (or simply \(m\)), can take any integer value from \( -l \) to \( +l \), including zero.
Possible values of \(m\) are: \( -l, (-l+1), ..., 0, ..., (l-1), +l \).
Step 3: Detailed Explanation:
To find the total number of these values (n\(_m\)), we can count them. The number of values is given by:
\[ n_m = (last value) - (first value) + 1 \] \[ n_m = (l) - (-l) + 1 \] \[ n_m = l + l + 1 \] \[ n_m = 2l + 1 \]
Now we have the relationship \(n_m = 2l + 1\). The question provides options where this relationship is rearranged. We need to find the option that is equivalent to our derived formula. Let's rearrange our formula to solve for \(l\):
\[ n_m - 1 = 2l \] \[ l = \frac{n_m - 1}{2} \]
Step 4: Final Answer:
This rearranged formula matches option (2). Therefore, the correct relation is given in option (2).
Quick Tip: For any subshell `l`, the number of orbitals is always \(2l+1\). For s-subshell (\(l=0\)), number of orbitals = \(2(0)+1 = 1\). For p-subshell (\(l=1\)), number of orbitals = \(2(1)+1 = 3\). For d-subshell (\(l=2\)), number of orbitals = \(2(2)+1 = 5\). This formula is fundamental to understanding electron configurations.
Intermolecular forces are forces of attraction and repulsion between interacting particles that will include :
A. dipole - dipole forces.
B. dipole - induced dipole forces.
C. hydrogen bonding.
D. covalent bonding.
E. dispersion forces.
Choose the most appropriate answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the listed forces are classified as intermolecular forces. Intermolecular forces are forces that exist between molecules.
Step 2: Detailed Explanation:
Let's analyze each force type:
A. dipole - dipole forces: These are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. They are a type of intermolecular force.
B. dipole - induced dipole forces: These forces arise when a polar molecule induces a temporary dipole in a nonpolar molecule, leading to a weak attraction. They are a type of intermolecular force (a category of van der Waals forces).
C. hydrogen bonding: This is a special, strong type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (N, O, or F) and another nearby electronegative atom. It is a very important intermolecular force.
D. covalent bonding: This is the force that holds atoms together \textit{within a molecule by the sharing of electrons. It is an intramolecular force, not an intermolecular force. Intramolecular forces are much stronger than intermolecular forces.
E. dispersion forces (London forces): These are weak intermolecular forces caused by temporary fluctuations in electron distribution within atoms or molecules, creating temporary dipoles. They exist between all atoms and molecules.
Step 3: Final Answer:
The forces A, B, C, and E are all types of intermolecular forces. Force D, covalent bonding, is an intramolecular force. Therefore, the correct combination is A, B, C, and E. This corresponds to option (4).
Quick Tip: Remember the distinction: \textbf{Intermolecular forces are "between" molecules (like an \textbf{inter}national flight is between nations), while \textbf{intra}molecular forces are "within" a molecule (like an \textbf{intra}mural sport is within a school). Covalent, ionic, and metallic bonds are intramolecular.
Homoleptic complex from the following complexes is :
View Solution
Step 1: Understanding the Question:
We need to identify the homoleptic complex from the given list of coordination compounds.
Step 2: Key Formula or Approach:
- Homoleptic complexes are those in which the central metal atom or ion is coordinated to only one type of ligand.
- Heteroleptic complexes are those in which the central metal atom or ion is coordinated to more than one type of ligand.
We will analyze the ligands attached to the central metal ion in each complex.
Step 3: Detailed Explanation:
1. Triamminetriaquachromium (III) chloride: The central metal is Chromium (Cr\(^{3+}\)). The ligands are 'triammine' (\(3 \times NH_3\)) and 'triaqua' (\(3 \times H_2O\)). Since there are two different types of ligands (ammine and aqua), this is a heteroleptic complex.
2. Potassium trioxalatoaluminate (III): The central metal is Aluminate (Al\(^{3+}\)). The ligand is 'trioxalato' (\(3 \times C_2O_4^{2-}\)). Since only one type of ligand (oxalato) is attached to the central metal, this is a homoleptic complex. The formula is \(K_3[Al(C_2O_4)_3]\).
3. Diamminechloridonitrito-N-platinum (II): The central metal is Platinum (Pt\(^{2+}\)). The ligands are 'diammine' (\(2 \times NH_3\)), 'chlorido' (\(Cl^-\)), and 'nitrito-N' (\(NO_2^-\)). Since there are three different types of ligands, this is a heteroleptic complex.
4. Pentaamminecarbonatocobalt (III) chloride: The central metal is Cobalt (Co\(^{3+}\)). The ligands are 'pentaammine' (\(5 \times NH_3\)) and 'carbonato' (\(CO_3^{2-}\)). Since there are two different types of ligands, this is a heteroleptic complex.
Step 4: Final Answer:
The only homoleptic complex among the options is Potassium trioxalatoaluminate (III).
Quick Tip: To identify a homoleptic complex, look at the name. If the name mentions only one type of ligand (e.g., 'hexaammine', 'tetracarbonyl', 'trioxalato'), it's homoleptic. If it lists multiple ligand names (e.g., 'diamminedichlorido'), it's heteroleptic.
Which of the following reactions will NOT give primary amine as the product?
View Solution
Step 1: Understanding the Question:
We need to identify which of the given reactions does not yield a primary amine as the main product.
Step 2: Key Formula or Approach:
We need to know the outcome of several named organic reactions for the synthesis of amines.
- Reduction of amides: LiAlH\(_4\) reduces the carbonyl group (C=O) of an amide to a methylene group (CH\(_2\)).
- Hoffmann bromamide degradation: An amide is treated with Br\(_2\) and KOH to produce a primary amine with one less carbon atom.
- Reduction of nitriles (cyanides): LiAlH\(_4\) reduces the C\(\equiv\)N group to a CH\(_2\)NH\(_2\) group.
- Reduction of isonitriles (isocyanides): LiAlH\(_4\) reduces the N\(\equiv\)C group to an NHCH\(_3\) group.
Step 3: Detailed Explanation:
Let's analyze each reaction:
1. CH\(_3\)CONH\(_2\) + LiAlH\(_4\): This is the reduction of acetamide. The C=O group is reduced to CH\(_2\).
\[ CH_3CONH_2 \xrightarrow{LiAlH_4} CH_3CH_2NH_2 \]
The product is ethylamine, which is a primary amine.
2. CH\(_3\)CONH\(_2\) + Br\(_2\)/KOH: This is the Hoffmann bromamide degradation of acetamide. The amide is converted to a primary amine with one carbon atom less.
\[ CH_3CONH_2 \xrightarrow{Br_2/KOH} CH_3NH_2 \]
The product is methylamine, which is a primary amine.
3. CH\(_3\)CN + LiAlH\(_4\): This is the reduction of acetonitrile (a nitrile). The triple bond is fully reduced.
\[ CH_3CN \xrightarrow{LiAlH_4} CH_3CH_2NH_2 \]
The product is ethylamine, which is a primary amine.
4. CH\(_3\)NC + LiAlH\(_4\): This is the reduction of methyl isocyanide (an isonitrile).
\[ CH_3NC \xrightarrow{LiAlH_4} CH_3NHCH_3 \]
The product is dimethylamine, which is a secondary amine.
Therefore, the reduction of an isonitrile (isocyanide) does not produce a primary amine.
Step 4: Final Answer:
The reaction of CH\(_3\)NC with LiAlH\(_4\) will NOT give a primary amine as the product.
Quick Tip: A key distinction for amine synthesis: - Reduction of \textbf{nitriles} (-C\(\equiv\)N) gives \textbf{primary} amines (-CH\(_2\)NH\(_2\)). - Reduction of \textbf{isonitriles} (-N\(\equiv\)C) gives \textbf{secondary} amines (-NHCH\(_3\)).
Which one of the following statements is correct?
View Solution
Step 1: Understanding the Question:
The question asks to identify the single correct statement among the four options concerning the biological roles and requirements of Magnesium (Mg) and Calcium (Ca).
Step 2: Detailed Explanation:
Let's evaluate each statement:
(1) Mg plays roles in neuromuscular function and interneuronal transmission. This statement is correct. Mg\(^{2+}\) ions act as a physiological antagonist to Ca\(^{2+}\) ions at the neuromuscular junction and are crucial for nerve impulse transmission and muscle relaxation.
(2) The daily requirement of Mg and Ca in the human body is estimated to be 0.2 - 0.3 g. This statement is presented as the correct answer. The value 0.2 - 0.3 g (or 200 - 300 mg) corresponds to the estimated daily requirement for Magnesium. However, the daily requirement for Calcium is much higher, around 1.0 - 1.2 g (1000 - 1200 mg). The phrasing "Mg and Ca" makes the statement scientifically imprecise. However, in the context of multiple-choice questions based on specific textbook lines (like NCERT), which states "The daily requirement in the human body has been estimated to be 200 – 300 mg" in a section discussing both elements, this option may be considered correct by the exam setters.
(3) All enzymes that utilise ATP in phosphate transfer require Ca as the cofactor. This statement is incorrect. The vast majority of enzymes that use ATP for phosphate transfer, such as kinases, require Magnesium (Mg\(^{2+}\)) as a cofactor. Mg\(^{2+}\) forms a complex with ATP (MgATP\(^{2-}\)) which is the true substrate for these enzymes.
(4) The bone in human body is an inert and unchanging substance. This statement is incorrect. Bone is a dynamic, living tissue that is constantly undergoing remodeling (resorption and formation). It also serves as the body's primary reservoir for calcium and phosphate.
Step 3: Final Answer:
While statement (1) is biologically accurate, the provided answer key points to (2). The justification for (2) relies on the fact that the given range (200-300 mg) is the approximate daily requirement for Mg, and the question might be flawed by including Ca. Given the clear incorrectness of options (3) and (4), and the potential ambiguity in interpreting the question's intent for (2), it is selected as the intended answer.
Quick Tip: When answering biology-related chemistry questions, be aware that exam questions can sometimes be based on specific, and occasionally ambiguous, statements from prescribed textbooks. While Ca\(^{2+}\) is famous for muscle contraction and nerve signals, Mg\(^{2+}\) is the key cofactor for almost all ATP-related enzymes.
Amongst the given options which of the following molecules / ion acts as a Lewis acid?
View Solution
Step 1: Understanding the Question:
We need to identify the Lewis acid among the given chemical species.
Step 2: Key Formula or Approach:
According to the Lewis theory of acids and bases:
- A Lewis acid is a species that can accept a pair of electrons. These are typically electron-deficient species (e.g., have an incomplete octet or a positive charge).
- A Lewis base is a species that can donate a pair of electrons. These are typically electron-rich species (e.g., have lone pairs of electrons or a negative charge).
Step 3: Detailed Explanation:
Let's analyze each option:
1. OH\(^-\): The hydroxide ion has a negative charge and lone pairs of electrons on the oxygen atom. It is electron-rich and readily donates an electron pair, making it a strong Lewis base.
2. NH\(_3\): The ammonia molecule has a lone pair of electrons on the nitrogen atom. It can donate this pair to form a coordinate bond, so it acts as a Lewis base.
3. H\(_2\)O: The water molecule has two lone pairs of electrons on the oxygen atom. It can donate one of these pairs, acting as a Lewis base.
4. BF\(_3\): In boron trifluoride, the central boron atom is bonded to three fluorine atoms. Boron has only 3 valence electrons, so after forming 3 bonds, it has only 6 electrons in its valence shell (an incomplete octet). To complete its octet, it has a strong tendency to accept a pair of electrons, making it a classic example of a Lewis acid.
Step 4: Final Answer:
BF\(_3\) is the species that acts as a Lewis acid.
Quick Tip: To quickly identify Lewis acids, look for molecules with central atoms having an incomplete octet (like compounds of B, Al, Be) or simple cations (like H\(^+\), Ag\(^+\)). For Lewis bases, look for anions or molecules with atoms having lone pairs (like compounds of N, O, F, P, S).
Which amongst the following options is correct graphical representation of Boyle's Law?
View Solution
Step 1: Understanding the Question:
We need to identify the graph that correctly represents Boyle's Law, including the effect of temperature on the graph.
Step 2: Key Formula or Approach:
Boyle's Law states that for a fixed mass of an ideal gas at constant temperature, the pressure (P) is inversely proportional to the volume (V). \[ P \propto \frac{1}{V} \quad or \quad PV = constant \, (k) \]
From the ideal gas equation, \(PV = nRT\), the constant \(k = nRT\). This shows that the value of the constant is directly proportional to the absolute temperature (T).
Step 3: Detailed Explanation:
Let's analyze the types of plots for Boyle's Law:
- P vs. V plot: A plot of P versus V should be a rectangular hyperbola. The curves are called isotherms. Since \(k = nRT\), a higher temperature corresponds to an isotherm that is further away from the origin. Graph (2) shows hyperbolas, but the isotherm T\(_3\) (highest temperature) is closest to the origin, which is incorrect.
- P vs. 1/V plot: The relationship is \(P = k \times (\frac{1}{V})\). This is of the form \(y = mx\), which represents a straight line passing through the origin. The slope of the line is \(m = k = nRT\).
- This means the slope of the P vs. 1/V graph is directly proportional to the temperature T.
- A higher temperature will result in a line with a greater slope.
Now let's examine the relevant graphs:
- Graph (3): This shows plots of P vs 1/V. They are straight lines passing through the origin, which is correct. The lines are for temperatures T\(_1\), T\(_2\), and T\(_3\). The slope of the line for T\(_3\) is the steepest, followed by T\(_2\), and then T\(_1\). This corresponds to the relationship \(slope_{T3} > slope_{T2} > slope_{T1}\), which correctly implies \(T_3 > T_2 > T_1\). This graph is a correct representation.
- Graph (4): This is a P vs. 1/V plot, but the curves are hyperbolic, which is incorrect. It should be a straight line.
- Graph (1): This is a P vs T plot, which represents Gay-Lussac's Law (at constant volume).
Step 4: Final Answer:
Graph (3) is the correct graphical representation of Boyle's Law.
Quick Tip: To analyze gas law graphs, always relate the plotted variables to the ideal gas equation \(PV=nRT\). Rearrange the equation to match the form of a straight line (\(y=mx+c\)) if possible. This helps in correctly interpreting the slope and intercept and their dependence on other variables like temperature.
Match List - I with List - II :
List - I & List - II
A. Coke & I. & Carbon atoms are
& & & sp\(^3\) hybridised.
B. Diamond & II. & Used as a dry
& & & lubricant
C. Fullerene & III. & Used as a
& & & reducing agent
D. Graphite & IV. & Cage like
& & & molecules
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question requires matching the substances in List-I (allotropes/forms of carbon) with their corresponding properties or uses in List-II.
Step 2: Detailed Explanation:
Let's analyze each item in List-I and find its correct match in List-II.
A. Coke: Coke is a high-carbon content fuel derived from coal. In metallurgy, particularly in blast furnaces for iron extraction, it acts as a crucial reducing agent, reducing iron oxides to molten iron. Therefore, Coke matches with III. Used as a reducing agent.
B. Diamond: Diamond is an allotrope of carbon where each carbon atom is covalently bonded to four other carbon atoms, forming a tetrahedral lattice. This type of bonding corresponds to sp\(^3\) hybridization. Therefore, Diamond matches with I. Carbon atoms are sp\(^3\) hybridised.
C. Fullerene: Fullerenes are allotropes of carbon that consist of molecules composed entirely of carbon, forming hollow spheres, ellipsoids, or tubes. The most famous example, Buckminsterfullerene (C\(_{60}\)), has a structure resembling a soccer ball, which is a cage-like molecule. Therefore, Fullerene matches with IV. Cage like molecules.
D. Graphite: Graphite is another allotrope of carbon with a layered structure. The layers are held by weak van der Waals forces, allowing them to slide easily over one another. This property makes graphite an excellent solid or dry lubricant. Therefore, Graphite matches with II. Used as a dry lubricant.
Step 3: Final Answer:
Based on the analysis, the correct matching is:
A \(\rightarrow\) III
B \(\rightarrow\) I
C \(\rightarrow\) IV
D \(\rightarrow\) II
This combination corresponds to option (4).
Quick Tip: Remembering the structure and key applications of carbon allotropes is crucial. Diamond's hardness comes from its sp\(^3\) tetrahedral network, Graphite's slipperiness from its sp\(^2\) layered structure, and Fullerenes are known for their unique cage-like shapes.
The element expected to form largest ion to achieve the nearest noble gas configuration is :
View Solution
Step 1: Understanding the Question:
We need to compare the sizes of the stable ions formed by Na, O, F, and N and identify which one is the largest.
Step 2: Key Formula or Approach:
1. First, determine the stable ion each element forms to achieve a noble gas electron configuration.
2. The species formed are Na\(^+\), O\(^{2-}\), F\(^-\), and N\(^{3-}\). Notice that all these ions have 10 electrons, making them an isoelectronic series.
3. For isoelectronic species (ions with the same number of electrons), the ionic radius decreases as the nuclear charge (atomic number, Z) increases. This is because a stronger pull from the nucleus on the same number of electrons contracts the electron cloud.
Step 3: Detailed Explanation:
- Na (Z=11) loses one electron to form Na\(^+\) (10 e\(^-\)).
- O (Z=8) gains two electrons to form O\(^{2-}\) (10 e\(^-\)).
- F (Z=9) gains one electron to form F\(^-\) (10 e\(^-\)).
- N (Z=7) gains three electrons to form N\(^{3-}\) (10 e\(^-\)).
All ions have 10 electrons. We now compare their nuclear charges (Z):
- N: Z=7
- O: Z=8
- F: Z=9
- Na: Z=11
The ion with the lowest nuclear charge will have the weakest attraction for the 10 electrons, resulting in the largest electron cloud and thus the largest ionic radius.
The order of nuclear charge is N < O < F < Na.
Therefore, the order of ionic size will be the reverse: N\(^{3-}\) > O\(^{2-}\) > F\(^-\) > Na\(^+\).
The largest ion is N\(^{3-}\), which is formed from the element Nitrogen (N).
Step 4: Final Answer:
Nitrogen (N) is the element that forms the largest ion among the given options.
Quick Tip: For isoelectronic species, the rule is simple: higher nuclear charge (more protons) = smaller radius. Anions are always larger than their parent atoms, and cations are always smaller.
Given below are two statements :
Statement I : A unit formed by the attachment of a base to 1' position of sugar is known as nucleoside
Statement II : When nucleoside is linked to phosphorous acid at 5'-position of sugar moiety, we get nucleotide.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question presents two statements related to the fundamental components of nucleic acids, nucleosides and nucleotides. We need to evaluate the correctness of each statement.
Step 2: Detailed Explanation:
Analysis of Statement I:
A nucleoside is a glycosylamine that consists of a nitrogenous base (a purine like Adenine or Guanine, or a pyrimidine like Cytosine, Thymine, or Uracil) linked to a sugar (ribose or deoxyribose). The bond forms between the anomeric carbon of the sugar (C1') and a nitrogen atom of the base. This definition matches Statement I exactly.
Therefore, Statement I is true.
Analysis of Statement II:
A nucleotide is formed when a phosphate group is attached to a nucleoside. Specifically, a phosphate group derived from phosphoric acid (H\(_3\)PO\(_4\)) is linked to the 5'-hydroxyl group of the sugar moiety of a nucleoside via a phosphoester bond. Statement II incorrectly states that the linkage is to phosphorous acid (H\(_3\)PO\(_3\)). Phosphorous acid and phosphoric acid are different chemical compounds.
Therefore, Statement II is false.
Step 3: Final Answer:
Since Statement I is true and Statement II is false, the correct option is (4).
Quick Tip: To remember the difference: a nucleo\textbf{s}ide is just \textbf{s}ugar + base. A nucleo\textbf{t}ide has an extra component, the phospha\textbf{t}e group. Pay close attention to chemical names; "phosphoric acid" and "phosphorous acid" are not interchangeable.
Which one is an example of heterogenous catalysis?
View Solution
Step 1: Understanding the Question:
We need to identify the example of heterogeneous catalysis from the given options.
Step 2: Key Formula or Approach:
Catalysis is classified based on the physical phases of the reactants and the catalyst:
- Homogeneous Catalysis: The reactants and the catalyst are in the same phase (e.g., all are gases, or all are in the same liquid solution).
- Heterogeneous Catalysis: The reactants and the catalyst are in different phases (e.g., gaseous reactants and a solid catalyst).
Step 3: Detailed Explanation:
Let's analyze the phases in each reaction:
1. Haber's process for ammonia:
\[ N_2(g) + 3H_2(g) \xrightarrow{Fe(s)} 2NH_3(g) \]
The reactants (N\(_2\), H\(_2\)) are in the gas phase, while the catalyst (iron) is in the solid phase. Since the phases are different, this is an example of heterogeneous catalysis.
2. Lead chamber process for H\(_2\)SO\(_4\):
\[ 2SO_2(g) + O_2(g) \xrightarrow{NO(g)} 2SO_3(g) \]
The reactants (SO\(_2\), O\(_2\)) and the catalyst (NO) are all in the gas phase. This is homogeneous catalysis.
3. Hydrolysis of sugar (sucrose):
\[ C_{12}H_{22}O_{11}(aq) + H_2O(l) \xrightarrow{H^+(aq)} C_6H_{12}O_6(aq) + C_6H_{12}O_6(aq) \]
The reactant (sucrose) and the catalyst (H\(^+\) ions) are both in the aqueous solution phase. This is homogeneous catalysis.
4. Decomposition of ozone:
\[ 2O_3(g) \xrightarrow{NO(g)} 3O_2(g) \]
The reactant (O\(_3\)) and the catalyst (NO) are both in the gas phase. This is homogeneous catalysis.
Step 4: Final Answer:
The formation of ammonia in the Haber's process is the correct example of heterogeneous catalysis.
Quick Tip: Most industrial catalytic processes use heterogeneous catalysts because they are easier to separate from the products, making the process more economical and efficient. Surface catalysis, like in the Haber's process, is a hallmark of heterogeneous catalysis.
The given compound
View Solution
Step 1: Understanding the Question:
The question requires us to classify the given organic halide based on the position of the halogen atom (X) in the molecule.
Step 2: Detailed Explanation:
Let's first define the different types of halides given in the options:
Aryl halide: The halogen atom is directly bonded to an sp\(^2\)-hybridized carbon atom of an aromatic ring.
Vinylic halide: The halogen atom is directly bonded to an sp\(^2\)-hybridized carbon atom of a carbon-carbon double bond.
Benzylic halide: The halogen atom is bonded to an sp\(^3\)-hybridized carbon atom which is directly attached to an aromatic ring.
Allylic halide: The halogen atom is bonded to an sp\(^3\)-hybridized carbon atom which is adjacent to a carbon-carbon double bond.
Now, let's analyze the given structure: \( C_6H_5-CH=CH-\overset{X}{\overset{|}{CH-CH_2CH_3 \)
1. The halogen atom (X) is bonded to a carbon atom.
2. This carbon atom is singly bonded to its neighbours (another C and an H), so it is sp\(^3\)-hybridized.
3. This sp\(^3\)-hybridized carbon atom is directly attached to a carbon atom which is part of a carbon-carbon double bond (\(-CH=\)).
This fits the definition of an allylic halide. The presence of the phenyl group (\(C_6H_5\)) does not change this classification, as the immediate environment of the C-X bond defines the type.
Step 3: Final Answer:
The compound is an example of an allylic halide. Therefore, option (4) is correct.
Quick Tip: To classify organic halides, always focus on the carbon atom directly bonded to the halogen. Check its hybridization (sp\(^3\), sp\(^2\)) and what it is attached to (aromatic ring, C=C double bond, etc.). This systematic check will lead you to the correct classification.
In Lassaigne's extract of an organic compound, both nitrogen and sulphur are present, which gives blood red colour with Fe\(^{3+}\) due to the formation of -
View Solution
Step 1: Understanding the Question:
The question asks to identify the chemical species responsible for the blood-red coloration observed in Lassaigne's test when both nitrogen and sulphur are present in the organic compound.
Step 2: Key Formula or Approach:
Lassaigne's test involves fusing the organic compound with sodium metal to convert covalently bonded elements like N, S, and halogens into ionic sodium salts.
1. If both N and S are present: \(Na + C + N + S \xrightarrow{\Delta} NaSCN\) (Sodium thiocyanate).
2. The sodium extract is then treated with a neutral or slightly acidic solution of Ferric chloride (FeCl\(_3\)), which provides Fe\(^{3+}\) ions.
3. The Fe\(^{3+}\) ions react with the thiocyanate ions (SCN\(^-\)) to form a complex ion which has a characteristic blood-red color.
Step 3: Detailed Explanation:
The reaction sequence is as follows:
- Fusion: Organic Compound (containing C, N, S) + Na \(\rightarrow\) NaSCN
- Test: The aqueous extract containing SCN\(^-\) ions is treated with Fe\(^{3+}\).
\[ Fe^{3+} (aq) + SCN^{-} (aq) \rightarrow [Fe(SCN)(H_2O)_5]^{2+} (aq) \]
This complex ion, ferric thiocyanate (or more accurately, pentaaquathiocyanatoiron(III)), is responsible for the intense blood-red color. For simplicity, it is often written as \([Fe(SCN)]^{2+}\).
Let's analyze the other options:
- (B) \(Fe_4[Fe(CN)_6]_3\): This is Ferric ferrocyanide (Prussian blue), which is formed when only nitrogen is present, not when both N and S are present.
- (C) NaSCN: This is the salt formed in the sodium extract, but it is colorless. The color appears only after reacting with Fe\(^{3+}\).
- (D) \([Fe(CN)_5 NOS]^{4-}\): This is the sodium nitroprusside complex, which is used to test for sulphur (as sulphide ions), not for the combined presence of N and S.
Step 4: Final Answer:
The blood-red color is due to the formation of the \([Fe(SCN)]^{2+}\) complex.
Quick Tip: Remember the characteristic colors in Lassaigne's test: - N only: Prussian blue with Fe\(^{2+}\)/Fe\(^{3+}\). - S only: Violet color with sodium nitroprusside. - N and S together: Blood-red color with Fe\(^{3+}\).
Amongst the given options which of the following molecules / ion acts as a Lewis acid?
View Solution
Step 1: Understanding the Question:
We need to identify the Lewis acid among the given chemical species.
Step 2: Key Formula or Approach:
According to the Lewis theory of acids and bases:
- A Lewis acid is a species that can accept a pair of electrons. These are typically electron-deficient species (e.g., have an incomplete octet or a positive charge).
- A Lewis base is a species that can donate a pair of electrons. These are typically electron-rich species (e.g., have lone pairs of electrons or a negative charge).
Step 3: Detailed Explanation:
Let's analyze each option:
1. OH\(^-\): The hydroxide ion has a negative charge and lone pairs of electrons on the oxygen atom. It is electron-rich and readily donates an electron pair, making it a strong Lewis base.
2. NH\(_3\): The ammonia molecule has a lone pair of electrons on the nitrogen atom. It can donate this pair to form a coordinate bond, so it acts as a Lewis base.
3. H\(_2\)O: The water molecule has two lone pairs of electrons on the oxygen atom. It can donate one of these pairs, acting as a Lewis base.
4. BF\(_3\): In boron trifluoride, the central boron atom is bonded to three fluorine atoms. Boron has only 3 valence electrons, so after forming 3 bonds, it has only 6 electrons in its valence shell (an incomplete octet). To complete its octet, it has a strong tendency to accept a pair of electrons, making it a classic example of a Lewis acid.
Step 4: Final Answer:
BF\(_3\) is the species that acts as a Lewis acid.
Quick Tip: To quickly identify Lewis acids, look for molecules with central atoms having an incomplete octet (like compounds of B, Al, Be) or simple cations (like H\(^+\), Ag\(^+\)). For Lewis bases, look for anions or molecules with atoms having lone pairs (like compounds of N, O, F, P, S).
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : In equation \(\Delta_r G = -nFE_{cell}\), value of \(\Delta_r G\) depends on n.
Reason R : \(E_{cell}\) is an intensive property and \(\Delta_r G\) is an extensive property.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question consists of an Assertion (A) and a Reason (R) related to the thermodynamic properties of an electrochemical cell. We need to determine if A and R are true and if R correctly explains A.
Step 2: Detailed Explanation:
Analysis of Assertion A:
The equation for the Gibbs free energy change of a cell reaction is \(\Delta_r G = -nFE_{cell}\), where:
- \(\Delta_r G\) is the Gibbs free energy change.
- \(n\) is the number of moles of electrons transferred in the balanced redox reaction.
- \(F\) is the Faraday constant (charge per mole of electrons).
- \(E_{cell}\) is the cell potential.
From the equation, it is clear that \(\Delta_r G\) is directly proportional to \(n\). Therefore, the value of \(\Delta_r G\) depends on \(n\). Assertion A is true.
Analysis of Reason R:
- An intensive property does not depend on the amount of matter in the system (e.g., temperature, pressure, density, cell potential \(E_{cell}\)). The potential of a cell is the same regardless of its size.
- An extensive property depends on the amount of matter (e.g., mass, volume, energy, Gibbs free energy \(\Delta_r G\)). The total energy released depends on how many reactants are converted.
So, the statement that \(E_{cell}\) is an intensive property and \(\Delta_r G\) is an extensive property is correct. Reason R is true.
Connecting Reason and Assertion:
The reason why \(\Delta_r G\) depends on \(n\) (the amount of reaction) is precisely because \(\Delta_r G\) is an extensive property. The equation \(\Delta_r G = -nFE_{cell}\) shows how the extensive property (\(\Delta_r G\)) is calculated from the intensive property (\(E_{cell}\)) by multiplying it by factors related to the amount of substance (\(nF\)). Thus, R provides the correct fundamental explanation for A.
Step 3: Final Answer:
Both Assertion A and Reason R are true, and Reason R is the correct explanation for Assertion A. Therefore, option (2) is the correct answer.
Quick Tip: Remember: Intensive properties are intrinsic to the substance (like its color or melting point), while extensive properties depend on how much of it you have (like its mass or total energy). Energy is almost always extensive. Potentials (like voltage) are intensive.
A compound is formed by two elements A and B. The element B forms cubic close packed structure and atoms of A occupy 1/3 of tetrahedral voids. If the formula of the compound is A\(_x\)B\(_y\), then the value of x + y is in option
View Solution
Step 1: Understanding the Question:
We are given information about the crystal lattice of an ionic compound A\(_x\)B\(_y\) and need to determine its empirical formula and then calculate x+y.
Step 2: Key Formula or Approach:
1. In a cubic close-packed (ccp) structure, which is equivalent to a face-centered cubic (fcc) lattice, the effective number of atoms per unit cell is 4.
2. For N atoms forming a close-packed structure, there are N octahedral voids and 2N tetrahedral voids.
3. We will determine the effective number of atoms of A and B in one unit cell and then find their simplest whole-number ratio to get the formula.
Step 3: Detailed Explanation:
- Element B forms the ccp structure. So, the effective number of atoms of B per unit cell is \(N_B = 4\).
- In a ccp lattice with \(N_B = 4\) atoms, the number of tetrahedral voids (TV) is \(2 \times N_B = 2 \times 4 = 8\).
- Atoms of element A occupy 1/3 of these tetrahedral voids.
- So, the effective number of atoms of A per unit cell is \(N_A = \frac{1}{3} \times (Number of TVs) = \frac{1}{3} \times 8 = \frac{8}{3}\).
Now we have the ratio of atoms A : B in the unit cell as:
\[ N_A : N_B = \frac{8}{3} : 4 \]
To get the simplest whole-number ratio, we can multiply both sides by 3:
\[ (\frac{8}{3} \times 3) : (4 \times 3) = 8 : 12 \]
Now, divide by the greatest common divisor, which is 4:
\[ \frac{8}{4} : \frac{12}{4} = 2 : 3 \]
So, the empirical formula of the compound is A\(_2\)B\(_3\).
By comparing this to A\(_x\)B\(_y\), we have \(x = 2\) and \(y = 3\).
The value of \(x + y\) is \(2 + 3 = 5\).
Step 4: Final Answer:
The value of x + y is 5.
Quick Tip: Remember the key numbers for close-packed structures (ccp/fcc and hcp): - Atoms per unit cell (Z) = 4 for fcc/ccp. - Octahedral voids = Z. - Tetrahedral voids = 2Z. Knowing these relationships is essential for solving solid-state stoichiometry problems.
Amongst the following, the total number of species NOT having eight electrons around central atom in its outer most shell, is NH\(_3\), AlCl\(_3\), BeCl\(_2\), CCl\(_4\), PCl\(_5\):
View Solution
Step 1: Understanding the Question:
We need to examine a list of molecules and identify how many of them do not follow the octet rule, meaning the central atom does not have exactly eight valence electrons.
Step 2: Key Formula or Approach:
We will draw the Lewis structure for each molecule and count the total number of electrons (from bonds and lone pairs) around the central atom. A single covalent bond contributes 2 electrons to the valence shell of both atoms involved.
Step 3: Detailed Explanation:
Let's analyze each species:
1. NH\(_3\): The central atom is Nitrogen (N). It forms 3 single bonds with H atoms and has 1 lone pair. Total electrons = (3 bonds \(\times\) 2 e\(^-\)/bond) + 2 e\(^-\) (lone pair) = 6 + 2 = 8 electrons. (Obeys octet rule)
2. AlCl\(_3\): The central atom is Aluminum (Al). It forms 3 single bonds with Cl atoms. Al is in group 13 and has 3 valence electrons. Total electrons = 3 bonds \(\times\) 2 e\(^-\)/bond = 6 electrons. (Incomplete octet, does NOT obey)
3. BeCl\(_2\): The central atom is Beryllium (Be). It forms 2 single bonds with Cl atoms. Be is in group 2 and has 2 valence electrons. Total electrons = 2 bonds \(\times\) 2 e\(^-\)/bond = 4 electrons. (Incomplete octet, does NOT obey)
4. CCl\(_4\): The central atom is Carbon (C). It forms 4 single bonds with Cl atoms. Total electrons = 4 bonds \(\times\) 2 e\(^-\)/bond = 8 electrons. (Obeys octet rule)
5. PCl\(_5\): The central atom is Phosphorus (P). It forms 5 single bonds with Cl atoms. Total electrons = 5 bonds \(\times\) 2 e\(^-\)/bond = 10 electrons. (Expanded octet, does NOT obey)
The species that do not have eight electrons around the central atom are AlCl\(_3\), BeCl\(_2\), and PCl\(_5\).
The total number of such species is 3.
Step 4: Final Answer:
There are 3 species that do not follow the octet rule.
Quick Tip: Exceptions to the octet rule are common. - Incomplete Octet: Often seen with elements like Be (4 e\(^-\)), B, and Al (6 e\(^-\)). - Expanded Octet: Possible for elements in the 3rd period and below (like P, S, Cl, Xe) as they can use their empty d-orbitals for bonding. - Odd-Electron Molecules: Molecules with an odd total number of valence electrons (e.g., NO).
Which of the following statements are NOT correct?
A. Hydrogen is used to reduce heavy metal oxides to metals.
B. Heavy water is used to study reaction mechanism.
C. Hydrogen is used to make saturated fats from oils.
D. The H-H bond dissociation enthalpy is lowest as compared to a single bond between two atoms of any element.
E. Hydrogen reduces oxides of metals that are more active than iron.
Choose the most appropriate answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to identify the statements that are incorrect regarding hydrogen and its compounds.
Step 2: Detailed Explanation:
Let's analyze each statement:
A. Hydrogen is used to reduce heavy metal oxides to metals. This statement is correct. Hydrogen is a good reducing agent and is used in metallurgy to reduce oxides of less reactive metals like copper, lead, and zinc to their respective metals (e.g., \( CuO + H_2 \rightarrow Cu + H_2O \)).
B. Heavy water is used to study reaction mechanism. This statement is correct. Heavy water (D\(_2\)O) is used as a tracer to study the mechanisms of chemical and biological reactions. The different mass of deuterium compared to hydrogen can lead to a kinetic isotope effect, which provides insights into reaction pathways.
C. Hydrogen is used to make saturated fats from oils. This statement is correct. The process, known as hydrogenation, involves adding hydrogen across the double bonds of unsaturated fats (in oils) using a catalyst (like Ni, Pd, or Pt) to produce saturated fats (like vanaspati ghee or margarine).
D. The H-H bond dissociation enthalpy is lowest as compared to a single bond between two atoms of any element. This statement is incorrect. The H-H bond dissociation enthalpy (\( \approx 436 kJ/mol \)) is actually the highest for a single bond between two atoms of any element. For example, the C-C bond enthalpy is \( \approx 348 kJ/mol \), and the F-F bond enthalpy is \( \approx 159 kJ/mol \).
E. Hydrogen reduces oxides of metals that are more active than iron. This statement is incorrect. Hydrogen can only reduce the oxides of metals that are less reactive (less electropositive) than itself. The reactivity series is K \(>\) Na \(>\) Ca \(>\) Mg \(>\) Al \(>\) Zn \(>\) Fe \(>\) H \(>\) Cu \(>\) Ag \(>\) Au. Since metals like Al, Zn, and Fe are more reactive than hydrogen, hydrogen cannot reduce their oxides under standard conditions.
Step 3: Final Answer:
Statements D and E are incorrect. Therefore, the correct option is (4).
Quick Tip: Be careful with keywords like "NOT correct," "incorrect," or "false" in the question stem. After analyzing all statements, re-read the question to ensure you are selecting the incorrect ones. Remember the position of Hydrogen in the electrochemical/reactivity series to answer questions about its reducing properties.
Identify the product in the following reaction:
View Solution
Step 1: Understanding the Question:
We are given a multi-step reaction starting from benzenediazonium chloride and need to identify the final product.
Step 2: Key Formula or Approach:
We need to analyze each step of the reaction sequence:
- Step (i): Sandmeyer Reaction: Reaction of a diazonium salt with cuprous halide (Cu\(_2\)X\(_2\)) in the presence of the corresponding halogen acid (HX). This reaction replaces the diazonium group (\(-N_2^+\)) with a halogen atom (\(-X\)).
- Step (ii): Grignard Reagent Formation: Reaction of an aryl halide with magnesium metal in dry ether.
- Step (iii): Reaction of Grignard Reagent with Water: Grignard reagents are strong bases and react readily with any source of protons (like water) to form a hydrocarbon.
Step 3: Detailed Explanation:
Step (i): The starting material is benzenediazonium chloride. It is treated with Cu\(_2\)Br\(_2\)/HBr. This is a Sandmeyer reaction which will replace the \(-N_2^+Cl^-\) group with a \(-Br\) atom. \[ C_6H_5N_2^+Cl^- \xrightarrow{Cu_2Br_2/HBr} C_6H_5Br + N_2 + CuCl \]
The product of the first step is bromobenzene.
Step (ii): Bromobenzene is treated with Mg in dry ether. This reaction forms a Grignard reagent. \[ C_6H_5Br + Mg \xrightarrow{dry ether} C_6H_5MgBr \]
The product is phenylmagnesium bromide.
Step (iii): Phenylmagnesium bromide is treated with water (H\(_2\)O). The Grignard reagent is a very strong base (the C\(_6\)H\(_5\)\(^-\) part is a carbanion). It will abstract a proton from water. \[ C_6H_5MgBr + H_2O \rightarrow C_6H_6 + Mg(OH)Br \]
The organic product is benzene (C\(_6\)H\(_6\)).
Looking at the options, option (3) represents the structure of benzene.
Step 4: Final Answer:
The final product of the reaction sequence is benzene.
Quick Tip: Grignard reagents are extremely useful but must be handled under anhydrous (dry) conditions. This is because they react with any protic solvent (water, alcohols, etc.) to form the corresponding alkane or arene, which is often an unwanted side reaction but is the main reaction in this specific problem.
The conductivity of centimolar solution of KCl at 25°C is 0.0210 ohm\(^{-1}\) cm\(^{-1}\) and the resistance of the cell containing the solution at 25°C is 60 ohm. The value of cell constant is -
View Solution
Step 1: Understanding the Question:
We are given the conductivity (\(\kappa\)) and resistance (R) of an electrolyte solution in a conductivity cell and asked to calculate the cell constant (\(G^*\)).
Step 2: Key Formula or Approach:
The relationship between conductivity (\(\kappa\)), resistance (R), and the cell constant (\(G^*\)) is given by the formula:
\[ \kappa = \frac{1}{R} \times G^* \]
The cell constant is a property of the conductivity cell, defined as the ratio of the distance between the electrodes (l) to their area of cross-section (A), i.e., \(G^* = l/A\).
Rearranging the formula to find the cell constant:
\[ G^* = \kappa \times R \]
Step 3: Detailed Explanation:
Given values:
- Conductivity, \(\kappa = 0.0210 \, \Omega^{-1} \, cm^{-1}\).
- Resistance, \(R = 60 \, \Omega\).
Substitute these values into the rearranged formula:
\[ G^* = (0.0210 \, \Omega^{-1} \, cm^{-1}) \times (60 \, \Omega) \] \[ G^* = 1.26 \, cm^{-1} \]
The ohm (\(\Omega\)) and inverse ohm (\(\Omega^{-1}\)) units cancel out, leaving the unit for the cell constant as cm\(^{-1}\).
Step 4: Final Answer:
The value of the cell constant is 1.26 cm\(^{-1}\).
Quick Tip: Remember the fundamental relationships in conductivity: - Resistance \(R = \rho \frac{l}{A}\) - Conductance \(G = \frac{1}{R} = \kappa \frac{A}{l}\) - Conductivity \(\kappa = \frac{1}{\rho}\) From these, you can derive the key formula used here: \(\kappa = G \times \frac{l}{A} = \frac{1}{R} \times G^*\).
The number of \(\sigma\) bonds, \(\pi\) bonds and lone pair of electrons in pyridine, respectively are:
View Solution
Step 1: Understanding the Question:
We need to determine the count of sigma bonds, pi bonds, and lone pairs of electrons in a molecule of pyridine.
Step 2: Key Formula or Approach:
First, we must know the structure of pyridine. Pyridine (C\(_5\)H\(_5\)N) is a six-membered heterocyclic aromatic compound, similar to benzene but with one CH group replaced by a nitrogen atom. We then count the bonds and lone pairs directly from the structure.
- Every single bond is one \(\sigma\) bond.
- Every double bond consists of one \(\sigma\) bond and one \(\pi\) bond.
- Every triple bond consists of one \(\sigma\) bond and two \(\pi\) bonds.
- We count the non-bonding valence electron pairs as lone pairs.
Step 3: Detailed Explanation:
The structure of pyridine is a hexagonal ring with alternating double bonds. The ring contains 5 carbon atoms and 1 nitrogen atom. Each of the 5 carbon atoms is bonded to one hydrogen atom.
Let's count the bonds:
1. \(\sigma\) bonds:
- There are 5 C-H single bonds. (5 \(\sigma\) bonds)
- Within the ring, there are bonds between the 6 atoms (4 C-C bonds and 2 C-N bonds). These form the framework of the ring. So there are 6 \(\sigma\) bonds within the ring.
- Total \(\sigma\) bonds = 5 (C-H) + 6 (in-ring) = 11 \(\sigma\) bonds.
2. \(\pi\) bonds:
- Pyridine is an aromatic system, analogous to benzene. There are 3 delocalized \(\pi\) bonds within the ring. ( 3 \(\pi\) bonds).
3. Lone pairs:
- Each carbon atom uses all 4 of its valence electrons in bonding (2 in the ring, 1 with H, 1 in the pi system). So, no lone pairs on carbon.
- The nitrogen atom (Group 15) has 5 valence electrons. It uses one electron for a \(\sigma\) bond with one carbon, one electron for a \(\sigma\) bond with another carbon, and one electron for the \(\pi\) system. This leaves two electrons as a non-bonding pair.
- Total lone pairs = 1 lone pair on the nitrogen atom.
The final count is 11 \(\sigma\) bonds, 3 \(\pi\) bonds, and 1 lone pair.
Step 4: Final Answer:
The number of \(\sigma\) bonds, \(\pi\) bonds, and lone pairs are 11, 3, and 1, respectively.
Quick Tip: For cyclic compounds, a quick way to count \(\sigma\) bonds is to count all the atoms in the molecule and add the number of rings, then subtract 1. Pyridine has 11 atoms (5C, 5H, 1N) and 1 ring. So, \(\sigma\) bonds = (11+1)-1 = 11. This shortcut works for many structures but always double-check by drawing.
Taking stability as the factor, which one of the following represents correct relationship?
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct stability relationship between compounds of Group 13 elements in different oxidation states. This is related to the inert pair effect.
Step 2: Key Formula or Approach:
The inert pair effect is the tendency of the two electrons in the outermost atomic s-orbital to remain unshared or un-ionised in compounds of post-transition metals. This effect becomes more prominent as we move down a group in the p-block.
For Group 13 (B, Al, Ga, In, Tl), the general outer electronic configuration is ns\(^2\)np\(^1\). They can exhibit +3 and +1 oxidation states.
- For lighter elements (Al, Ga, In), the +3 oxidation state is more stable.
- For the heaviest element, Thallium (Tl), the inert pair effect is very strong, making the +1 oxidation state more stable than the +3 oxidation state.
Step 3: Detailed Explanation:
Let's analyze the stability based on the oxidation state of the metal:
1. TlI vs TlI\(_3\): In TlI, Thallium is in the +1 oxidation state. In TlI\(_3\), Thallium is in the +3 oxidation state. Due to the strong inert pair effect, Tl\(^+\) is significantly more stable than Tl\(^{3+}\). Therefore, TlI is more stable than TlI\(_3\). This statement is correct.
2. TlCl\(_3\) vs TlCl: This is the opposite of the first statement. TlCl (+1 state) is more stable than TlCl\(_3\) (+3 state). This statement is incorrect.
3. InI\(_3\) vs InI: For Indium (In), the +3 oxidation state is more stable than the +1 state, although the inert pair effect starts to become noticeable. So, InI\(_3\) should be more stable than InI. The statement is given as InI\(_3\) > InI. This is correct in terms of thermodynamic stability. However, the question asks for the best representation of the trend. The most dramatic and classic example is Thallium. Let's re-evaluate the options. The provided answer key states (1) is correct. Let's assume the question asks for the most pronounced effect.
4. AlCl vs AlCl\(_3\): For Aluminium (Al), the +3 oxidation state is overwhelmingly more stable. AlCl\(_3\) is much more stable than AlCl. This statement is incorrect.
Comparing the options, the most definitive and correct relationship representing the inert pair effect is the superior stability of Tl(+1) compounds over Tl(+3) compounds.
Step 4: Final Answer:
The correct stability relationship is TlI \(>\) TlI\(_3\).
Quick Tip: Remember the stability trend for oxidation states in Group 13, 14, and 15 due to the inert pair effect: - Group 13: Al\(^{3+}\) > Ga\(^{3+}\) > In\(^{3+}\) > Tl\(^{3+}\) (Stability of +3 decreases) - Group 13: Tl\(^+\) > In\(^+\) > Ga\(^+\) > Al\(^+\) (Stability of +1 increases) The same logic applies to Group 14 (+4 vs +2) and Group 15 (+5 vs +3).
Identify product (A) in the following reaction:
A diketone is reacted with Zn-Hg / conc. HCl to give product (A). The diketone is 1-(4-acetylphenyl)cyclohexan-1-one.
View Solution
Step 1: Understanding the Question:
The question asks to identify the product (A) of a reaction involving a diketone with zinc amalgam (Zn-Hg) and concentrated hydrochloric acid (conc. HCl).
Step 2: Key Formula or Approach:
The reagent system, Zn-Hg / conc. HCl, is used for the Clemmensen reduction. This reaction specifically reduces a carbonyl group (C=O) of an aldehyde or a ketone to a methylene group (-CH\(_2\)-), effectively converting the carbonyl compound into an alkane. It does not reduce carboxylic acids or their derivatives and does not affect carbon-carbon double or triple bonds.
\[ R-CO-R' \xrightarrow{Zn-Hg, conc. HCl} R-CH_2-R' \]
Step 3: Detailed Explanation:
The starting material has two ketone groups:
1. An acetyl group (\(-COCH_3\)) attached to the benzene ring.
2. A carbonyl group within the cyclohexanone ring.
The Clemmensen reduction will reduce both of these carbonyl groups.
The acetyl group (\(-C(=O)CH_3\)) will be reduced to an ethyl group (\(-CH_2CH_3\)).
The carbonyl group in the cyclohexanone ring will be reduced to a methylene group (\(-CH_2-\)), converting the cyclohexanone ring into a cyclohexane ring.
The overall transformation is from 1-(4-acetylphenyl)cyclohexan-1-one to 1-ethyl-4-cyclohexylbenzene.
Step 4: Final Answer:
Let's examine the options, which represent the structures shown in the image:
Option (1) corresponds to 1-Ethyl-4-(1-hydroxycyclohexyl)benzene, which is incorrect.
Option (2) corresponds to 1-Cyclohexyl-4-ethylbenzene, the correct product of a double Clemmensen reduction.
Option (3) corresponds to 1-(4-(1-hydroxyethyl)phenyl)cyclohexan-1-ol, which is a diol, a typical result from reagents like NaBH\(_4\) or LiAlH\(_4\), not Clemmensen reduction.
Option (4) shows incorrect reduction products.
Thus, the correct product corresponds to structure (2).
Quick Tip: Recognizing named reactions and their specific reagents is key in organic chemistry. Clemmensen (Zn-Hg/HCl) and Wolff-Kishner (N\(_2\)H\(_4\)/KOH) both reduce C=O to CH\(_2\). Remember that Clemmensen reduction uses acidic conditions, while Wolff-Kishner uses basic conditions. Choose the reagent based on the presence of other acid- or base-sensitive groups in the molecule.
For a certain reaction, the rate = k[A]\(^2\)[B], when the initial concentration of A is tripled keeping concentration of B constant, the initial rate would
View Solution
Step 1: Understanding the Question:
We are given the rate law for a reaction and asked how the initial rate of reaction changes when the concentration of one reactant is changed.
Step 2: Key Formula or Approach:
The rate law is given as: Rate = k[A]\(^2\)[B].
We need to compare the initial rate with the new rate after changing the concentration of A.
Let the initial rate be \(r_1\) and the new rate be \(r_2\).
Step 3: Detailed Explanation:
Initial State:
Let the initial concentrations be [A] and [B].
The initial rate is: \[ r_1 = k[A]^2[B] \]
New State:
The concentration of A is tripled, so the new concentration is [A'] = 3[A].
The concentration of B is kept constant, so [B'] = [B].
The new rate is: \[ r_2 = k[A']^2[B'] = k(3[A])^2[B] \] \[ r_2 = k(9[A]^2)[B] = 9(k[A]^2[B]) \]
By substituting \(r_1 = k[A]^2[B]\), we get: \[ r_2 = 9 \times r_1 \]
This means the new rate is 9 times the initial rate. The rate increases by a factor of nine.
Step 4: Final Answer:
The initial rate would increase by a factor of nine.
Quick Tip: To quickly determine the effect of a concentration change on the reaction rate, look at the order of the reaction with respect to that reactant. If the concentration is changed by a factor of 'x', the rate will change by a factor of 'x\(^n\)', where 'n' is the order with respect to that reactant. Here, [A] is tripled (x=3) and the order is 2, so the rate changes by a factor of 3\(^2\) = 9.
Consider the following reaction and identify the product (P).
\( CH_3-CH(CH_3)-CH(OH)-CH_3 \xrightarrow{HBr} Product (P) \)
3-Methylbutan-2-ol
View Solution
Step 1: Understanding the Question:
The question shows the reaction of a secondary alcohol, 3-methylbutan-2-ol, with hydrogen bromide (HBr). We need to predict the major product (P) of this reaction. This is a nucleophilic substitution reaction.
Step 2: Key Formula or Approach:
The reaction of an alcohol with HBr proceeds via an S\(_N\)1 mechanism, especially for secondary and tertiary alcohols, which involves the formation of a carbocation intermediate. Carbocation intermediates can undergo rearrangement to form a more stable carbocation. The order of carbocation stability is: tertiary (\(3^{\circ}\)) \(>\) secondary (\(2^{\circ}\)) \(>\) primary (\(1^{\circ}\)).
Step 3: Detailed Explanation:
Mechanism:
1. Protonation of the alcohol: The lone pair of electrons on the oxygen atom of the hydroxyl group attacks the proton (H\(^+\)) from HBr, forming a protonated alcohol (an oxonium ion). This makes the hydroxyl group a good leaving group (water).
\[ CH_3-\underset{CH_3}{\underset{|}{CH-\underset{OH}{\underset{|}{CH-CH_3 + H^+ \rightleftharpoons CH_3-\underset{CH_3}{\underset{|}{CH-\underset{OH_2^+}{\underset{|}{CH-CH_3 \]
2. Formation of carbocation: The C-O bond breaks, and the water molecule leaves, resulting in the formation of a secondary (\(2^{\circ}\)) carbocation.
\[ CH_3-\underset{CH_3}{\underset{|}{CH-\underset{OH_2^+}{\underset{|}{CH-CH_3 \rightarrow CH_3-\underset{CH_3}{\underset{|}{CH-\underset{+}{\underset{|}{CH-CH_3 + H_2O \]
(This is 3-methylbutan-2-yl cation, a \(2^{\circ}\) carbocation)
3. Carbocation rearrangement: The secondary carbocation can rearrange to a more stable tertiary (\(3^{\circ}\)) carbocation. A hydrogen atom from the adjacent carbon (C-3) shifts with its pair of electrons to the positively charged carbon (C-2). This is called a 1,2-hydride shift.
\[ \underset{\(2^{\circ\) Carbocation (less stable){CH_3-\underset{H}{\underset{|}{\overset{CH_3}{\overset{|}{C-\overset{+}{C}H-CH_3} \xrightarrow{1,2-Hydride shift} \underset{\(3^{\circ\) Carbocation (more stable){CH_3-\overset{+}{\underset{CH_3}{\underset{|}{C}-CH_2-CH_3} \]
4. Nucleophilic attack: The bromide ion (Br\(^-\)), which is a good nucleophile, attacks the more stable tertiary carbocation to form the final product.
\[ CH_3-\overset{+}{\underset{CH_3}{\underset{|}{C}-CH_2-CH_3 + Br^- \rightarrow CH_3-\underset{Br}{\underset{|}{\overset{CH_3}{\overset{|}{C-CH_2-CH_3 \]
Step 4: Final Answer:
The major product formed is 2-bromo-2-methylbutane. This corresponds to option (2). Option (4) would be the product formed without rearrangement, which is the minor product.
Quick Tip: Whenever a reaction involves a carbocation intermediate (like S\(_N\)1, E1, or acid-catalyzed hydration/dehydration), always check for the possibility of rearrangement (1,2-hydride or 1,2-methyl shift) to form a more stable carbocation. The major product will always arise from the most stable carbocation.
Which amongst the following options is the correct relation between change in enthalpy and change in internal energy?
View Solution
Step 1: Understanding the Question:
The question asks for the fundamental thermodynamic relationship between enthalpy change (\(\Delta\)H) and internal energy change (\(\Delta\)U).
Step 2: Key Formula or Approach:
The definition of enthalpy (H) is given by the equation: \[ H = U + PV \]
where U is the internal energy, P is the pressure, and V is the volume.
Step 3: Detailed Explanation:
For a change in the state of the system, the change in enthalpy (\(\Delta\)H) can be written as: \[ \Delta H = \Delta U + \Delta (PV) \]
For a process occurring at constant pressure, this simplifies to: \[ \Delta H = \Delta U + P\Delta V \]
For chemical reactions involving gases, we often assume they behave ideally. According to the ideal gas law: \[ PV = nRT \]
If the reaction involves a change in the number of moles of gas, \(\Delta n_g\), at constant temperature (T) and pressure (P), then the change in volume is related to the change in moles of gas: \[ P\Delta V = (\Delta n_g)RT \]
Here, \(\Delta n_g\) = (total moles of gaseous products) - (total moles of gaseous reactants).
Substituting this back into the enthalpy equation gives the desired relationship: \[ \Delta H = \Delta U + \Delta n_g RT \]
Step 4: Final Answer:
Comparing this derived equation with the given options, we find that option (3) is the correct relation.
Quick Tip: A simple way to remember the sign in the \(\Delta H = \Delta U + \Delta n_g RT\) equation is to think of enthalpy as the "total heat content". It includes the internal energy (\(\Delta U\)) plus the work the system has to do on the surroundings to make space for itself (\(P\Delta V \approx \Delta n_g RT\)). So, you add the work term to the internal energy.
The equilibrium concentrations of the species in the reaction A + B \(\rightleftharpoons\) C + D are 2, 3, 10 and 6 mol L\(^{-1}\), respectively at 300 K. \(\Delta\)G\(^\circ\) for the reaction is (R = 2 cal / mol K)
View Solution
Step 1: Understanding the Question:
The question provides equilibrium concentrations for a reaction and asks to calculate the standard Gibbs free energy change (\(\Delta\)G\(^\circ\)).
Step 2: Key Formula or Approach:
1. First, calculate the equilibrium constant (\(K_c\)) from the given concentrations.
2. Then, use the thermodynamic relationship between \(\Delta\)G\(^\circ\) and \(K_c\):
\[ \Delta G^\circ = -RT \ln K_c \]
This can also be written as:
\[ \Delta G^\circ = -2.303 RT \log_{10} K_c \]
Step 3: Detailed Explanation:
Calculation of Equilibrium Constant (\(K_c\)):
The reaction is: A + B \(\rightleftharpoons\) C + D
The equilibrium concentrations are:
[A] = 2 mol L\(^{-1}\)
[B] = 3 mol L\(^{-1}\)
[C] = 10 mol L\(^{-1}\)
[D] = 6 mol L\(^{-1}\)
The expression for \(K_c\) is: \[ K_c = \frac{[C][D]}{[A][B]} \] \[ K_c = \frac{(10)(6)}{(2)(3)} = \frac{60}{6} = 10 \]
Calculation of \(\Delta\)G\(^\circ\):
Given values are:
R = 2 cal / mol K
T = 300 K
K\(_c\) = 10
Using the formula: \[ \Delta G^\circ = -RT \ln K_c \] \[ \Delta G^\circ = -(2 cal / mol K) \times (300 K) \times \ln(10) \]
We know that \(\ln(10) \approx 2.303\). \[ \Delta G^\circ = -600 \times 2.303 cal/mol \] \[ \Delta G^\circ = -1381.8 cal/mol \]
Step 4: Final Answer:
The standard Gibbs free energy change for the reaction is -1381.80 cal. This corresponds to option (4).
Quick Tip: Remember the relationship between the sign of \(\Delta G^\circ\) and the value of K. If K > 1, then ln(K) is positive, and \(\Delta G^\circ\) is negative (reaction is spontaneous under standard conditions). If K < 1, then ln(K) is negative, and \(\Delta G^\circ\) is positive. Here K=10, so we expect a negative \(\Delta G^\circ\).
Identify the major product obtained in the following reaction :
View Solution
Step 1: Understanding the Question:
The question asks for the major product of the reaction between the given reactant and Tollens' reagent ([Ag(NH\(_3\))\(_2\)]\(^+\)) in a basic medium, followed by heating. The reactant shown is 1,3-indandione, which has an active methylene group at position 2.
Step 2: Key Formula or Approach:
The reaction appears to be a non-standard oxidation. Tollens' reagent is a mild oxidizing agent. 1,3-Dicarbonyl compounds, like the reactant, have a highly acidic methylene group (\(-CH_2-\)) between the two carbonyls. In the presence of a base (OH\(^-\)), this group can be deprotonated to form an enolate. This enolate might be susceptible to oxidative cleavage. The product shown in the correct option (4) is the salt of 2-acetylbenzoic acid. This product indicates that the five-membered ring has been opened oxidatively.
Step 3: Detailed Explanation:
The question as presented has some inconsistencies, as the conversion of 1,3-indandione to 2-acetylbenzoic acid with Tollens' reagent is not a standard textbook reaction. However, we must deduce the transformation based on the given answer.
Reactant: 1,3-Indandione.
Reagent: Tollens' reagent ([Ag(NH\(_3\))\(_2\)]\(^+\) / OH\(^-\)), which is an oxidizing agent.
Product (from answer key): Salt of 2-acetylbenzoic acid.
This transformation requires the cleavage of one of the C-C bonds in the five-membered ring and rearrangement. Let's assume the active methylene group is first oxidized to a carbonyl group, forming 1,2,3-indantrione. This highly reactive intermediate, in the presence of a strong base, can undergo a benzilic acid-type rearrangement and further cleavage.
The overall transformation can be rationalized as an oxidative ring cleavage of the active methylene compound. The C1-C2 bond breaks, and the C2 and C3 atoms are rearranged and oxidized to form an acetyl group (\(-COCH_3\)) and a carboxylate group (\(-COO^-\)), respectively, attached to the benzene ring.
Although the mechanism is complex and not straightforward, the net result is the conversion of the fused ring system into the ortho-substituted benzene derivative shown in option (4).
Step 4: Final Answer:
Given the options, the reaction represents an oxidative cleavage of the 1,3-dicarbonyl system, leading to the formation of the carboxylate salt of 2-acetylbenzoic acid. This corresponds to structure (4).
Quick Tip: In complex organic reactions where the mechanism is not immediately obvious, analyze the change in the carbon skeleton and functional groups between the reactant and product. Here, a fused ring system is converted to a single ring with two functional groups, indicating a ring-opening reaction, which in this case is oxidative.
Pumice stone is an example of -
View Solution
Step 1: Understanding the Question:
The question asks to classify pumice stone based on the type of colloidal system it represents. A colloidal system is defined by its dispersed phase and dispersion medium.
Step 2: Key Formula or Approach:
Let's define the components of pumice stone and the different types of colloids listed:
Pumice stone: It is a porous volcanic rock formed when super-heated, highly pressurized rock is rapidly ejected from a volcano. The porous texture is due to gas bubbles being trapped in the rock as it cooled. So, the dispersed phase is a gas, and the dispersion medium is a solid.
Foam: Dispersed phase = Gas, Dispersion medium = Liquid. (e.g., whipped cream).
Sol: Dispersed phase = Solid, Dispersion medium = Liquid. (e.g., paint).
Gel: Dispersed phase = Liquid, Dispersion medium = Solid. (e.g., cheese, jelly).
Solid Sol: This term can be used for two types of colloids: Solid in Solid (e.g., colored glass) or Gas in Solid. The latter is more precisely called a "solid foam".
Step 3: Detailed Explanation:
Based on its structure, pumice stone is a system where a gas is dispersed in a solid medium. This type of colloid is technically called a solid foam. However, among the given options, "solid sol" is often used in textbooks as a classification that can include gas-in-solid systems. Given the choices, "solid sol" is the intended answer representing the dispersion of gas bubbles within the solid rock matrix.
Step 4: Final Answer:
Pumice stone is an example of a gas dispersed in a solid. In the context of the given options, this is classified as a solid sol. Therefore, option (4) is correct.
Quick Tip: Memorize the eight types of colloidal systems by creating a table of dispersed phase vs. dispersion medium. Remember key examples for each type. Pumice stone and styrofoam are classic examples of a gas-in-solid colloid, which may be called either a solid foam or a solid sol depending on the classification system used.
On balancing the given redox reaction,
a Cr\(_2\)O\(_7\)\(^{2-}\) + b SO\(_3\)\(^{2-}\)(aq) + c H\(^+\)(aq) \(\rightarrow\) 2a Cr\(^{3+}\)(aq) + b SO\(_4\)\(^{2-}\)(aq) + \(\frac{c}{2}\)H\(_2\)O(l)
the coefficients a, b and c are found to be, respectively -
View Solution
Step 1: Understanding the Question:
The question asks to find the stoichiometric coefficients (a, b, and c) for the given redox reaction in an acidic medium.
Step 2: Key Formula or Approach:
We will use the ion-electron (or half-reaction) method to balance the equation.
Step 3: Detailed Explanation:
1. Identify and balance the half-reactions:
Reduction half-reaction: Dichromate(VI) is reduced to Chromium(III).
\[ Cr_2O_7^{2-} \rightarrow Cr^{3+} \]
- Balance Cr atoms: \( Cr_2O_7^{2-} \rightarrow 2Cr^{3+} \)
- Balance O atoms with H\(_2\)O: \( Cr_2O_7^{2-} \rightarrow 2Cr^{3+} + 7H_2O \)
- Balance H atoms with H\(^+\): \( Cr_2O_7^{2-} + 14H^+ \rightarrow 2Cr^{3+} + 7H_2O \)
- Balance charge with electrons (e\(^-\)): L.H.S charge = (-2) + (+14) = +12. R.H.S charge = 2(+3) = +6. Add 6e\(^-\) to L.H.S.
\[ Cr_2O_7^{2-} + 14H^+ + 6e^- \rightarrow 2Cr^{3+} + 7H_2O \quad \cdots(i) \]
Oxidation half-reaction: Sulfite(IV) is oxidized to Sulfate(VI).
\[ SO_3^{2-} \rightarrow SO_4^{2-} \]
- S atoms are balanced.
- Balance O atoms with H\(_2\)O: \( SO_3^{2-} + H_2O \rightarrow SO_4^{2-} \)
- Balance H atoms with H\(^+\): \( SO_3^{2-} + H_2O \rightarrow SO_4^{2-} + 2H^+ \)
- Balance charge with electrons (e\(^-\)): L.H.S charge = -2. R.H.S charge = (-2) + (+2) = 0. Add 2e\(^-\) to R.H.S.
\[ SO_3^{2-} + H_2O \rightarrow SO_4^{2-} + 2H^+ + 2e^- \quad \cdots(ii) \]
2. Combine the half-reactions:
To make the electrons equal, multiply equation (ii) by 3. \[ 3SO_3^{2-} + 3H_2O \rightarrow 3SO_4^{2-} + 6H^+ + 6e^- \]
Now add this to equation (i): \[ Cr_2O_7^{2-} + 14H^+ + 6e^- + 3SO_3^{2-} + 3H_2O \rightarrow 2Cr^{3+} + 7H_2O + 3SO_4^{2-} + 6H^+ + 6e^- \]
3. Simplify the final equation:
Cancel species that appear on both sides (6e\(^-\), 6H\(^+\), 3H\(_2\)O). \[ Cr_2O_7^{2-} + 3SO_3^{2-} + 8H^+ \rightarrow 2Cr^{3+} + 3SO_4^{2-} + 4H_2O \]
Step 4: Final Answer:
Comparing the balanced equation with the given format `a \(Cr_2O_7^{2-}\) + b \(SO_3^{2-}\) + c H+ ...`, we find:
a = 1
b = 3
c = 8
These coefficients correspond to option (2).
Quick Tip: Always double-check your final balanced redox equation by ensuring that both the atoms of each element and the total charge are balanced on both sides of the reaction. This final check can catch simple arithmetic errors.
What fraction of one edge centred octahedral void lies in one unit cell of fcc?
View Solution
Step 1: Understanding the Question:
The question asks for the contribution of a single octahedral void located at the edge center of a face-centered cubic (fcc) unit cell to that specific unit cell.
Step 2: Key Formula or Approach:
In a crystal lattice, atoms or voids located at different positions (corners, faces, edges, body center) are shared by multiple adjacent unit cells. The contribution of a particle at a specific position to a single unit cell is given by:
Corner: shared by 8 cells, contribution = 1/8
Face center: shared by 2 cells, contribution = 1/2
Edge center: shared by 4 cells, contribution = 1/4
Body center: shared by 1 cell, contribution = 1
Step 3: Detailed Explanation:
In a face-centered cubic (fcc) lattice, there are octahedral voids at two types of locations:
1. One void at the body center of the cube.
2. One void at the center of each of the 12 edges of the cube.
The question specifically asks about an "edge centred octahedral void". An edge of a cubic unit cell is shared by four other unit cells. Therefore, a void located at the center of an edge is also shared by those four unit cells.
The fraction of this void that lies within one particular unit cell is \(\frac{1}{4}\).
Step 4: Final Answer:
The contribution of one edge-centered octahedral void to a single unit cell is \(\frac{1}{4}\). This corresponds to option (4).
Quick Tip: To easily remember contributions, visualize the unit cell in a 3D lattice. An edge is a line shared by the 4 cubes that meet at that line. Anything in the middle of that edge must also be shared by those 4 cubes.
Given below are two statements :
Statement I : The nutrient deficient water bodies lead to eutrophication.
Statement II : Eutrophication leads to decrease in the level of oxygen in the water bodies.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to evaluate two statements about eutrophication, a process of water pollution, and determine their correctness.
Step 2: Detailed Explanation:
Analysis of Statement I:
"The nutrient deficient water bodies lead to eutrophication."
This statement is incorrect. Eutrophication is the process of nutrient enrichment of a water body. It is caused by an excess of nutrients, particularly nitrates and phosphates, from sources like agricultural runoff (fertilizers) and sewage. Nutrient deficiency describes an oligotrophic water body, which is the opposite of a eutrophic one.
Analysis of Statement II:
"Eutrophication leads to decrease in the level of oxygen in the water bodies."
This statement is correct. The excess nutrients in a eutrophic water body cause a massive growth of algae and other aquatic plants, a phenomenon known as an algal bloom. When this large mass of algae dies, it sinks to the bottom and is decomposed by aerobic bacteria. This decomposition process consumes large amounts of dissolved oxygen from the water. The resulting depletion of oxygen (hypoxia or anoxia) can lead to the death of fish and other aquatic organisms.
Step 3: Final Answer:
Based on the analysis, Statement I is incorrect, and Statement II is true. This corresponds to option (1).
Quick Tip: Remember that "eu-" is a prefix meaning "good" or "well," and "trophic" relates to nutrition. So, eutrophication literally means "well-nourished," implying an excess of nutrients, not a deficiency. This excess leads to a cascade of negative effects, including oxygen depletion.
Which amongst the following will be most readily dehydrated under acidic conditions ?
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given alcohols will undergo dehydration most easily (i.e., at the fastest rate) under acidic conditions.
Step 2: Key Formula or Approach:
The acid-catalyzed dehydration of alcohols typically proceeds via an E1 mechanism. The mechanism involves three steps:
1. Protonation of the hydroxyl group.
2. Loss of a water molecule to form a carbocation intermediate. This is the rate-determining step.
3. Deprotonation of an adjacent carbon to form an alkene.
The rate of the reaction is determined by the stability of the carbocation intermediate formed in the second step. The more stable the carbocation, the lower the activation energy, and the faster the reaction.
Step 3: Detailed Explanation:
Let's analyze the stability of the carbocation formed from each alcohol:
(1) Dehydration of 4-nitrohexan-2-ol forms a secondary carbocation. The electron-withdrawing nitro (-NO\(_2\)) group is far away (at C4), so its destabilizing inductive effect is weak.
(2) Dehydration of 3-methyl-4-nitropentan-2-ol forms a secondary carbocation. Here, the electron-withdrawing -NO\(_2\) group is closer (at C4), exerting a stronger destabilizing inductive (-I) effect on the carbocation at C2.
(3) Dehydration of butane-2,3-diol. Let's consider the dehydration of the OH at C2. It forms a secondary carbocation at C2: CH\(_3\)-C\(^+\)H-CH(OH)-CH\(_3\). This carbocation is exceptionally stable because the lone pair of electrons on the oxygen atom of the adjacent hydroxyl group can donate electron density through resonance, which is a very powerful stabilizing effect.
(4) Dehydration of 1-nitropropan-2,3-diol. Dehydration of the secondary OH at C2 would form a carbocation directly adjacent to the carbon bearing the very strong electron-withdrawing -NO\(_2\) group. This would be an extremely unstable carbocation.
Comparing the stabilities, the carbocation formed from alcohol (3) is by far the most stable due to resonance stabilization from the adjacent -OH group. Therefore, this alcohol will be dehydrated most readily.
Step 4: Final Answer:
Alcohol (3), butane-2,3-diol, forms the most stable carbocation intermediate, and thus will be dehydrated most readily. This corresponds to option (3).
Quick Tip: When assessing carbocation stability, remember the order of stabilizing effects: Resonance > Hyperconjugation > Inductive effect. A carbocation adjacent to an atom with a lone pair (like O or N) is significantly stabilized by resonance. Conversely, a carbocation near a strong electron-withdrawing group (like -NO\(_2\)) is significantly destabilized.
Match List - I with List - II :
List - I (Oxoacids of Sulphur) & List - II (Bonds)
A. Peroxodisulphuric acid & I. & Two S-OH, Four S=O,
& & & One S-O-S
B. Sulphuric acid & II. & Two S-OH, One S=O
C. Pyrosulphuric acid & III. & Two S-OH, Four S=O,
& & & One S-O-O-S
D. Sulphurous acid & IV. & Two S-OH, Two S=O
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching four oxoacids of sulfur with the correct description of the chemical bonds present in their structures.
Step 2: Key Formula or Approach:
To solve this, one needs to know the molecular structures of the given oxoacids.
Step 3: Detailed Explanation:
Let's determine the structure and bonds for each acid in List-I:
A. Peroxodisulphuric acid (H\(_2\)S\(_2\)O\(_8\)): Also known as Marshall's acid. Its structure is HO-SO\(_2\)-O-O-SO\(_2\)-OH. It contains a peroxide linkage (-O-O-). Counting the bonds:
Two S-OH single bonds.
Four S=O double bonds.
One S-O-O-S linkage.
This matches with description III in List-II. So, A-III.
B. Sulphuric acid (H\(_2\)SO\(_4\)): Its structure is HO-SO\(_2\)-OH. Counting the bonds:
Two S-OH single bonds.
Two S=O double bonds.
This matches with description IV in List-II. So, B-IV.
C. Pyrosulphuric acid (H\(_2\)S\(_2\)O\(_7\)): Also known as oleum. Its structure is HO-SO\(_2\)-O-SO\(_2\)-OH. It contains an S-O-S linkage. Counting the bonds:
Two S-OH single bonds.
Four S=O double bonds.
One S-O-S linkage.
This matches with description I in List-II. So, C-I.
D. Sulphurous acid (H\(_2\)SO\(_3\)): Its structure is HO-SO-OH, with a lone pair on the sulfur atom. Counting the bonds:
Two S-OH single bonds.
One S=O double bond.
This matches with description II in List-II. So, D-II.
Step 4: Final Answer:
The correct set of matches is A-III, B-IV, C-I, D-II. This corresponds to option (3).
Quick Tip: Being able to draw the structures of common oxoacids (of sulfur, phosphorus, chlorine) is a very useful skill for competitive exams. Key structural features to remember are peroxide linkages (-O-O-) in "peroxo" acids and direct M-O-M linkages in "pyro" acids.
Identify the final product [D] obtained in the following sequence of reactions.
View Solution
Step 1: Understanding the Question:
The question presents a multi-step reaction sequence and asks for the structure of the final product [D].
Step 2: Key Formula or Approach:
We need to identify the product of each step in the sequence. The key reactions are reduction of an aldehyde, dehydration of an alcohol, addition of HBr to an alkene, and a Wurtz-Fittig reaction.
Step 3: Detailed Explanation:
Step 1: CH\(_3\)CHO \(\xrightarrow{i) LiAlH_4 ii) H_3O^+}\) [A]
Lithium aluminium hydride (LiAlH\(_4\)) is a strong reducing agent that reduces the aldehyde ethanal (CH\(_3\)CHO) to a primary alcohol.
\[ [A] is CH_3CH_2OH (Ethanol) \]
Step 2: [A] \(\xrightarrow{H_2SO_4, \Delta}\) [B]
Ethanol is dehydrated by concentrated sulfuric acid upon heating to form an alkene.
\[ [B] is CH_2=CH_2 (Ethene) \]
Step 3: [B] \(\xrightarrow{HBr}\) [C]
Ethene undergoes electrophilic addition with hydrogen bromide.
\[ [C] is CH_3CH_2Br (Bromoethane) \]
Step 4: [C] + Bromobenzene \(\xrightarrow{Na/dry ether}\) [D]
This is a Wurtz-Fittig reaction. Bromoethane (an alkyl halide) reacts with bromobenzene (an aryl halide) and sodium metal in dry ether to form an alkylbenzene. The ethyl group from bromoethane attaches to the phenyl ring from bromobenzene.
\[ CH_3CH_2Br + C_6H_5Br + 2Na \xrightarrow{dry ether} C_6H_5-CH_2CH_3 + 2NaBr \]
\[ [D] is Ethylbenzene \]
Step 4: Final Answer:
The final product [D] is ethylbenzene, which is represented by the structure in option (2).
Quick Tip: Pay close attention to the layout of reaction schemes. The reaction of an alkyl halide and an aryl halide with sodium in ether is a specific named reaction called the Wurtz-Fittig reaction, which is used to synthesize alkylbenzenes.
Which complex compound is most stable?
View Solution
Step 1: Understanding the Question:
The question asks to identify the most stable coordination compound from the given list. The stability of a complex is a key concept in coordination chemistry.
Step 2: Key Formula or Approach:
A major factor contributing to the stability of coordination complexes is the chelate effect. The chelate effect states that complexes formed by polydentate ligands (ligands that can bind to the central metal ion through more than one donor atom, forming a ring) are significantly more stable than complexes with analogous monodentate ligands.
Step 3: Detailed Explanation:
Let's analyze the ligands present in each complex:
(1) [Co(NH\(_3\))\(_6\)]\(_2\)(SO\(_4\))\(_3\): The ligand is ammonia (NH\(_3\)), which is a monodentate ligand. It does not form a chelate ring.
(2) [Co(NH\(_3\))\(_4\)(H\(_2\)O)Br](NO\(_3\))\(_2\): The ligands are ammonia (NH\(_3\)), water (H\(_2\)O), and bromide (Br\(^-\)). All are monodentate ligands. No chelation occurs.
(3) [Co(NH\(_3\))\(_3\)(NO\(_3\))\(_3\): The ligands are ammonia (NH\(_3\)) and nitrate (NO\(_3\)\(^{-}\)). Both are acting as monodentate ligands here. No chelation occurs.
(4) [CoCl\(_2\)(en)\(_2\)]NO\(_3\): The ligands are chloride (Cl\(^-\)) and ethylenediamine (en). Ethylenediamine (H\(_2\)N-CH\(_2\)-CH\(_2\)-NH\(_2\)) is a bidentate ligand. It binds to the cobalt ion through its two nitrogen atoms, forming a stable five-membered chelate ring.
Because the complex in option (4) contains a chelating ligand (ethylenediamine), it benefits from the chelate effect, which leads to a large increase in thermodynamic stability compared to the other complexes which only contain monodentate ligands.
Step 4: Final Answer:
The complex [CoCl\(_2\)(en)\(_2\)]NO\(_3\) is the most stable due to the chelate effect. This corresponds to option (4).
Quick Tip: When asked to compare the stability of complexes, the first thing to look for is the presence of chelating (polydentate) ligands like 'en', 'edta', or 'ox'. Complexes with these ligands are almost always more stable than those without them.
Consider the following reaction :
Identify products A and B.
View Solution
Step 1: Understanding the Question:
The question asks to predict the products (A and B) of the reaction between benzyl phenyl ether and hydrogen iodide (HI) with heat. This is a classic ether cleavage reaction.
Step 2: Key Formula or Approach:
The cleavage of ethers by hydrogen halides (like HI or HBr) follows specific rules:
1. The oxygen atom of the ether is first protonated by the acid.
2. The halide ion (I\(^-\)) then acts as a nucleophile and attacks one of the carbon atoms attached to the oxygen, displacing the other part of the molecule.
3. The crucial rule for mixed ethers (with different alkyl/aryl groups) is that an aryl-oxygen bond (C\(_aryl\)-O) is very strong due to resonance and the sp\(^2\) character of the carbon, and it does not break. The cleavage always occurs at the alkyl-oxygen bond.
Step 3: Detailed Explanation:
The starting material is benzyl phenyl ether: \( C_6H_5-O-CH_2-C_6H_5 \).
The oxygen is bonded to a phenyl group (\(C_6H_5\)) and a benzyl group (\(-CH_2-C_6H_5\)).
As per the rule, the C\(_phenyl\)-O bond will not break.
Therefore, cleavage must occur at the O-\(CH_2\) (benzyl) bond.
The iodide ion (I\(^-\)) will attack the benzylic carbon (\(-CH_2-\)).
The other fragment will be the phenoxide ion, which will be protonated by the acid to form phenol.
The overall reaction is: \[ C_6H_5-O-CH_2-C_6H_5 + HI \xrightarrow{\Delta} C_6H_5-OH + I-CH_2-C_6H_5 \]
So, the products A and B are phenol and benzyl iodide.
Step 4: Final Answer:
Matching the products with the given options, we find that option (4) correctly identifies A as benzyl iodide (\(CH_2I\) attached to a benzene ring) and B as phenol (OH attached to a benzene ring).
Quick Tip: A key rule for ether cleavage with HX: the bond between an sp\(^2\) carbon (from a phenyl or vinyl group) and the ether oxygen is never broken. This means that if a phenyl group is present, phenol will always be one of the products.
Which of the following statements are INCORRECT?
A. All the transition metals except scandium form MO oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc\(_2\)O\(_3\) to Mn\(_2\)O\(_7\).
C. Basic character increases from V\(_2\)O\(_3\) to V\(_2\)O\(_4\) to V\(_2\)O\(_5\).
D. V\(_2\)O\(_4\) dissolves in acids to give VO\(^{3+}\) salts.
E. CrO is basic but Cr\(_2\)O\(_3\) is amphoteric.
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question requires us to identify the incorrect statements from a list of five statements about the properties of transition metals and their oxides.
Step 2: Detailed Explanation:
Let's analyze each statement:
A. All the transition metals except scandium form MO oxides which are ionic. This is a broad generalization and is not entirely correct. While many MO oxides (like FeO, MnO) are ionic, others (like ZnO) have significant covalent character. Also, higher oxides are covalent. So, this statement is factually weak but let's evaluate others which might be more clearly incorrect.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc\(_2\)O\(_3\) to Mn\(_2\)O\(_7\). This statement is correct. Sc (Group 3) shows +3. Ti (Group 4) shows +4 (in TiO\(_2\)). V (Group 5) shows +5 (in V\(_2\)O\(_5\)). Cr (Group 6) shows +6 (in CrO\(_3\)). Mn (Group 7) shows +7 (in Mn\(_2\)O\(_7\)). This trend holds true up to manganese.
C. Basic character increases from V\(_2\)O\(_3\) to V\(_2\)O\(_4\) to V\(_2\)O\(_5\). This statement is incorrect. The acidic character of metal oxides increases with an increase in the oxidation state of the metal. For vanadium oxides: V\(_2\)O\(_3\) (+3) is basic, V\(_2\)O\(_4\) (+4) is amphoteric, and V\(_2\)O\(_5\) (+5) is acidic. Thus, the basic character decreases, not increases.
D. V\(_2\)O\(_4\) dissolves in acids to give VO\(^{3+}\) salts. This statement is incorrect. V\(_2\)O\(_4\) contains vanadium in the +4 oxidation state. When it dissolves in acid, it forms the vanadyl ion, which is VO\(^{2+}\), not VO\(^{3+}\). The VO\(^{3+}\) ion would correspond to a +5 oxidation state.
E. CrO is basic but Cr\(_2\)O\(_3\) is amphoteric. This statement is correct. Following the trend with oxidation states, CrO (+2) is basic, Cr\(_2\)O\(_3\) (+3) is amphoteric, and CrO\(_3\) (+6) is acidic.
Step 3: Final Answer:
The incorrect statements are C and D. Therefore, the correct option is (4).
Quick Tip: A crucial trend for metal oxides is that their acidity increases with the oxidation state of the metal. Low oxidation states yield basic oxides, intermediate states yield amphoteric oxides, and high oxidation states yield acidic oxides.
Consider the following compounds/species:
The number of compounds/species which obey Huckel's rule is _____________.
View Solution
Step 1: Understanding the Question:
The question asks us to count how many of the given seven species are aromatic based on Hückel's rule.
Step 2: Key Formula or Approach:
Hückel's rule states that for a species to be aromatic, it must satisfy four conditions:
1. It must be cyclic.
2. It must be planar.
3. It must be completely conjugated (every atom in the ring must have a p-orbital).
4. It must contain (4n + 2) \(\pi\) electrons, where n is a non-negative integer (0, 1, 2, ...).
Step 3: Detailed Explanation:
Let's analyze each species:
i. Naphthalene: It is cyclic, planar, and fully conjugated. It has 10 \(\pi\) electrons. For 4n + 2 = 10, 4n = 8, so n = 2. It obeys Hückel's rule. (Aromatic)
ii. Cyclopentadienyl anion: It is cyclic, planar, and fully conjugated. It has 6 \(\pi\) electrons (4 from double bonds, 2 from the lone pair/negative charge). For 4n + 2 = 6, 4n = 4, so n = 1. It obeys Hückel's rule. (Aromatic)
iii. Cyclopropenyl cation: It is a three-membered ring with a positive charge. It is cyclic, planar, and fully conjugated. It has 2 \(\pi\) electrons. For 4n + 2 = 2, 4n = 0, so n = 0. It obeys Hückel's rule. (Aromatic)
iv. Bicyclo[1.1.0]butane: This is a bicyclic, non-planar molecule. It is not aromatic. (Non-aromatic)
v. Cyclopropenyl cation: This appears to be the same as species iii. Assuming it's a distinct species intended, like cyclobutadiene, which has 4\(\pi\) electrons (anti-aromatic), or some other non-aromatic species, it doesn't add to the count. Let's assume it is just a repeated structure.
vi. Cyclooctatetraene (COT): It is cyclic and has 8 \(\pi\) electrons (a 4n system, where n=2). To avoid the instability of being anti-aromatic, it adopts a non-planar, tub-like shape. Since it's not planar, it is not aromatic. (Non-aromatic)
vii. Anthracene: It is cyclic, planar, and fully conjugated. It has 14 \(\pi\) electrons. For 4n + 2 = 14, 4n = 12, so n = 3. It obeys Hückel's rule. (Aromatic)
Step 4: Final Answer:
The species that are aromatic are i, ii, iii, and vii. Counting these, we find there are 4 aromatic species. This corresponds to option (2).
Quick Tip: When applying Hückel's rule, remember to check all four criteria: Cyclic, Planar, Conjugated, and (4n+2) \(\pi\) electrons. A species failing even one criterion is not aromatic. Common non-aromatic examples include molecules with sp\(^3\) carbons in the ring or non-planar systems like Cyclooctatetraene.
The reaction that does NOT take place in a blast furnace between 900 K to 1500 K temperature range during extraction of iron is :
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given chemical reactions does not occur in the higher temperature zone (900 K - 1500 K) of a blast furnace used for iron extraction.
Step 2: Key Formula or Approach:
The blast furnace has different temperature zones, and specific reactions occur in each zone.
Lower Temperature Zone (500 K - 800 K): This is the upper part of the furnace. Here, the iron oxides are reduced by carbon monoxide.
Higher Temperature Zone (900 K - 1500 K): This is the lower part of the furnace. Here, the final reduction of iron oxide occurs, and slag is formed. Also, the reducing agent CO is regenerated.
Step 3: Detailed Explanation:
Let's analyze the reactions based on the temperature zones:
(1) CaO + SiO\(_2\) \(\rightarrow\) CaSiO\(_3\): This is the formation of slag (calcium silicate). The limestone (CaCO\(_3\)) decomposes to CaO at high temperatures, which then reacts with the silica (SiO\(_2\)) impurity. This process occurs at about 1200 K, which is within the 900 K - 1500 K range.
(2) Fe\(_2\)O\(_3\) + CO \(\rightarrow\) 2FeO + CO\(_2\): This is one of the initial reduction steps of hematite ore. This reaction takes place in the upper part of the furnace at lower temperatures, typically around 500 K - 800 K. Therefore, it does NOT occur in the 900 K - 1500 K range.
(3) FeO + CO \(\rightarrow\) Fe + CO\(_2\): This is the final reduction step where iron(II) oxide is reduced to molten iron. This occurs at higher temperatures, around 1075 K, well within the specified range.
(4) C + CO\(_2\) \(\rightarrow\) 2CO: This is the Boudouard reaction, where hot coke reduces carbon dioxide to produce carbon monoxide, the main reducing agent. This reaction is favored at high temperatures (above 1075 K) and occurs in the lower part of the furnace.
Step 4: Final Answer:
The reaction Fe\(_2\)O\(_3\) + CO \(\rightarrow\) 2FeO + CO\(_2\) occurs at lower temperatures and not in the 900 K - 1500 K range. Hence, option (2) is the correct answer.
Quick Tip: Remember the temperature gradient in a blast furnace is hottest at the bottom and cooler at the top. The initial reduction of the ore (Fe\(_2\)O\(_3\)) happens at the top (cooler region), while the final reduction to Fe and slag formation happens at the bottom (hotter region).
Which micronutrient is required for splitting of water molecule during photosynthesis?
View Solution
Step 1: Understanding the Question:
The question asks to identify the specific micronutrient that plays a crucial role in the photolysis (splitting) of water during the light-dependent reactions of photosynthesis.
Step 3: Detailed Explanation:
The splitting of water molecules occurs during the light-dependent reactions of photosynthesis, a process known as photolysis.
This reaction takes place in Photosystem II (PS II) and is catalyzed by the Oxygen Evolving Complex (OEC).
The OEC is a metalloenzyme complex that contains a cluster of four manganese ions (\(Mn\)) and one calcium ion (\(Ca^{2+}\)), which are essential for its catalytic activity.
The overall reaction is: \[ 2H_2O \rightarrow 4H^+ + 4e^- + O_2 \]
Magnesium (\(Mg^{2+}\)) is a central atom of the chlorophyll molecule, but it is not directly involved in water splitting.
Copper and molybdenum are also essential micronutrients but have different roles in plant metabolism.
Step 4: Final Answer:
Therefore, manganese is the correct micronutrient required for the splitting of the water molecule.
Quick Tip: Remember the key roles of important micronutrients in photosynthesis: Manganese (Mn) is for photolysis of water, and Magnesium (Mg) is the central atom in chlorophyll. Don't confuse the two.
Unequivocal proof that DNA is the genetic material was first proposed by
View Solution
Step 1: Understanding the Question:
The question asks to identify the scientist(s) who provided the definitive, or "unequivocal," proof that DNA, and not protein, is the genetic material.
Step 3: Detailed Explanation:
Frederick Griffith (1928): His experiment on Streptococcus pneumoniae demonstrated the "transforming principle" but did not identify what it was.
Avery, Macleoid, and McCarthy (1944): They expanded on Griffith's work and showed that DNA was the transforming principle. However, their findings were not universally accepted by the scientific community at the time.
Alfred Hershey and Martha Chase (1952): They conducted the "blender experiment" using bacteriophages (viruses that infect bacteria). They radioactively labeled the DNA of the phage with \(^{32}P\) and the protein coat with \(^{35}S\). They found that only the radioactive DNA entered the bacterial cells, providing clear and unequivocal evidence that DNA is the genetic material.
Wilkins and Franklin: Their X-ray diffraction work was crucial for determining the structure of DNA, but did not prove it was the genetic material.
Step 4: Final Answer:
The Hershey-Chase experiment is considered the conclusive proof that DNA is the genetic material.
Quick Tip: Remember the timeline and contribution of each experiment: Griffith discovered transformation, Avery et al. identified the transforming substance as DNA, and Hershey-Chase provided the definitive proof using radioactive tracers.
Given below are two statements :
Statement I: Endarch and exarch are the terms often used for describing the position of secondary xylem in the plant body.
Statement II: Exarch condition is the most common feature of the root system.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question presents two statements about vascular tissue arrangement in plants and asks to evaluate their correctness.
Step 3: Detailed Explanation:
Statement I Analysis:
The terms 'endarch' and 'exarch' describe the arrangement of primary xylem, not secondary xylem.
Endarch: Protoxylem (the first formed primary xylem) is towards the center (pith), and metaxylem is towards the periphery. This is characteristic of stems.
Exarch: Protoxylem is towards the periphery, and metaxylem is towards the center. This is characteristic of roots.
Since the statement refers to secondary xylem, Statement I is incorrect.
Statement II Analysis:
As explained above, the exarch condition, where the protoxylem is located on the outer side and metaxylem on the inner side, is the defining characteristic of the vascular bundles in the root system of plants. Therefore, Statement II is true.
Step 4: Final Answer:
Statement I is incorrect, and Statement II is true.
Quick Tip: Remember: "Ex" in Exarch is like "Exit" (outward), which helps recall that protoxylem is towards the periphery, a feature of roots. "En" in Endarch is like "Enter" (inward), for protoxylem towards the center, a feature of stems.
Cellulose does not form blue colour with Iodine because
View Solution
Step 1: Understanding the Question:
The question asks for the reason why cellulose does not give a positive iodine test (i.e., does not turn blue), unlike starch.
Step 3: Detailed Explanation:
The iodine test for starch is based on the ability of the starch molecule to trap iodine molecules within its structure.
Starch consists of two components: amylose and amylopectin. Amylose has a helical secondary structure. The iodine molecules (\(I_2\)) fit perfectly inside these helices, forming a charge-transfer complex that appears blue-black.
Cellulose, on the other hand, is a polysaccharide made of \(\beta\)-glucose units linked by \(\beta\)-1,4 glycosidic bonds. This results in a straight, linear chain structure. These chains are arranged in parallel to form fibrils.
Cellulose does not form the complex helical structures found in amylose. Without these helices, there is no space for the iodine molecules to be trapped. Therefore, no blue colour is produced.
Option (B) is incorrect because cellulose is a polysaccharide, not a disaccharide.
Step 4: Final Answer:
Cellulose has a linear structure without complex helices, so it cannot hold iodine molecules to produce the characteristic blue colour.
Quick Tip: Associate the helical structure with the iodine test. Starch (specifically amylose) is helical and turns blue with iodine. Cellulose is linear and does not. This structural difference is key.
Family Fabaceae differs from Solanaceae and Liliaceae. With respect to the stamens, pick out the characteristics specific to family Fabaceae but not found in Solanaceae or Liliaceae.
View Solution
Step 1: Understanding the Question:
The question asks to identify a characteristic of stamens that is unique to the family Fabaceae when compared to Solanaceae and Liliaceae.
Step 3: Detailed Explanation:
Let's analyze the characteristics of stamens in these families:
Fabaceae (Pea family): The stamens are typically ten. A key feature is that they are often diadelphous, meaning they are fused into two bundles (commonly in a (9)+1 arrangement, where 9 are fused into a tube and one is free). The anthers are dithecous (having two lobes).
Solanaceae (Potato family): Stamens are typically five and epipetalous (fused to the petals). Anthers are dithecous.
Liliaceae (Lily family): Stamens are typically six (3+3) and can be epiphyllous or epitepalous (fused to the tepals). Anthers are dithecous.
Comparing the options:
(A) Epiphyllous condition is found in Liliaceae.
(B) Diadelphous condition is characteristic of Fabaceae. Dithecous anthers are common to all three, but the diadelphous arrangement is specific to Fabaceae among the choices.
(C) Polyadelphous (fused into more than two bundles) is found in families like Rutaceae (Citrus). Epipetalous is found in Solanaceae.
(D) Monoadelphous (fused into one bundle) is found in Malvaceae (China rose). Monothecous anthers are also found in Malvaceae.
Step 4: Final Answer:
The diadelphous condition of stamens is a specific characteristic of the family Fabaceae that distinguishes it from Solanaceae and Liliaceae.
Quick Tip: Memorize the key floral characteristics for major plant families. For Fabaceae, remember the butterfly-like (papilionaceous) corolla and the diadelphous ((9)+1) stamen arrangement.
The reaction centre in PS II has an absorption maxima at
View Solution
Step 1: Understanding the Question:
The question asks for the specific wavelength of light at which the reaction center of Photosystem II (PS II) shows its maximum absorption.
Step 3: Detailed Explanation:
In photosynthesis, there are two photosystems, Photosystem I (PS I) and Photosystem II (PS II). Each photosystem consists of a reaction center surrounded by light-harvesting complexes.
The reaction center is a special pair of chlorophyll 'a' molecules that can become photo-oxidized.
The reaction center of Photosystem II (PS II) absorbs light most effectively at a wavelength of 680 nm and is therefore called P680.
The reaction center of Photosystem I (PS I) absorbs light most effectively at a wavelength of 700 nm and is therefore called P700.
Step 4: Final Answer:
Based on this information, the reaction centre in PS II has an absorption maximum at 680 nm.
Quick Tip: A simple way to remember is that the photosystems are named in the order of their discovery, not the order they function in the Z-scheme. PS II (P680) comes before PS I (P700) in the electron flow. Remember II comes before I, and 680 comes before 700.
Upon exposure to UV radiation, DNA stained with ethidium bromide will show
View Solution
Step 1: Understanding the Question:
The question asks about the appearance of DNA when it is stained with ethidium bromide (EtBr) and then viewed under ultraviolet (UV) radiation. This is a standard technique in molecular biology.
Step 3: Detailed Explanation:
Ethidium bromide (EtBr) is a fluorescent dye that is commonly used as a stain for visualizing nucleic acids (DNA and RNA) in molecular biology laboratories.
EtBr works by intercalating, or inserting itself, between the base pairs of the DNA double helix.
When this DNA-EtBr complex is exposed to ultraviolet (UV) light, the EtBr fluoresces. The light emitted is in the orange part of the visible spectrum.
This allows for the visualization of DNA bands in an agarose gel after electrophoresis. The unbound dye does not fluoresce significantly.
Step 4: Final Answer:
Therefore, DNA stained with ethidium bromide appears as bright orange bands under UV radiation.
Quick Tip: Remember the combination: DNA + Ethidium Bromide + UV light = Bright Orange. This is a fundamental visualization technique in gel electrophoresis for DNA.
The phenomenon of pleiotropism refers to
View Solution
Step 1: Understanding the Question:
The question asks for the definition of the genetic term "pleiotropism" (or pleiotropy).
Step 3: Detailed Explanation:
Let's analyze the options:
Pleiotropy is the phenomenon where one single gene influences multiple, often unrelated, phenotypic traits. For example, the gene causing phenylketonuria (PKU) in humans affects mental development, skin pigmentation, and hair colour. So, option (D) is the correct definition.
Option (A) describes polygenic inheritance, where a single trait (like height or skin colour) is controlled by multiple genes.
Options (B) and (C) are incorrect descriptions of genetic principles. Multiple alleles refer to a gene having more than two allelic forms in a population, but they still control a single character.
Step 4: Final Answer:
The correct definition of pleiotropism is a single gene affecting multiple phenotypic expressions.
Quick Tip: To avoid confusion, remember: \textbf{Pleiotropy:} One gene \(\rightarrow\) Many traits. \textbf{Polygenic Inheritance:} Many genes \(\rightarrow\) One trait. The prefix "pleio-" means "more," referring to more effects. "Poly-" means "many," referring to many genes.
The historic Convention on Biological Diversity, 'The Earth Summit' was held in Rio de Janeiro in the year :
View Solution
Step 1: Understanding the Question:
The question asks for the year in which the Earth Summit, formally known as the United Nations Conference on Environment and Development (UNCED), was held in Rio de Janeiro. This summit led to the Convention on Biological Diversity (CBD).
Step 3: Detailed Explanation:
The United Nations Conference on Environment and Development (UNCED), famously known as the Earth Summit, was a major international conference held in Rio de Janeiro, Brazil, from June 3 to 14, 1992.
One of the key agreements adopted at the summit was the Convention on Biological Diversity (CBD). The objectives of the CBD are the conservation of biological diversity, the sustainable use of its components, and the fair and equitable sharing of benefits arising out of the utilization of genetic resources.
The other years listed are significant for other environmental events:
2002: The World Summit on Sustainable Development was held in Johannesburg.
1985: The Vienna Convention for the Protection of the Ozone Layer was adopted.
1986: The Environment Protection Act was enacted in India.
Step 4: Final Answer:
The Earth Summit in Rio de Janeiro was held in 1992.
Quick Tip: Associate "Rio Summit" or "Earth Summit" with the year 1992 and the Convention on Biological Diversity (CBD). This is a landmark event in global environmental policy.
During the purification process for recombinant DNA technology, addition of chilled ethanol precipitates out
View Solution
Step 1: Understanding the Question:
The question asks what biological macromolecule precipitates out of a solution when chilled ethanol is added during the purification steps of recombinant DNA technology.
Step 3: Detailed Explanation:
The process of isolating DNA from cells involves several steps:
1. Lysis: Breaking open the cells to release their contents, including DNA, RNA, proteins, and lipids.
2. Enzymatic Digestion: Using enzymes like proteases to break down proteins (like histones) and ribonucleases (RNase) to break down RNA.
3. Precipitation: After removing other macromolecules, the DNA needs to be isolated from the aqueous solution. DNA is insoluble in ethanol. Adding chilled ethanol causes the DNA to precipitate out of the solution, as it is dehydrated and aggregates.
The precipitated DNA can then be seen as a collection of fine white threads. This process is called ethanol precipitation.
Polysaccharides may also precipitate to some extent, but the primary target and result of this specific step in DNA isolation is the precipitation of DNA.
Step 4: Final Answer:
The addition of chilled ethanol causes the purified DNA to precipitate out of the solution.
Quick Tip: Remember the key principle: DNA is soluble in water (aqueous solution) but insoluble in alcohol (like ethanol). Adding chilled ethanol is the standard final step to concentrate and purify DNA from a lysate.
Frequency of recombination between gene pairs on same chromosome as a measure of the distance between genes to map their position on chromosome, was used for the first time by
View Solution
Step 1: Understanding the Question:
The question asks to identify the scientist who first used recombination frequency to measure the distance between genes and create a genetic map.
Step 3: Detailed Explanation:
Thomas Hunt Morgan: He performed experiments with Drosophila melanogaster (fruit flies) and discovered concepts like linkage and recombination. He proposed that the frequency of recombination was related to the distance between genes.
Alfred Sturtevant: He was a student of Thomas Hunt Morgan. He took Morgan's idea a step further. In 1913, Sturtevant realized that if the frequency of recombination between two genes is related to their physical distance, then this frequency could be used as a unit of measurement. He used recombination data to construct the very first genetic map of a chromosome. He defined one map unit (or centimorgan, cM) as a 1% recombination frequency.
Sutton and Boveri: They proposed the Chromosomal Theory of Inheritance, which states that genes are located on chromosomes.
Henking: He discovered the X chromosome.
While Morgan laid the groundwork, it was Alfred Sturtevant who first used the concept of recombination frequency to map gene positions.
Step 4: Final Answer:
Alfred Sturtevant was the first to use recombination frequency to create a genetic map.
Quick Tip: Associate the scientists with their key contributions: \textbf{Morgan:} Linkage and recombination. \textbf{Sturtevant (Morgan's student):} Genetic mapping using recombination frequency. \textbf{Sutton \& Boveri:} Chromosomal Theory of Inheritance.
Given below are two statements : One is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : The first stage of gametophyte in the life cycle of moss is protonema stage.
Reason R : Protonema develops directly from spores produced in capsule.
In the light of the above statements, choose the most appropriate answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question presents an Assertion (A) and a Reason (R) related to the life cycle of a moss. We need to evaluate if both statements are true and if the Reason correctly explains the Assertion.
Step 3: Detailed Explanation:
Assertion A Analysis:
In the life cycle of a moss (a bryophyte), the dominant phase is the gametophyte. The life cycle begins with a haploid spore. When this spore germinates, it develops into a filamentous, creeping, green structure called the protonema. This protonema is the juvenile gametophyte, or the first stage of the gametophyte. From this protonema, buds arise that develop into the mature, leafy gametophyte. So, Assertion A is correct.
Reason R Analysis:
The spores of mosses are produced in a structure called the capsule, which is part of the sporophyte. These haploid spores are released and, under favorable conditions, they germinate to form the protonema. Thus, the protonema develops directly from the spore. So, Reason R is also correct.
Explanation Analysis:
The Assertion states that the protonema is the first stage of the gametophyte. The Reason explains how this protonema is formed—by direct development from a spore produced in the capsule. This explanation is accurate and directly clarifies why the protonema is considered the initial stage of the gametophytic generation. Therefore, R is the correct explanation for A.
Step 4: Final Answer:
Both Assertion A and Reason R are correct, and R provides the correct explanation for A.
Quick Tip: Remember the moss life cycle sequence: Spore (n) \(\rightarrow\) Protonema (n, juvenile gametophyte) \(\rightarrow\) Leafy gametophyte (n, mature) \(\rightarrow\) Gametes (n) \(\rightarrow\) Zygote (2n) \(\rightarrow\) Sporophyte (2n, with capsule) \(\rightarrow\) Spore (n). This flow clarifies the role of the protonema.
Among eukaryotes, replication of DNA takes place in
View Solution
Step 1: Understanding the Question:
The question asks to identify the specific phase of the eukaryotic cell cycle during which DNA replication occurs.
Step 3: Detailed Explanation:
The eukaryotic cell cycle is divided into two main stages: Interphase and M phase (Mitotic phase).
Interphase is further subdivided into three phases:
\(G_1\) phase (Gap 1): The cell grows and carries out its normal metabolic functions. It prepares for DNA replication.
S phase (Synthesis): This is the phase where DNA replication occurs. The amount of DNA in the cell doubles (from 2C to 4C), but the chromosome number remains the same (each chromosome now consists of two sister chromatids).
\(G_2\) phase (Gap 2): The cell continues to grow and prepares for mitosis. Proteins required for cell division are synthesized.
M phase (Mitosis): This is where the cell divides its nucleus (mitosis) and cytoplasm (cytokinesis) to form two daughter cells.
Step 4: Final Answer:
DNA replication specifically takes place during the S phase of the cell cycle.
Quick Tip: Remember the mnemonic "Go, Sally, Go, Make Children" for the cell cycle phases: \(G_1\), S (Synthesis of DNA), \(G_2\), M (Mitosis), C (Cytokinesis). This helps recall that DNA synthesis happens in the S phase.
What is the function of tassels in the corn cob?
View Solution
Step 1: Understanding the Question:
The question asks for the function of the tassels found on a corn cob. However, there's a common confusion in the terminology of the question. Tassels are the male flowers at the top of the corn plant that *produce* pollen. The silky threads emerging from the top of the cob are the styles/stigmas of the female flowers. The question likely refers to these silky threads, not the tassels. Let's assume it means the silky threads (silks) on the cob.
Step 3: Detailed Explanation:
Let's clarify the parts of a corn plant (Maize):
Tassel: This is the male inflorescence located at the apex of the plant. Its function is to produce and disperse pollen grains.
Ear (Cob): This is the female inflorescence. It consists of multiple female flowers arranged on a central axis.
Silks: The long, silky threads that emerge from the top of the ear are the elongated styles and stigmas of the female flowers. Each silk is connected to a potential kernel (ovule).
Corn is wind-pollinated (anemophilous). The tassels release pollen into the wind, and the long, feathery silks of the cob have a large surface area designed to trap these airborne pollen grains. When a pollen grain lands on a silk, it germinates and a pollen tube grows down the silk to fertilize the ovule, which then develops into a corn kernel.
Based on the options, the question is definitely referring to the corn silks on the cob, not the tassels at the top of the plant. The function of these silks is to trap pollen.
Step 4: Final Answer:
The function of the silks (incorrectly called tassels in the question) on the corn cob is to trap pollen grains for fertilization.
Quick Tip: Be aware of the common confusion between corn tassels and silks. Tassel = Top, Male, Pollen Producer. Silk = on the Ear/Cob, Female, Pollen Catcher. In exams, if the question links "tassels" with the "cob," it's almost certainly referring to the silks and their function in trapping pollen.
In the equation
GPP – R = NPP
GPP is Gross Primary Productivity
NPP is Net Primary Productivity
R here is
View Solution
Step 1: Understanding the Question:
The question asks to identify what the term 'R' represents in the ecological equation relating Gross Primary Productivity (GPP) and Net Primary Productivity (NPP).
Step 2: Key Formula or Approach:
The fundamental equation for energy flow at the producer level is: \[ NPP = GPP - R \]
Step 3: Detailed Explanation:
Gross Primary Productivity (GPP): This is the total rate at which solar energy is captured and converted into chemical energy (organic matter) by producers (e.g., plants) through photosynthesis. It's the total amount of food produced.
Producers need to use some of this captured energy for their own life processes, such as growth, maintenance, and reproduction. The main metabolic process that releases this energy is cellular respiration.
R (Respiratory Loss): This term represents the energy consumed by the producers for their own respiration. This energy is lost as heat and is not available to the next trophic level.
Net Primary Productivity (NPP): This is the rate at which producers create biomass that is available to consumers (herbivores). It is the GPP minus the energy lost through respiration (R). It represents the net amount of stored energy.
Step 4: Final Answer:
In the equation GPP – R = NPP, R stands for Respiratory loss.
Quick Tip: Think of it like a salary. GPP is your gross salary (total earnings). R is the tax and deductions (your expenses). NPP is your net salary or take-home pay (what you have left to spend or save).
Which hormone promotes internode/petiole elongation in deep water rice?
View Solution
Step 1: Understanding the Question:
The question asks to identify the plant hormone responsible for promoting rapid stem (internode) and leaf stalk (petiole) elongation in rice plants that are submerged in deep water.
Step 3: Detailed Explanation:
Deep water rice is a variety of rice that has adapted to grow in flooded conditions. When the plant is submerged, it needs to rapidly elongate its stems to keep its leaves above the water surface for photosynthesis and gas exchange.
This rapid elongation response is primarily mediated by the gaseous plant hormone ethylene.
When parts of the plant are submerged, the diffusion of ethylene out of the plant is blocked by the water. This leads to an accumulation of ethylene within the plant tissues.
This increased concentration of ethylene stimulates cell division and elongation in the internodes, causing the stem to grow quickly and escape the water. Although Gibberellins (\(GA_3\)) also promote stem elongation (bolting), in the specific case of deep water rice, ethylene is the primary trigger that enhances the sensitivity of the cells to gibberellins, leading to the rapid growth. Therefore, ethylene is the direct promoter of this specific response.
2, 4-D is a synthetic auxin, often used as a herbicide.
\(GA_3\) (Gibberellic acid) generally promotes stem elongation.
Kinetin is a cytokinin, which promotes cell division.
Step 4: Final Answer:
Ethylene is the hormone that promotes internode elongation in deep water rice plants.
Quick Tip: Associate ethylene with a wide range of stress responses and senescence, including fruit ripening, abscission, and specific adaptations like stem elongation in submerged plants.
Axile placentation is observed in
View Solution
Step 1: Understanding the Question:
The question asks to identify the group of plants from the given options that exhibit axile placentation. Placentation refers to the arrangement of ovules within the ovary.
Step 3: Detailed Explanation:
In axile placentation, the ovary is partitioned into two or more chambers (locules) by septa. The placenta, where the ovules are attached, is located in the central axis where the septa meet.
Let's analyze the placentation types in the plants mentioned:
China rose (Hibiscus), Petunia, Lemon, Tomato: All these plants have a syncarpous (fused carpels), multilocular ovary with ovules attached to the central axis. This is the definition of axile placentation.
Mustard: Has parietal placentation.
Cucumber: Has parietal placentation.
Primrose, Dianthus: Have free-central placentation.
Beans, Lupin, Pea: These are members of the Fabaceae family and have marginal placentation.
Now let's check the options:
(A) China rose, Petunia, and Lemon all exhibit axile placentation.
(B) Contains Mustard and Primrose which do not have axile placentation.
(C) Contains Beans and Lupin which have marginal placentation.
(D) Contains Dianthus and Pea which do not have axile placentation.
Step 4: Final Answer:
The group of plants that all show axile placentation is China rose, Petunia, and Lemon.
Quick Tip: A good way to remember examples for axile placentation is to think of fruits you slice and see a central column with seeds attached in segments, like a tomato, lemon, or orange.
The process of appearance of recombination nodules occurs at which sub stage of prophase I in meiosis?
View Solution
Step 1: Understanding the Question:
The question asks to identify the specific sub-stage of Prophase I of meiosis where recombination nodules are formed.
Step 3: Detailed Explanation:
Prophase I of meiosis is a long and complex phase divided into five sub-stages:
Leptotene: Chromosomes condense and become visible.
Zygotene: Homologous chromosomes pair up in a process called synapsis, forming bivalents. The synaptonemal complex begins to form.
Pachytene: Synapsis is complete. The paired chromosomes (bivalents) are clearly visible. At this stage, crossing over occurs between non-sister chromatids of homologous chromosomes. The sites of crossing over are marked by the appearance of proteinaceous structures called recombination nodules. These nodules contain the enzymes required for recombination.
Diplotene: The synaptonemal complex dissolves, and the homologous chromosomes begin to separate, except at the sites of crossing over. These X-shaped structures are called chiasmata.
Diakinesis: Chromosomes become fully condensed, and the chiasmata terminalize (move to the ends). The nuclear envelope breaks down.
Step 4: Final Answer:
Recombination nodules, which are the sites of crossing over, appear during the Pachytene sub-stage of Prophase I.
Quick Tip: Use the mnemonic "Lazy Zebra Pounces On Dingoes" to remember the stages of Prophase I: Leptotene, Zygotene, Pachytene, Diplotene, Diakinesis. Associate "Pounces" (Pachytene) with the "pact" or crossing over event, where recombination nodules are found.
The thickness of ozone in a column of air in the atmosphere is measured in terms of :
View Solution
Step 1: Understanding the Question:
The question asks for the unit of measurement used to quantify the thickness or concentration of the ozone layer in the atmosphere.
Step 3: Detailed Explanation:
The concentration of ozone in the stratosphere (the ozone layer) is measured in Dobson Units (DU).
One Dobson Unit is defined as the thickness (in units of 10 µm) that the ozone column would occupy if it were compressed to standard temperature and pressure (0°C and 1 atm).
For example, a column of ozone with a thickness of 300 DU would form a 3 mm thick layer at the Earth's surface under standard conditions.
Let's look at the other options:
Kilobase (kb): A unit of length for DNA or RNA molecules, equal to 1000 base pairs.
Decibels (dB): A logarithmic unit used to measure sound intensity.
Decameter (dam): A unit of length equal to 10 meters.
Step 4: Final Answer:
The thickness of the ozone layer is measured in Dobson units.
Quick Tip: Associate "Ozone" with "Dobson." The name itself is distinctive and specifically linked to measuring the ozone layer. This is a common factual question in environmental science topics.
Given below are two statements :
Statement I: The forces generated by transpiration can lift a xylem-sized column of water over 130 meters height.
Statement II : Transpiration cools leaf surfaces sometimes 10 to 15 degrees, by evaporative cooling.
In the light of the above statements, choose the most appropriate answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question presents two statements related to the process of transpiration in plants. We need to evaluate the correctness of both statements.
Step 3: Detailed Explanation:
Statement I Analysis:
This statement refers to the cohesion-tension theory of water transport in plants. Transpiration creates a negative pressure potential, or tension, in the xylem. This "transpirational pull" is the main driving force for lifting water from the roots to the top of the plant. The forces of cohesion (attraction between water molecules) and adhesion (attraction of water molecules to xylem walls) create an unbroken water column. This mechanism is powerful enough to lift water to the tops of the tallest trees, which can exceed 100 meters in height. A lift of over 130 meters is within the theoretical capacity of this mechanism. Therefore, Statement I is correct.
Statement II Analysis:
Transpiration is the evaporation of water from the plant's surface, primarily from the leaves. Evaporation is a cooling process because it requires energy (latent heat of vaporization) to change water from a liquid to a gaseous state. This energy is taken from the leaf, thereby lowering its temperature. This evaporative cooling can prevent leaves from overheating in direct sunlight, and the cooling effect can indeed be in the range of 10 to 15 degrees Celsius. Therefore, Statement II is also correct.
Step 4: Final Answer:
Both statements accurately describe important aspects of transpiration. Statement I describes its role in water transport, and Statement II describes its role in thermoregulation. Thus, both statements are correct.
Quick Tip: Remember the two main functions of transpiration: 1. **Transport:** Creates the "pull" to lift water and minerals from roots to leaves. 2. **Cooling:** Prevents leaf damage from high temperatures through evaporative cooling.
Which of the following stages of meiosis involves division of centromere?
View Solution
Step 1: Understanding the Question:
The question asks to identify the specific stage of meiosis where the centromeres, which hold the sister chromatids together, divide.
Step 3: Detailed Explanation:
Let's review the key events in the stages of meiosis:
Meiosis I (Reductional Division):
Prophase I, Metaphase I, Anaphase I, Telophase I: The main event of Anaphase I is the separation of homologous chromosomes. The sister chromatids remain attached at their centromeres. The centromeres do not divide in Meiosis I.
Meiosis II (Equational Division): This phase is very similar to mitosis.
Prophase II, Metaphase II: Chromosomes, each composed of two sister chromatids, align at the metaphase plate.
Anaphase II: This is the critical stage where the centromeres finally divide. The sister chromatids separate and are now considered individual chromosomes. They move towards opposite poles of the cell.
Telophase II: The separated chromosomes arrive at the poles, and nuclear envelopes reform, resulting in four haploid cells.
Step 4: Final Answer:
The division of the centromere and separation of sister chromatids occurs during Anaphase II of meiosis.
Quick Tip: Remember the key difference between Anaphase I and Anaphase II: \textbf{Anaphase I:} Separation of \textbf{homologous chromosomes}. Centromeres do not split. \textbf{Anaphase II:} Separation of \textbf{sister chromatids}. Centromeres split. (Similar to mitotic Anaphase).
Identify the correct statements :
A. Detrivores perform fragmentation.
B. The humus is further degraded by some microbes during mineralization.
C. Water soluble inorganic nutrients go down into the soil and get precipitated by a process called leaching.
D. The detritus food chain begins with living organisms.
E. Earthworms break down detritus into smaller particles by a process called catabolism.
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements regarding the process of decomposition. Decomposition involves several steps: fragmentation, leaching, catabolism, humification, and mineralization.
Step 3: Detailed Explanation:
Let's analyze each statement:
A. Detrivores perform fragmentation. This is correct. Detritivores, like earthworms, break down detritus (dead organic matter) into smaller particles. This process is called fragmentation.
B. The humus is further degraded by some microbes during mineralization. This is correct. Humus is a dark amorphous substance that is highly resistant to microbial action. It is degraded very slowly, and this process, called mineralization, releases inorganic nutrients.
C. Water soluble inorganic nutrients go down into the soil and get precipitated by a process called leaching. This is correct. Leaching is the process by which water-soluble nutrients percolate through the soil horizons and can be precipitated as unavailable salts.
D. The detritus food chain begins with living organisms. This is incorrect. The detritus food chain (DFC) begins with dead organic matter (detritus). The grazing food chain (GFC) begins with living producers.
E. Earthworms break down detritus into smaller particles by a process called catabolism. This is incorrect. The physical breakdown of detritus by earthworms is fragmentation. Catabolism refers to the enzymatic degradation of detritus into simpler inorganic substances by bacteria and fungi.
Thus, statements A, B, and C are correct.
Step 4: Final Answer:
The correct option includes statements A, B, and C only.
Quick Tip: Remember the sequence of decomposition: Fragmentation (by detritivores) \(\rightarrow\) Leaching (water-soluble nutrients move down) \(\rightarrow\) Catabolism (bacterial/fungal enzymes) \(\rightarrow\) Humification (forms humus) \(\rightarrow\) Mineralization (releases inorganic nutrients).
In angiosperm, the haploid, diploid and triploid structures of a fertilized embryo sac sequentially are :
View Solution
Step 1: Understanding the Question:
The question asks to identify a set of structures from a fertilized embryo sac that are, in order, haploid (n), diploid (2n), and triploid (3n).
Step 3: Detailed Explanation:
Let's determine the ploidy of the structures in a fertilized embryo sac:
Haploid (n) structures: After fertilization, the synergids and antipodal cells degenerate, but they were haploid. Let's assume the question refers to their ploidy level just after fertilization starts. Synergids are haploid (n), and antipodals are haploid (n).
Diploid (2n) structure: The zygote is formed by the fusion of one male gamete (n) with the egg cell (n). Thus, the zygote is diploid (2n).
Triploid (3n) structure: The Primary Endosperm Nucleus (PEN) is formed by the fusion of the second male gamete (n) with the two polar nuclei (n + n). This process is called triple fusion, resulting in a triploid (3n) nucleus, which develops into the endosperm.
Now let's check the sequence in the options:
(A) Synergids (n), antipodals (n), Polar nuclei (n+n, not yet 3n). Incorrect sequence.
(B) Synergids (n), Primary endosperm nucleus (3n), zygote (2n). The ploidy sequence is n, 3n, 2n. Incorrect.
(C) Antipodals (n), synergids (n), primary endosperm nucleus (3n). The ploidy sequence starts with two haploid structures. Incorrect.
(D) Synergids (n), Zygote (2n), and Primary endosperm nucleus (3n). The ploidy sequence is n, 2n, 3n. This is the correct sequential order.
Step 4: Final Answer:
The correct sequence of haploid, diploid, and triploid structures is Synergids, Zygote, and Primary endosperm nucleus.
Quick Tip: Remember the key events of double fertilization: Syngamy: Male gamete (n) + Egg (n) \(\rightarrow\) Zygote (2n). Triple Fusion: Male gamete (n) + 2 Polar Nuclei (n+n) \(\rightarrow\) PEN (3n). This helps recall the ploidy of the resulting structures.
Among 'The Evil Quartet', which one is considered the most important cause driving extinction of species?
View Solution
Step 1: Understanding the Question:
The question asks to identify the primary cause of species extinction from the four major causes, collectively known as 'The Evil Quartet'.
Step 3: Detailed Explanation:
'The Evil Quartet' is a term used to describe the four major causes of biodiversity loss:
Habitat loss and fragmentation: This is the destruction or division of natural habitats due to activities like deforestation, urbanization, and agriculture. It affects the largest number of species by removing the places they live and find food, making it the single most important cause of extinction.
Over-exploitation: This refers to harvesting species from the wild at rates faster than natural populations can recover (e.g., overfishing, overhunting).
Alien species invasions: When non-native species are introduced into an ecosystem, they can outcompete, prey upon, or introduce diseases to native species, leading to their decline.
Co-extinctions: This occurs when the extinction of one species causes the extinction of another species that depended on it (e.g., a specific pollinator for a plant).
While all four are significant threats, scientific consensus holds that habitat loss and fragmentation is the leading driver of species extinction worldwide.
Step 4: Final Answer:
Habitat loss and fragmentation is considered the most important cause driving species extinction.
Quick Tip: Remember that destroying an organism's home is the most direct and widespread way to threaten its survival. That's why habitat loss is consistently ranked as the number one threat to biodiversity.
In tissue culture experiments, leaf mesophyll cells are put in a culture medium to form callus. This phenomenon may be called as:
View Solution
Step 1: Understanding the Question:
The question describes a process in plant tissue culture where specialized (differentiated) leaf mesophyll cells are induced to become an unspecialized (undifferentiated) mass of cells called a callus. We need to identify the correct term for this process.
Step 3: Detailed Explanation:
Let's define the terms:
Differentiation: The process by which a less specialized cell becomes a more specialized cell type. For example, a meristematic cell differentiating into a mesophyll cell.
Dedifferentiation: The process by which mature, differentiated, non-dividing cells revert to a meristematic (undifferentiated) state and regain the ability to divide. This is exactly what happens when permanent mesophyll cells form a callus.
Redifferentiation: The process by which dedifferentiated cells (like callus cells) divide and then differentiate again to form new, specialized cells, tissues, or organs.
Senescence: The process of aging in plants.
The phenomenon described in the question, where specialized mesophyll cells form an undifferentiated callus, is a classic example of dedifferentiation.
Step 4: Final Answer:
The formation of callus from leaf mesophyll cells is called dedifferentiation.
Quick Tip: Remember the cycle in tissue culture: Start with a differentiated cell (explant). It undergoes \textbf{dedifferentiation} to form a callus. The callus undergoes \textbf{redifferentiation} to form a new plantlet.
In gene gun method used to introduce alien DNA into host cells, microparticles of __________ metal are used.
View Solution
Step 1: Understanding the Question:
The question asks about the type of metal microparticles used in the gene gun (biolistics) method for genetic transformation.
Step 3: Detailed Explanation:
The gene gun method, also known as biolistic particle delivery system, is a technique for introducing foreign DNA into cells.
The method involves coating microscopic particles of a heavy metal with the DNA of interest. These DNA-coated microprojectiles are then accelerated to high velocity and fired into the target cells or tissues.
The particles must be dense enough to penetrate the cell walls and membranes but also be biologically inert to not harm the cells.
The metals of choice for these microparticles are gold (Au) or tungsten (W) due to their high density and chemical inertness.
Step 4: Final Answer:
Microparticles of tungsten or gold are used in the gene gun method.
Quick Tip: Associate the "gene gun" with valuable projectiles. Gold and Tungsten are heavy, dense metals, making them suitable "bullets" for this technique. This is a common fact-based question in biotechnology.
Large, colourful, fragrant flowers with nectar are seen in :
View Solution
Step 1: Understanding the Question:
The question describes a set of floral characteristics (large size, colourful, fragrant, with nectar) and asks to identify the type of pollination associated with them.
Step 3: Detailed Explanation:
These characteristics are adaptations to attract animal pollinators. Let's analyze the options:
Wind pollinated plants (anemophily): Flowers are typically small, inconspicuous, not colourful, and lack fragrance and nectar, as they do not need to attract pollinators.
Insect pollinated plants (entomophily): Flowers have evolved to attract insects. Large size and bright colours provide visual cues, fragrance provides an olfactory cue, and nectar serves as a food reward. The combination of all these traits is characteristic of insect pollination.
Bird pollinated plants (ornithophily): Flowers are often large and brightly coloured (especially red or orange), with abundant nectar, but they are typically odorless because birds have a poor sense of smell.
Bat pollinated plants (chiropterophily): Flowers are usually large, pale or white, open at night, and emit a strong, musky or fruity odor. They also produce copious nectar.
The combination of being large, colourful, fragrant, and having nectar is the classic suite of traits for flowers pollinated by insects like bees and butterflies.
Step 4: Final Answer:
The described floral characteristics are adaptations for insect pollination.
Quick Tip: Remember that different pollinators perceive different cues. Insects see colour and smell fragrance. Birds see colour but don't smell well. Bats are nocturnal and rely on strong smells and pale colours visible at night.
Expressed Sequence Tags (ESTs) refers to
View Solution
Step 1: Understanding the Question:
The question asks for the definition of Expressed Sequence Tags (ESTs).
Step 3: Detailed Explanation:
Expressed Sequence Tags (ESTs) are a tool used in genomics to identify gene transcripts. The process involves:
Isolating all the messenger RNA (mRNA) from a cell or tissue.
Using the enzyme reverse transcriptase to create complementary DNA (cDNA) from the mRNA templates.
Sequencing short fragments (tags) from one or both ends of these cDNAs.
Since the process starts with mRNA, which is the product of gene transcription (expression), ESTs represent parts of genes that are being expressed as RNA in that specific cellular context. Therefore, the collection of ESTs from a sample represents "all genes that are expressed as RNA".
Option (A) is too restrictive; ESTs identify all expressed genes, not just "important" ones.
Option (C) is incorrect because ESTs are derived from RNA transcripts. Not all RNAs are translated into proteins.
Option (D) is incorrect because ESTs are by definition derived from expressed genes only.
Step 4: Final Answer:
ESTs refer to all genes that are expressed as RNA.
Quick Tip: Break down the name: "Expressed" means it comes from transcribed genes (mRNA). "Sequence Tag" means it's a short piece of sequence used to identify a longer transcript. This helps recall that ESTs are markers for genes expressed as RNA.
Movement and accumulation of ions across a membrane against their concentration gradient can be explained by
View Solution
Step 1: Understanding the Question:
The question asks to identify the transport mechanism that allows ions to move and accumulate against a concentration gradient (i.e., from a region of lower concentration to a region of higher concentration).
Step 3: Detailed Explanation:
Let's analyze the transport mechanisms:
Passive Transport: This is the movement of substances down the concentration gradient (from high to low concentration) and does not require energy. Simple diffusion and facilitated diffusion are types of passive transport.
Facilitated Diffusion: A type of passive transport where substances move down the concentration gradient with the help of membrane proteins (channels or carriers). No energy is expended.
Osmosis: The specific movement of water across a selectively permeable membrane from a region of high water potential to low water potential. It is a type of passive transport.
Active Transport: This is the movement of substances against the concentration gradient. This process is "uphill" and requires the cell to expend metabolic energy (usually from ATP). It is carried out by specific membrane proteins called pumps.
The key phrase in the question is "against their concentration gradient," which is the defining feature of active transport.
Step 4: Final Answer:
The movement of ions against a concentration gradient is explained by Active Transport.
Quick Tip: Think of concentration gradients like a hill. Passive transport is like rolling a ball downhill (no energy needed). Active transport is like pushing a ball uphill (energy is required). "Against the gradient" means "uphill."
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R :
Assertion A: ATP is used at two steps in glycolysis.
Reason R: First ATP is used in converting glucose into glucose-6-phosphate and second ATP is used in conversion of fructose-6-phosphate into fructose-1-6-diphosphate.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question presents an Assertion (A) and a Reason (R) about the consumption of ATP during the process of glycolysis. We need to evaluate their truthfulness and the relationship between them.
Step 3: Detailed Explanation:
Assertion A Analysis:
Glycolysis is a 10-step process. In the initial "preparatory" or "investment" phase, energy is consumed.
Step 1: Glucose is phosphorylated to glucose-6-phosphate. This reaction consumes one molecule of ATP.
Step 3: Fructose-6-phosphate is phosphorylated to fructose-1,6-bisphosphate. This reaction consumes a second molecule of ATP.
No other steps in glycolysis consume ATP. Therefore, the statement "ATP is used at two steps in glycolysis" is correct. Assertion A is true.
Reason R Analysis:
The reason states the specific steps where ATP is used: "First ATP is used in converting glucose into glucose-6-phosphate and second ATP is used in conversion of fructose-6-phosphate into fructose-1,6-diphosphate." As detailed above, these are precisely the two ATP-consuming steps. Therefore, Reason R is also true.
Explanation Analysis:
Reason R provides the exact details that validate Assertion A. It explains *which* two steps use ATP, thus serving as a direct and correct explanation for the assertion.
Step 4: Final Answer:
Both Assertion A and Reason R are true, and R is the correct explanation of A.
Quick Tip: Remember glycolysis as having an "investment phase" and a "payoff phase." In the investment phase, you spend 2 ATP (at steps 1 and 3). In the payoff phase, you generate 4 ATP and 2 NADH. The net gain is 2 ATP and 2 NADH.
Spraying of which of the following phytohormone on juvenile conifers helps in hastening the maturity period, that leads to early seed production?
View Solution
Step 1: Understanding the Question:
The question asks which plant hormone can be used to speed up the transition from the juvenile to the mature phase in conifers, thereby promoting earlier seed production.
Step 3: Detailed Explanation:
Let's review the functions of the listed phytohormones:
Abscisic Acid (ABA): Generally acts as a growth inhibitor. It promotes dormancy and stomatal closure during stress. It does not hasten maturity.
Indole-3-butyric Acid (IBA): An auxin that primarily promotes root initiation in stem cuttings.
Gibberellic Acid (GA): Gibberellins have many roles, including promoting stem elongation (bolting), seed germination, and flowering. A significant commercial application is spraying juvenile conifers with GAs to overcome the juvenile phase and induce early flowering and seed production. This shortens the breeding cycle.
Zeatin: A type of cytokinin that promotes cell division, delays senescence, and overcomes apical dominance. It does not have a primary role in hastening maturity.
The specific effect of promoting early maturity in juvenile conifers is a well-known function of gibberellins.
Step 4: Final Answer:
Spraying with Gibberellic Acid helps in hastening the maturity period of juvenile conifers.
Quick Tip: Associate Gibberellins (GA) with "growing up" and "growing tall" in plants. They cause bolting (stem elongation before flowering) and help plants mature faster, especially in conifers.
Identify the pair of heterosporous pteridophytes among the following :
View Solution
Step 1: Understanding the Question:
The question asks to identify a pair of pteridophytes that are both heterosporous. Heterosporous plants produce two distinct types of spores: smaller microspores (which develop into male gametophytes) and larger megaspores (which develop into female gametophytes).
Step 3: Detailed Explanation:
Let's classify the given pteridophytes as either homosporous (producing one type of spore) or heterosporous:
Equisetum: Homosporous.
Lycopodium: Homosporous.
Psilotum: Homosporous.
Selaginella: Heterosporous.
Salvinia: Heterosporous.
Other examples of heterosporous pteridophytes include Azolla, \textit{Marsilea, and \textit{Isoetes.
Now let's evaluate the options:
(A) Equisetum (homosporous) and Salvinia (heterosporous). Not a pair of heterosporous plants.
(B) Lycopodium (homosporous) and Selaginella (heterosporous). Not a pair of heterosporous plants.
(C) Selaginella (heterosporous) and Salvinia (heterosporous). This is a correct pair of heterosporous pteridophytes.
(D) Psilotum (homosporous) and Salvinia (heterosporous). Not a pair of heterosporous plants.
Step 4: Final Answer:
Both Selaginella and Salvinia are heterosporous pteridophytes.
Quick Tip: Most pteridophytes are homosporous. Remember the key exceptions that are heterosporous: \textit{Selaginella, Salvinia, Azolla, and Marsilea. The "S" genera (Selaginella, Salvinia) are the most common examples in exams.
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R :
Assertion A: Late wood has fewer xylary elements with narrow vessels.
Reason R: Cambium is less active in winters.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question provides an Assertion (A) about the characteristics of late wood and a Reason (R) about the activity of cambium in winter. We need to determine if they are true and if R explains A.
Step 3: Detailed Explanation:
Assertion A Analysis:
In temperate regions, the vascular cambium's activity changes with the seasons, leading to the formation of annual rings.
Spring wood (early wood): Formed during spring when the cambium is very active. It consists of a large number of xylary elements with wider vessels.
Autumn wood (late wood): Formed during autumn/winter when the cambium is less active. It has fewer xylary elements, and the vessels are narrow and thick-walled. This wood is denser.
So, the statement "Late wood has fewer xylary elements with narrow vessels" is correct. Assertion A is true.
Reason R Analysis:
The activity of the vascular cambium is regulated by physiological and environmental factors. During the unfavorable conditions of winter (low temperature, short daylight), the cambium becomes less active or dormant. Therefore, the statement "Cambium is less active in winters" is correct. Reason R is true.
Explanation Analysis:
The reduced activity of the cambium in winter (Reason R) is the direct cause of the structural features of late wood described in Assertion A (fewer elements, narrow vessels). Because the cambium is less active, it produces fewer cells, and the cells that are produced do not expand as much, resulting in narrow lumens. Thus, R is the correct explanation for A.
Step 4: Final Answer:
Both Assertion A and Reason R are true, and R is the correct explanation of A.
Quick Tip: Think of it like this: Spring = favorable conditions, high activity, big/wide cells (spring wood). Winter = unfavorable conditions, low activity, small/narrow cells (late wood). The difference between these two creates the annual ring.
What is the role of RNA polymerase III in the process of transcription in Eukaryotes?
View Solution
Step 1: Understanding the Question:
The question asks for the specific function of RNA polymerase III in eukaryotic transcription.
Step 3: Detailed Explanation:
In eukaryotic nuclei, transcription is carried out by three distinct RNA polymerases, each with a specific set of genes to transcribe:
RNA Polymerase I: Located in the nucleolus, it transcribes the genes for the large ribosomal RNAs (rRNAs): 28S, 18S, and 5.8S rRNA.
RNA Polymerase II: Located in the nucleoplasm, it transcribes the precursor of messenger RNA (called pre-mRNA or hnRNA) and most small nuclear RNAs (snRNAs).
RNA Polymerase III: Located in the nucleoplasm, it transcribes the genes for transfer RNA (tRNA), the 5S ribosomal RNA (a small component of the large ribosomal subunit), and some small nuclear RNAs (e.g., U6 snRNA).
Based on this division of labor:
Option (A) is incorrect.
Option (B) describes the function of RNA Polymerase I.
Option (C) correctly lists the main transcripts produced by RNA Polymerase III.
Option (D) describes the function of RNA Polymerase II.
Step 4: Final Answer:
The role of RNA polymerase III is the transcription of tRNA, 5S rRNA, and some snRNAs.
Quick Tip: Use the mnemonic "1, 2, 3 - R, M, T". RNA Pol \textbf{I} makes \textbf{r}RNA. RNA Pol \textbf{II} makes \textbf{m}RNA. RNA Pol \textbf{III} makes \textbf{t}RNA (and other small RNAs like 5S rRNA).
How many ATP and NADPH\(_2\) are required for the synthesis of one molecule of Glucose during Calvin cycle?
View Solution
Step 1: Understanding the Question:
The question asks for the total number of ATP and NADPH molecules required by the Calvin cycle to synthesize one molecule of glucose (\(C_6H_{12}O_6\)). (Note: NADPH\(_2\) is an older notation for NADPH + H\(^+\), and is equivalent to NADPH in this context).
Step 2: Key Formula or Approach:
The synthesis of one glucose molecule requires 6 turns of the Calvin cycle, as each turn fixes one molecule of \(CO_2\). We need to calculate the ATP and NADPH cost per turn and then multiply by 6.
Step 3: Detailed Explanation:
Let's analyze the energy requirements for one turn of the Calvin cycle (fixing 1 \(CO_2\)):
Reduction Phase: Two molecules of 3-PGA are converted to two molecules of glyceraldehyde-3-phosphate (G3P). This step requires 2 ATP and 2 NADPH.
Regeneration Phase: For every 3 turns of the cycle, 5 molecules of G3P are used to regenerate 3 molecules of RuBP. This process requires 3 ATP. Per turn, this averages to 1 ATP.
So, the total cost for one turn of the Calvin cycle is:
ATP: 2 (from reduction) + 1 (from regeneration) = 3 ATP
NADPH: 2 (from reduction) = 2 NADPH
To synthesize one molecule of glucose (\(C_6\)), the cycle must turn 6 times.
Total ATP required = 6 turns \(\times\) 3 ATP/turn = 18 ATP
Total NADPH required = 6 turns \(\times\) 2 NADPH/turn = 12 NADPH
Step 4: Final Answer:
The synthesis of one molecule of glucose requires 18 ATP and 12 NADPH.
Quick Tip: Remember the numbers 3 and 2 for the Calvin cycle. For every 1 \(CO_2\) fixed, you need \textbf{3 ATP} and \textbf{2 NADPH}. To make glucose (\(C_6\)), multiply everything by 6.
Match List I with List II :
List I & List II
A. Iron & I. & Synthesis of auxin
B. Zinc & II. & Component of nitrate reductase
C. Boron & III. & Activator of catalase
D. Molybdenum & IV. & Cell elongation and differentiation
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question requires matching the micronutrients in List I with their corresponding functions in plants from List II.
Step 3: Detailed Explanation:
Let's match each micronutrient with its role:
A. Iron (Fe): Iron is a crucial component of proteins involved in electron transport, such as cytochromes and ferredoxin. It is also required for chlorophyll formation and is an essential activator for the enzyme catalase. Thus, A matches with III.
B. Zinc (Zn): Zinc is required for the synthesis of the plant hormone auxin (as it activates enzymes involved in tryptophan synthesis, a precursor to auxin). It is also an activator of various other enzymes like carboxylases. Thus, B matches with I.
C. Boron (B): Boron is required for the uptake and utilization of \(Ca^{2+}\), membrane functioning, pollen germination, cell elongation, and cell differentiation. Thus, C matches with IV.
D. Molybdenum (Mo): Molybdenum is a component of several enzymes, most notably nitrate reductase and nitrogenase, both of which are critical for nitrogen metabolism. Thus, D matches with II.
The correct matching is: A-III, B-I, C-IV, D-II.
Step 4: Final Answer:
Looking at the options, option (D) provides the correct combination: A-III, B-I, C-IV, D-II.
Quick Tip: For mineral nutrition matching questions, memorize at least one key, unique function for each element. For example: Zinc \(\rightarrow\) Auxin synthesis; Molybdenum \(\rightarrow\) Nitrate reductase; Boron \(\rightarrow\) Pollen germination; Iron \(\rightarrow\) Catalase/Chlorophyll synthesis.
How many different proteins does the ribosome consist of?
View Solution
Step 1: Understanding the Question:
The question asks for the approximate number of different proteins that make up a ribosome. The question does not specify prokaryotic or eukaryotic, but typically in a general context, it refers to eukaryotes, and we should check which answer fits best.
Step 3: Detailed Explanation:
Ribosomes are complex macromolecular machines composed of ribosomal RNA (rRNA) and ribosomal proteins.
Prokaryotic Ribosomes (70S): The 30S subunit has about 21 proteins, and the 50S subunit has about 34 proteins, making a total of approximately 55 proteins.
Eukaryotic Ribosomes (80S): The 40S subunit has about 33 proteins, and the 60S subunit has about 49 proteins, making a total of approximately 82 proteins.
Looking at the given options:
(A) 20
(B) 80
(C) 60
(D) 40
The value 80 is the closest approximation to the number of proteins found in a eukaryotic ribosome (~82). The other options are significantly different from the protein counts in either prokaryotic or eukaryotic ribosomes.
Step 4: Final Answer:
A ribosome (eukaryotic) consists of approximately 80 different proteins.
Quick Tip: Remember the general composition of ribosomes. Eukaryotic ribosomes are larger (80S) and more complex than prokaryotic ones (70S). The number 80 in the option is a strong hint towards the 80S eukaryotic ribosome.
Match List I with List II :
List I & List II
A. Oxidative decarboxylation & I. & Citrate synthase
B. Glycolysis & II. & Pyruvate dehydrogenase
C. Oxidative phosphorylation & III. & Electron transport system
D. Tricarboxylic acid cycle & IV. & EMP pathway
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
This question requires matching metabolic processes from List I with their associated enzymes, pathways, or locations from List II.
Step 3: Detailed Explanation:
Let's match each process:
A. Oxidative decarboxylation: This refers to the link reaction where pyruvate is converted to Acetyl-CoA. This reaction is catalyzed by the pyruvate dehydrogenase complex. Thus, A matches with II.
B. Glycolysis: This is the initial pathway for glucose breakdown. It is also known as the Embden-Meyerhof-Parnas (EMP) pathway. Thus, B matches with IV.
C. Oxidative phosphorylation: This is the final stage of cellular respiration where the energy from NADH and FADH\(_2\) is used to produce ATP. This process occurs via the Electron Transport System (ETS) located on the inner mitochondrial membrane. Thus, C matches with III.
D. Tricarboxylic acid cycle (TCA cycle): Also known as the Krebs cycle or citric acid cycle. The cycle begins when acetyl-CoA combines with oxaloacetate to form citrate, a reaction catalyzed by the enzyme citrate synthase. Therefore, this enzyme is fundamentally linked to the cycle. Thus, D matches with I.
The correct matching is: A-II, B-IV, C-III, D-I.
Step 4: Final Answer:
Looking at the options, option (A) provides the correct combination: A-II, B-IV, C-III, D-I.
Quick Tip: Create a mental flowchart for cellular respiration: Glycolysis (EMP pathway) \(\rightarrow\) Pyruvate \(\rightarrow\) Oxidative Decarboxylation (by Pyruvate Dehydrogenase) \(\rightarrow\) Acetyl-CoA \(\rightarrow\) TCA Cycle (starts with Citrate Synthase) \(\rightarrow\) Electron Transport System (for Oxidative Phosphorylation). This flow helps to link the terms correctly.
Match List I with List II :
List I (Interaction) & List II (Species A and B)
A. Mutualism & I. & +(A), 0(B)
B. Commensalism & II. & -(A), 0(B)
C. Amensalism & III. & +(A), -(B)
D. Parasitism & IV. & +(A), +(B)
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question requires matching different types of population interactions (List I) with their symbolic representations (List II), where '+' denotes benefit, '-' denotes harm, and '0' denotes no significant effect.
Step 3: Detailed Explanation:
Let's analyze each interaction:
A. Mutualism: An interaction where both species benefit from each other. The representation is (+, +). Thus, A matches with IV.
B. Commensalism: An interaction where one species benefits, and the other is neither harmed nor benefited (unaffected). The representation is (+, 0). Thus, B matches with I.
C. Amensalism: An interaction where one species is harmed, and the other is unaffected. The representation is (-, 0). Thus, C matches with II.
D. Parasitism: An interaction where one species (the parasite) benefits at the expense of the other species (the host), which is harmed. The representation is (+, -). Thus, D matches with III.
The correct set of matches is A-IV, B-I, C-II, D-III.
Step 4: Final Answer:
Comparing our matches with the given options, option (C) correctly represents the relationships: A-IV, B-I, C-II, D-III.
Quick Tip: Create a simple table to memorize these interactions. Key pairs to remember are Mutualism (+,+) vs. Competition (-,-) and Commensalism (+,0) vs. Amensalism (-,0).
Given below are two statements :
Statement I: Gause's 'Competitive Exclusion Principle' states that two closely related species competing for the same resources cannot co-exist indefinitely and competitively inferior one will be eliminated eventually.
Statement II: In general, carnivores are more adversely affected by competition than herbivores.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to evaluate the correctness of two statements related to ecological competition.
Step 3: Detailed Explanation:
Statement I Analysis:
This statement provides the definition of Gause's 'Competitive Exclusion Principle'. The principle indeed states that when two species compete for the exact same limited resources, one species will be more efficient and will eventually outcompete and eliminate the other. This statement is a correct definition of the principle. Thus, Statement I is correct.
Statement II Analysis:
This statement makes a broad generalization that carnivores are more affected by competition than herbivores. Competition is a powerful evolutionary force at all trophic levels. Herbivores often face intense interspecific and intraspecific competition for limited plant resources. Carnivores also face intense competition for prey and territory. There is no universally accepted ecological rule that states that competition is inherently more adverse for carnivores than for herbivores. The intensity of competition depends on various factors like resource availability, niche overlap, and population density, not just the trophic level. Therefore, this generalization is considered incorrect. Thus, Statement II is false.
Step 4: Final Answer:
Statement I is correct, and Statement II is false.
Quick Tip: Remember Gause's principle with the phrase "complete competitors cannot coexist." For broad ecological statements, be cautious. Generalizations like "always" or "more than" are often incorrect because ecosystems are complex and context-dependent.
Identify the correct statements :
A. Lenticels are the lens-shaped openings permitting the exchange of gases.
B. Bark formed early in the season is called hard bark.
C. Bark is a technical term that refers to all tissues exterior to vascular cambium.
D. Bark refers to periderm and secondary phloem.
E. Phellogen is single-layered in thickness.
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements from a list concerning plant anatomy, specifically about bark and related structures.
Step 3: Detailed Explanation:
Let's evaluate each statement:
A. Lenticels are the lens-shaped openings permitting the exchange of gases. This is correct. Lenticels are pores in the periderm of woody plants that allow for gas exchange between the internal tissues and the atmosphere.
B. Bark formed early in the season is called hard bark. This is incorrect. Bark formed early in the season (spring) is called 'early' or 'soft' bark, while that formed late in the season is 'late' or 'hard' bark.
C. Bark is a technical term that refers to all tissues exterior to vascular cambium. This is a broad, non-technical definition of bark. While correct in a general sense, there is a more precise definition.
D. Bark refers to periderm and secondary phloem. This is the more precise, technical definition. Bark includes the secondary phloem (inner bark) and the periderm (outer bark). This statement is correct.
E. Phellogen is single-layered in thickness. This is incorrect. Phellogen, or cork cambium, is a meristematic tissue and is typically a few cell layers thick, not just a single layer.
The definitively correct statements are A and D. Statement C is a less precise definition but not entirely wrong, however, D is the better technical definition. Given the options, the combination of the two most accurate statements must be chosen.
Step 4: Final Answer:
Statements A and D are correct. Therefore, the correct option is (C).
Quick Tip: Remember that "bark" is a common word with a specific biological meaning. Technically, it's everything outside the vascular cambium. More specifically, it's composed of periderm and secondary phloem.
Main steps in the formation of Recombinant DNA are given below. Arrange these steps in a correct sequence.
A. Insertion of recombinant DNA into the host cell.
B. Cutting of DNA at specific location by restriction enzyme.
C. Isolation of desired DNA fragment.
D. Amplification of gene of interest using PCR.
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to arrange the given steps of creating recombinant DNA in the correct chronological order.
Step 3: Detailed Explanation:
The process of recombinant DNA technology involves a series of sequential steps:
The process begins with identifying and isolating the gene of interest. This corresponds to C. Isolation of desired DNA fragment.
Once the source DNA is isolated, restriction enzymes are used to cut out the specific gene of interest. This is B. Cutting of DNA at specific location by restriction enzyme.
To get a sufficient quantity of the gene for further steps, it is amplified using the Polymerase Chain Reaction (PCR). This is D. Amplification of gene of interest using PCR. (This step is often done after isolating the gene, before or after ligation into a vector).
The amplified gene of interest is then ligated into a vector to create recombinant DNA (this step is not listed, but is implied before A).
Finally, the recombinant DNA (vector + gene) is introduced into a suitable host cell for cloning or expression. This is A. Insertion of recombinant DNA into the host cell.
The logical sequence of the given steps is C \(\rightarrow\) B \(\rightarrow\) D \(\rightarrow\) A.
Step 4: Final Answer:
The correct sequence is C, B, D, A, which corresponds to option (D).
Quick Tip: Think of the process like a recipe: First, you get your special ingredient (C: Isolate gene). Then you prepare it (B: Cut with enzymes). Then you make more of it if needed (D: Amplify with PCR). Finally, you add it to the main dish (A: Insert into host).
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R :
Assertion A: In gymnosperms the pollen grains are released from the microsporangium and carried by air currents.
Reason R: Air currents carry the pollen grains to the mouth of the archegonia where the male gametes are discharged and pollen tube is not formed.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question provides an Assertion and a Reason related to pollination in gymnosperms. We need to evaluate their correctness and the relationship between them.
Step 3: Detailed Explanation:
Assertion A Analysis:
This statement describes the mode of pollination in gymnosperms. Gymnosperms are typically wind-pollinated (anemophilous). The pollen grains, which develop in the microsporangium, are lightweight and produced in large quantities to be dispersed by air currents. This statement is correct. So, Assertion A is true.
Reason R Analysis:
This statement describes the events after pollination. It has two parts. First, "Air currents carry the pollen grains to the mouth of the archegonia". The pollen grain lands on the micropyle of the ovule, not directly on the archegonium which is deep inside the ovule. Second, and more importantly, it claims "...pollen tube is not formed". This is incorrect. In most gymnosperms (like Pinus), the pollen grain germinates on the ovule to form a pollen tube, which grows towards the archegonium to deliver the male gametes. This process is called siphonogamy. Therefore, the statement that the pollen tube is not formed is false. So, Reason R is false.
Step 4: Final Answer:
Assertion A is true, but Reason R is false.
Quick Tip: Remember that the development of the pollen tube (siphonogamy) is a key evolutionary advancement seen in both gymnosperms and angiosperms, which allows for fertilization without the need for external water.
Which one of the following statements is NOT correct?
View Solution
Step 1: Understanding the Question:
The question asks to identify the incorrect statement among the four options related to water pollution and its ecological effects.
Step 3: Detailed Explanation:
Let's analyze each statement:
(A) This statement correctly describes the phenomenon of biomagnification, where the concentration of non-biodegradable toxic substances increases at each successive trophic level in a food chain.
(B) This statement correctly describes the consequences of high organic pollution. Decomposer microorganisms consume dissolved oxygen to break down organic waste, increasing the Biological Oxygen Demand (BOD). This depletion of oxygen can lead to hypoxia or anoxia, causing mass death of fish and other aquatic organisms.
(C) This statement is incorrect. Algal blooms are caused by nutrient enrichment (eutrophication), often from sewage and agricultural runoff. They severely degrade water quality by blocking sunlight, producing toxins, and causing severe oxygen depletion when they die and decompose. This leads to the death of fish and does not promote fisheries; it destroys them.
(D) This statement is correct. Water hyacinth (\textit{Eichhornia crassipes) is a notorious invasive weed that thrives in nutrient-rich (eutrophic) waters. Its rapid growth covers the water surface, blocking light and oxygen, which disrupts the aquatic ecosystem.
Step 4: Final Answer:
The incorrect statement is (C), as algal blooms are detrimental to water quality and aquatic life.
Quick Tip: Associate "algal bloom" and "eutrophication" with negative consequences: oxygen depletion, fish kills, and poor water quality. Any statement claiming they are beneficial is almost certainly incorrect.
Which of the following statements are correct about Klinefelter's Syndrome?
A. This disorder was first described by Langdon Down (1866).
B. Such an individual has overall masculine development. However, the feminine development is also expressed.
C. The affected individual is short statured.
D. Physical, psychomotor and mental development is retarded.
E. Such individuals are sterile.
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements about Klinefelter's Syndrome from the given list.
Step 3: Detailed Explanation:
Let's evaluate each statement regarding Klinefelter's Syndrome (genotype 47, XXY):
A. This disorder was first described by Langdon Down (1866). This is incorrect. Langdon Down described Down's Syndrome. Klinefelter's Syndrome was described by Dr. Harry Klinefelter in 1942.
B. Such an individual has overall masculine development. However, the feminine development is also expressed. This is correct. The presence of the Y chromosome leads to male development, but the extra X chromosome leads to the expression of some feminine characteristics, such as the development of breasts (gynaecomastia).
C. The affected individual is short statured. This is incorrect. Individuals with Klinefelter's Syndrome are often taller than average. Short stature is characteristic of Turner's Syndrome (45, X0).
D. Physical, psychomotor and mental development is retarded. This is incorrect. While some learning difficulties may be present, severe mental retardation is not a typical feature of Klinefelter's Syndrome. This description is more characteristic of Down's Syndrome.
E. Such individuals are sterile. This is correct. The extra X chromosome impairs the development of the testes, leading to low testosterone production and infertility.
The correct statements are B and E.
Step 4: Final Answer:
The combination of correct statements is B and E, which is given in option (D).
Quick Tip: To remember the key features of chromosomal disorders: \textbf{Klinefelter's (XXY):} A tall, sterile male with some female characteristics (gynaecomastia). \textbf{Turner's (XO):} A short, sterile female with underdeveloped secondary sexual characteristics. \textbf{Down's (Trisomy 21):} Short stature, characteristic facial features, and retarded development.
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : A flower is defined as modified shoot wherein the shoot apical meristem changes to floral meristem.
Reason R: Internode of the shoot gets condensed to produce different floral appendages laterally at successive nodes instead of leaves.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question presents an Assertion (A) defining a flower and a Reason (R) describing the morphological changes involved. We need to evaluate if both are true and if the reason explains the assertion.
Step 3: Detailed Explanation:
Assertion A Analysis:
The statement "A flower is defined as modified shoot wherein the shoot apical meristem changes to floral meristem" is the accepted botanical definition of a flower. The vegetative shoot apex transforms into a reproductive apex that produces floral parts instead of leaves. So, Assertion A is true.
Reason R Analysis:
The statement "Internode of the shoot gets condensed to produce different floral appendages laterally at successive nodes instead of leaves" explains the process of modification. The floral axis (thalamus) is a condensed stem where internodes do not elongate. The floral parts (sepals, petals, stamens, carpels) are homologous to leaves and arise from these condensed nodes. So, Reason R is also true.
Explanation Analysis:
The reason (R) provides the specific details of how a shoot is modified to become a flower—the condensation of internodes and the modification of leaves into floral appendages. This directly explains why a flower is considered a modified shoot (A). Therefore, R is the correct explanation for A.
Step 4: Final Answer:
Both Assertion A and Reason R are true, and R is the correct explanation of A.
Quick Tip: Remember the evidence for a flower being a modified shoot: the thalamus is a condensed stem, and floral parts like sepals and petals are structurally similar to leaves. This homology is a core concept in plant morphology.
Match List I with List II :
List I & List II
A. M Phase & I. & Proteins are synthesized
B. G\(_2\) Phase & II. & Inactive phase
C. Quiescent stage & III. & Interval between mitosis and
& & & initiation of DNA replication
D. G\(_1\) Phase & IV. & Equational division
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question requires matching the phases of the cell cycle (List I) with their corresponding descriptions or key events (List II).
Step 3: Detailed Explanation:
Let's match each phase with its description:
A. M Phase: This is the mitotic phase where the cell divides. Mitosis is known as equational division because the chromosome number in the daughter cells is the same as in the parent cell. Thus, A matches with IV.
B. G\(_2\) Phase: This is the second gap phase, occurring after DNA synthesis (S phase) and before mitosis (M phase). During G\(_2\), the cell prepares for division, which includes the synthesis of proteins like tubulin required for the spindle apparatus. Thus, B matches with I.
C. Quiescent stage (G\(_0\)): This is a non-dividing state where cells exit the cell cycle. Cells in G\(_0\) are metabolically active but do not proliferate. It can be considered an inactive phase with respect to cell division. Thus, C matches with II.
D. G\(_1\) Phase: This is the first gap phase, which is the interval between the end of mitosis (M phase) and the beginning of DNA synthesis (S phase). Thus, D matches with III.
The correct set of matches is A-IV, B-I, C-II, D-III.
Step 4: Final Answer:
Comparing our matches with the given options, option (D) correctly represents the relationships: A-IV, B-I, C-II, D-III.
Quick Tip: Visualize the cell cycle as a clock: M \(\rightarrow\) G\(_1\) \(\rightarrow\) S \(\rightarrow\) G\(_2\) \(\rightarrow\) M. G\(_1\) is the gap before S (Synthesis). G\(_2\) is the gap after S. M is the division itself. G\(_0\) is an exit ramp from G\(_1\).
Melonate inhibits the growth of pathogenic bacteria by inhibiting the activity of
View Solution
Step 1: Understanding the Question:
The question asks for the specific enzyme that is inhibited by malonate (spelled as "Melonate" in the question), leading to the inhibition of bacterial growth.
Step 3: Detailed Explanation:
Malonate is a classic example of a competitive inhibitor in biochemistry. Its molecular structure is very similar to that of succinate, the natural substrate for the enzyme succinic dehydrogenase.
Succinic dehydrogenase is a key enzyme in the Krebs cycle (citric acid cycle), where it catalyzes the oxidation of succinate to fumarate.
Because of its structural similarity, malonate binds to the active site of succinic dehydrogenase but cannot be acted upon by the enzyme. This binding blocks the real substrate, succinate, from accessing the active site.
By inhibiting this crucial step in the Krebs cycle, malonate disrupts aerobic respiration and ATP production, thereby inhibiting the growth and proliferation of organisms like pathogenic bacteria that rely on this pathway.
Step 4: Final Answer:
Malonate inhibits the enzyme succinic dehydrogenase.
Quick Tip: Remember the concept of competitive inhibition: "A similar-looking molecule competes for the same spot." Malonate looks like succinate, so it competes for the active site of succinic dehydrogenase. This is a frequently cited example.
Which of the following combinations is required for chemiosmosis?
View Solution
Step 1: Understanding the Question:
The question asks to identify the essential components required for the process of chemiosmosis, which is the mechanism for ATP synthesis in both photosynthesis and cellular respiration.
Step 3: Detailed Explanation:
According to Peter Mitchell's chemiosmotic theory, ATP synthesis is coupled to the movement of protons across a membrane. The essential requirements are:
A membrane: An intact, impermeable membrane (like the inner mitochondrial membrane or the thylakoid membrane) is necessary to separate two aqueous compartments and maintain a proton gradient.
A proton pump: This is typically the electron transport chain (ETC), which uses the energy from electron flow to actively pump protons (H\(^+\)) from one side of the membrane to the other.
A proton gradient: The pumping of protons creates an electrochemical potential difference across the membrane, also known as the proton motive force. This gradient is a form of stored energy.
ATP synthase: This is a large enzyme complex embedded in the membrane. It has a channel that allows protons to flow back across the membrane down their concentration gradient. This flow of protons drives the catalytic site of the enzyme to synthesize ATP from ADP and inorganic phosphate (Pi).
Option (B) includes all four of these essential components. The other options are incorrect because they either miss a component (like the membrane) or name the wrong enzyme (NADP synthase instead of ATP synthase) or gradient (electron gradient instead of proton gradient).
Step 4: Final Answer:
The combination required for chemiosmosis is a membrane, a proton pump, a proton gradient, and ATP synthase.
Quick Tip: Think of chemiosmosis like a hydroelectric dam. The \textbf{membrane} is the dam. The \textbf{proton pump} is the machinery that pumps water up to the reservoir. The \textbf{proton gradient} is the stored water in the reservoir. The \textbf{ATP synthase} is the turbine that generates energy (ATP) as the water flows back down.
Match List I with List II :
List I & List II
A. Cohesion & I. & More attraction in liquid phase
B. Adhesion & II. & Mutual attraction among water molecules
C. Surface tension & III. & Water loss in liquid phase
D. Guttation & IV. & Attraction towards polar surfaces
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question requires matching terms related to the physical properties of water and plant water relations (List I) with their correct definitions (List II).
Step 3: Detailed Explanation:
Let's match each term:
A. Cohesion: Refers to the attraction between molecules of the same substance. For water, it is the strong mutual attraction between water molecules due to hydrogen bonds. Thus, A matches with II.
B. Adhesion: Refers to the attraction between molecules of different substances. In plants, it is the attraction of water molecules to polar surfaces like the lignocellulosic walls of xylem vessels. Thus, B matches with IV.
C. Surface tension: A property of liquids that allows them to resist an external force, due to the cohesive nature of its molecules. Water molecules are more attracted to each other in the liquid phase than to water in the gas phase. This leads to high surface tension. Thus, C matches with I.
D. Guttation: The process of water exudation in liquid form (as droplets) from the hydathodes at the tips or margins of leaves. This is literally water loss in the liquid phase. Thus, D matches with III.
The correct set of matches is A-II, B-IV, C-I, D-III.
Step 4: Final Answer:
Comparing our matches with the given options, option (B) correctly represents the relationships: A-II, B-IV, C-I, D-III.
Quick Tip: Remember the "co-" in cohesion means "together" (like molecules sticking together). The "ad-" in adhesion means "to" (like molecules sticking to a surface). Differentiate guttation (liquid water loss) from transpiration (water vapor loss).
Broad palm with single palm crease is visible in a person suffering from-
View Solution
Step 1: Understanding the Question:
The question asks to identify the genetic disorder associated with the physical characteristic of a broad palm with a single transverse palmar crease.
Step 3: Detailed Explanation:
Down's syndrome, also known as Trisomy 21, is a chromosomal disorder caused by the presence of all or part of a third copy of chromosome 21.
It is characterized by a set of distinct physical features. One of the well-known features is a single transverse palmar crease (also called a simian crease) across the palm of the hand, along with broad palms and short fingers.
Other features include a small round head, a flattened facial profile, a furrowed tongue, and congenital heart defects.
Thalassemia is a blood disorder. Turner's syndrome (XO) and Klinefelter's syndrome (XXY) are chromosomal disorders with different sets of symptoms, but the single palmar crease is not a defining characteristic for them.
Step 4: Final Answer:
A broad palm with a single palmar crease is a characteristic feature of Down's syndrome.
Quick Tip: Associate the term "single palmar crease" or "simian crease" directly with Down's syndrome. It is a classic and frequently tested physical marker for this condition.
Given below are two statements:
Statement I: Low temperature preserves the enzyme in a temporarily inactive state whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
Statement II: When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to evaluate two statements. The first statement is about the effect of temperature on enzyme activity. The second statement defines competitive inhibition.
Step 3: Detailed Explanation:
Statement I Analysis:
Enzymes have an optimal temperature range for activity.
Low temperatures reduce the kinetic energy of both the enzyme and substrate molecules, causing a decrease in the reaction rate. The enzyme is preserved in a temporarily inactive state. If the temperature is raised back to the optimum, its activity is restored.
High temperatures increase kinetic energy, but beyond the optimum, the heat disrupts the weak hydrogen bonds that maintain the enzyme's specific three-dimensional structure. This change in shape, called denaturation, is irreversible and leads to a permanent loss of enzymatic activity.
Therefore, Statement I is correct.
Statement II Analysis:
This statement describes competitive inhibition. A competitive inhibitor is a molecule that is structurally similar to the substrate. It competes with the substrate for binding to the active site of the enzyme. When the inhibitor is bound, the substrate cannot bind, and the enzyme's activity is inhibited. This is the precise definition of a competitive inhibitor. Therefore, Statement II is correct.
Step 4: Final Answer:
Both Statement I and Statement II are true statements.
Quick Tip: Remember that low temperature is like putting an enzyme into 'hibernation' (inactive but recoverable), while high temperature 'cooks' it (denatured and destroyed). Competitive inhibitors 'compete' for the active site because they 'look like' the real substrate.
Which one of the following common sexually transmitted diseases is completely curable when detected early and treated properly?
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the listed sexually transmitted diseases (STDs) can be completely cured.
Step 3: Detailed Explanation:
The key to answering this question is to distinguish between bacterial and viral infections.
Gonorrhoea is a bacterial infection caused by \textit{Neisseria gonorrhoeae. Bacterial infections can generally be treated and completely cured with a course of antibiotics, especially if diagnosed early.
HIV Infection, Genital herpes (caused by Herpes Simplex Virus), and Hepatitis-B are all caused by viruses. As of now, there are no cures for these viral infections. Antiviral medications can manage the symptoms, reduce the viral load, and prevent transmission, but they cannot eliminate the virus from the body completely.
Step 4: Final Answer:
Among the given options, only Gonorrhoea, being a bacterial STD, is completely curable with proper antibiotic treatment.
Quick Tip: A general rule for STDs in exams: bacterial infections (like Gonorrhoea, Syphilis, Chlamydia) are considered curable, while viral infections (like HIV, Herpes, Hepatitis B, HPV) are generally not curable, only manageable.
Given below are two statements:
Statement I: Electrostatic precipitator is most widely used in thermal power plant.
Statement II: Electrostatic precipitator in thermal power plant removes ionising radiations
In the light of the above statements, choose the most appropriate answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to evaluate two statements regarding the use and function of an electrostatic precipitator in a thermal power plant.
Step 3: Detailed Explanation:
Statement I Analysis:
Thermal power plants, which burn fossil fuels like coal, produce large amounts of fly ash and other particulate matter in their exhaust gases. The electrostatic precipitator is a highly efficient (up to 99% efficiency) and widely used device to remove these particulate pollutants from the exhaust before it is released into the atmosphere. Therefore, Statement I is correct.
Statement II Analysis:
An electrostatic precipitator works by using high-voltage electrodes to create an electric field. This field charges the dust particles in the exhaust gas, which are then attracted to and collected on oppositely charged plates. Its function is to remove particulate matter, not ionising radiations. Ionising radiation is a concern associated with nuclear power plants, not thermal power plants. Therefore, Statement II is incorrect.
Step 4: Final Answer:
Statement I is a correct fact, but Statement II incorrectly describes the function of an electrostatic precipitator.
Quick Tip: Associate "electrostatic precipitator" with "particulate matter" (like dust and ash). It uses static electricity to clean smoke. It has nothing to do with radiation.
Given below are two statements:
Statement I: Vas deferens receives a duct from seminal vesicle and opens into urethra as the ejaculatory duct.
Statement II: The cavity of the cervix is called cervical canal which along with vagina forms birth canal.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to evaluate the correctness of two statements describing parts of the male and female reproductive systems.
Step 3: Detailed Explanation:
Statement I Analysis:
This statement describes the formation of the ejaculatory duct in the male reproductive system. The vas deferens, which carries sperm from the epididymis, ascends and loops over the urinary bladder. It then receives the duct from the seminal vesicle. The union of the vas deferens and the seminal vesicle duct forms the ejaculatory duct. This duct then passes through the prostate gland and opens into the urethra. This description is anatomically correct.
Statement II Analysis:
This statement describes the formation of the birth canal in the female reproductive system. The cervix is the lower, narrow part of the uterus that opens into the vagina. The cavity within the cervix is called the cervical canal. During childbirth (parturition), the baby passes from the uterus, through the cervical canal, and then through the vagina to the outside. Therefore, the cervical canal and the vagina together constitute the birth canal. This description is also anatomically correct.
Step 4: Final Answer:
Both statements are accurate descriptions of human reproductive anatomy.
Quick Tip: To remember the male duct system, trace the path of sperm: Epididymis \(\rightarrow\) Vas deferens + Seminal vesicle duct \(\rightarrow\) Ejaculatory duct \(\rightarrow\) Urethra. For the female system, remember the birth canal is the exit path: Uterus \(\rightarrow\) Cervical Canal + Vagina.
Which one of the following symbols represents mating between relatives in human pedigree analysis?
View Solution
Step 1: Understanding the Question:
The question asks to identify the standard symbol used in a human pedigree chart to represent a consanguineous mating, i.e., mating between close relatives.
Step 3: Detailed Explanation:
In standard pedigree nomenclature:
A square represents a male.
A circle represents a female.
A horizontal line connecting a square and a circle represents mating between them.
A double horizontal line connecting a square and a circle specifically indicates a consanguineous mating (mating between relatives).
Let's analyze the options (assuming standard symbols):
(A) Shows an affected female (filled circle) and an unaffected male (unfilled square) mating.
(B) Shows a normal mating between an unaffected male and an unaffected female.
(C) Shows a mating between an unaffected male and an unaffected female connected by a double line, which is the correct symbol for mating between relatives.
(D) Shows a mating between two affected individuals.
Step 4: Final Answer:
The symbol with the double line between the male and female represents mating between relatives.
Quick Tip: In pedigree charts, remember: Single line = normal mating; Double line = consanguineous (relative) mating. This is a crucial symbol for tracking recessive genetic disorders, which are more likely to appear in the offspring of such matings.
Match List I with List II.
List I & List II
A. Taenia & I. & Nephridia
B. Paramoecium & II. & Contractile vacuole
C. Periplaneta & III. & Flame cells
D. Pheretima & IV. & Urecose gland
Choose the correct answer from the options give below:
View Solution
Step 1: Understanding the Question:
The question requires matching organisms from List I with their corresponding excretory or osmoregulatory structures from List II.
Step 3: Detailed Explanation:
A. Taenia (Tapeworm): It belongs to the phylum Platyhelminthes. The excretory structures in platyhelminths are specialized cells called flame cells (protonephridia). So, A matches III.
B. Paramoecium: It is a single-celled protozoan. For osmoregulation (excretion of excess water), it uses a specialized organelle called the contractile vacuole. So, B matches II.
C. Periplaneta (Cockroach): It belongs to the phylum Arthropoda, class Insecta. The primary excretory organs are Malpighian tubules. However, insects also use fat bodies and urecose glands to store uric acid. So, C matches IV.
D. Pheretima (Earthworm): It belongs to the phylum Annelida. The excretory organs in annelids are coiled tubular structures called nephridia. So, D matches I.
The correct combination is A-III, B-II, C-IV, D-I.
Step 4: Final Answer:
The option that corresponds to the correct matching is (D).
Quick Tip: Memorizing excretory structures of major phyla is essential. Create a list: Protozoa \(\rightarrow\) Contractile vacuole Platyhelminthes \(\rightarrow\) Flame cells Annelida \(\rightarrow\) Nephridia Arthropoda (Insects) \(\rightarrow\) Malpighian tubules
Given below are two statements:
Statement I: In prokaryotes, the positively charged DNA is held with some negatively charged proteins in a region called nucleoid.
Statement II: In eukaryotes, the negatively charged DNA is wrapped around the positively charged histone octamer to form nucleosome.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to evaluate two statements describing DNA packaging in prokaryotes and eukaryotes, focusing on the charges of DNA and associated proteins.
Step 3: Detailed Explanation:
Statement I Analysis:
This statement has a fundamental error in the charges. DNA, due to its phosphate backbone (PO\(_4^{3-}\)), is negatively charged. In prokaryotes, this negatively charged DNA is organized in a region called the nucleoid, associated with some positively charged proteins (often called histone-like proteins). The statement says "positively charged DNA" and "negatively charged proteins," which is the reverse of the actual situation. Therefore, Statement I is incorrect.
Statement II Analysis:
This statement correctly describes the basic unit of DNA packaging in eukaryotes. The negatively charged DNA molecule wraps around a core of eight histone proteins (a histone octamer). Histones are rich in positively charged amino acids (lysine and arginine), making the histone octamer positively charged. This entire complex is called a nucleosome. Therefore, Statement II is correct.
Step 4: Final Answer:
Statement I is incorrect because it reverses the charges of DNA and its associated proteins, while Statement II is correct.
Quick Tip: Always remember: DNA is an acid (Deoxyribonucleic Acid) and is \textbf{negatively charged} due to its phosphate groups. For packaging, it must be associated with \textbf{positively charged} proteins. In eukaryotes, these are histones.
Which of the following functions is carried out by cytoskeleton in a cell?
View Solution
Step 1: Understanding the Question:
The question asks to identify a key function performed by the cytoskeleton.
Step 3: Detailed Explanation:
The cytoskeleton is a network of protein filaments (microfilaments, intermediate filaments, and microtubules) within the cytoplasm. Its major functions include:
Mechanical Support: Maintaining the shape of the cell.
Anchorage of Organelles: Holding organelles in place.
Intracellular Transport: Acting as tracks for motor proteins to move vesicles and organelles.
Motility: This is a crucial function. It includes the movement of the entire cell (e.g., amoeboid movement via microfilaments) and the movement of cilia and flagella (powered by microtubules).
Let's analyze the options:
(A) Transportation: While the cytoskeleton is involved in intracellular transport, "transportation" is a very broad term.
(B) Nuclear division: The cytoskeleton (specifically microtubules forming the spindle) is essential for chromosome separation during nuclear division, but "motility" is a more general and direct function.
(C) Protein synthesis: This is the function of ribosomes.
(D) Motility: This is a direct and defining function of the cytoskeleton, encompassing various forms of cellular movement.
Given the choices, motility is one of the most prominent and direct functions of the cytoskeleton.
Step 4: Final Answer:
Motility is a primary function carried out by the cytoskeleton.
Quick Tip: Think of the cytoskeleton as the cell's "skeleton and muscles." It provides structure and is responsible for all kinds of movement, both inside the cell and of the cell itself.
Given below are two statements:
Statement I: Ligaments are dense irregular tissue.
Statement II: Cartilage is dense regular tissue.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to evaluate the correctness of two statements that classify specific types of connective tissue.
Step 3: Detailed Explanation:
Statement I Analysis:
Ligaments are connective tissues that attach bone to bone. They are composed of collagen fibres oriented in a parallel fashion to withstand tensile stress in one direction. This parallel arrangement is characteristic of dense regular connective tissue, not dense irregular. Therefore, Statement I is false.
Statement II Analysis:
Cartilage is a type of specialized connective tissue, characterized by a firm, pliable matrix and chondrocyte cells. It is not classified under the categories of dense regular or dense irregular connective tissue, which are types of connective tissue proper. Therefore, Statement II is false.
Step 4: Final Answer:
Both statements incorrectly classify the tissues. Ligaments are dense regular, and cartilage is a specialized connective tissue.
Quick Tip: Remember the key examples for connective tissue proper: \textbf{Dense Regular:} Tendons (muscle to bone) and Ligaments (bone to bone). Think "regular" arrangement for strength in one direction. \textbf{Dense Irregular:} Dermis of the skin. Think "irregular" arrangement for strength in many directions. \textbf{Specialized:} Cartilage, Bone, and Blood are in their own categories.
Which of the following are NOT considered as the part of endomembrane system?
A. Mitochondria \hspace{1cm} B. Endoplasmic Reticulum
C. Chloroplasts \hspace{1cm} D. Golgi complex
E. Peroxisomes
Choose the most appropriate answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the listed organelles are not part of the endomembrane system.
Step 3: Detailed Explanation:
The endomembrane system is a group of membranes and organelles in eukaryotic cells that work together to modify, package, and transport lipids and proteins. The components are either directly connected or exchange material through vesicle transport.
The core components of the endomembrane system are:
Endoplasmic Reticulum (B)
Golgi complex (apparatus) (D)
Lysosomes
Vacuoles
The plasma membrane
The following organelles are not part of the endomembrane system because their functions are distinct and they do not originate from the ER-Golgi pathway:
Mitochondria (A): They are involved in cellular respiration and have their own DNA.
Chloroplasts (C): They are involved in photosynthesis and have their own DNA.
Peroxisomes (E): They are involved in metabolic processes, including breaking down fatty acids and detoxifying harmful substances.
Therefore, mitochondria, chloroplasts, and peroxisomes are not part of the endomembrane system.
Step 4: Final Answer:
The organelles not part of the endomembrane system are A, C, and E.
Quick Tip: Think of the endomembrane system as a cell's internal "postal service" for proteins and lipids: ER \(\rightarrow\) Golgi \(\rightarrow\) Vesicles. Mitochondria and chloroplasts are the cell's "power plants" and are functionally separate. Peroxisomes are the "detox centers."
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Amniocentesis for sex determination is one of the strategies of Reproductive and Child Health Care Programme.
Reason R: Ban on amniocentesis checks increasing menace of female foeticide.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question presents an Assertion and a Reason regarding amniocentesis, its use for sex determination, and its relation to government health programs and social issues.
Step 3: Detailed Explanation:
Assertion A Analysis:
Amniocentesis is a prenatal diagnostic technique used to detect genetic abnormalities in the fetus. Its use for determining the sex of the fetus is a misuse of the technology and is legally banned in India under the Pre-Conception and Pre-Natal Diagnostic Techniques (PCPNDT) Act, 1994. The Reproductive and Child Health Care (RCH) Programme is a government initiative to improve maternal and child health. Promoting a banned practice like sex determination is completely against the objectives of the RCH Programme. Therefore, Assertion A is false.
Reason R Analysis:
The primary reason for imposing a statutory ban on amniocentesis for sex determination was to curb the widespread practice of female foeticide, which has led to a skewed sex ratio in many parts of the country. By making it illegal to determine and disclose the sex of the fetus, the government aims to prevent the selective abortion of female fetuses. Therefore, Reason R is true.
Step 4: Final Answer:
The Assertion (A) is false, while the Reason (R) is true.
Quick Tip: Amniocentesis for checking genetic disorders = a valid medical procedure. Amniocentesis for sex determination = a banned, illegal act. Government health programs (like RCH) promote health and would never include a banned activity in their strategies.
Given below are statements: one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Nephrons are of two types: Cortical \& Juxta medullary, based on their relative position in cortex and medulla.
Reason R: Juxta medullary nephrons have short loop of Henle whereas, cortical nephrons have longer loop of Henle.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question presents an Assertion and a Reason about the types of nephrons and the length of their loops of Henle.
Step 3: Detailed Explanation:
Assertion A Analysis:
Nephrons are the functional units of the kidney. Based on their location within the kidney, they are classified into two main types:
Cortical nephrons: The majority of nephrons (about 85%), with their glomeruli located in the outer cortex.
Juxtamedullary nephrons: Their glomeruli are located deep in the cortex, close to the medulla (juxta = near).
This classification based on position is correct. Therefore, Assertion A is true.
Reason R Analysis:
This statement describes the loop of Henle length for the two types of nephrons. However, it states the facts incorrectly.
Cortical nephrons have a short loop of Henle that only extends slightly into the medulla.
Juxtamedullary nephrons have a very long loop of Henle that extends deep into the medulla. This long loop is crucial for creating the concentration gradient required to produce concentrated urine.
The Reason statement claims the opposite, so Reason R is false.
Step 4: Final Answer:
The Assertion (A) is true, but the Reason (R) is false.
Quick Tip: Remember: \textbf{Juxtamedullary} means "near the medulla." These nephrons dive deep into the medulla, so they need a \textbf{long} loop of Henle. Cortical nephrons stay mainly in the cortex and have \textbf{short} loops.
Match List I with List II.
List I (Type of Joint) & List II (Found between)
A. Cartilaginous Joint & I. & Between flat skull bones
B. Ball and Socket Joint & II. & Between adjacent vertebrae in
& & & vertebral column
C. Fibrous Joint & III. & Between carpal and metacarpal of
& & & thumb
D. Saddle Joint & IV. & Between Humerus and
& & & Pectoral girdle
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to match the type of joint in List I with its correct location in the human body from List II.
Step 3: Detailed Explanation:
A. Cartilaginous Joint: These joints have bones connected by cartilage and allow for limited movement. An example is the joint between adjacent vertebrae in the vertebral column. Thus, A matches with II.
B. Ball and Socket Joint: This is a type of synovial joint where the ball-shaped head of one bone fits into the cup-like socket of another, allowing for movement in many directions. An example is the joint between the humerus and the pectoral girdle (the shoulder joint). Thus, B matches with IV.
C. Fibrous Joint: These joints have bones connected by dense fibrous connective tissue and allow for no movement. The sutures between the flat skull bones are examples of fibrous joints. Thus, C matches with I.
D. Saddle Joint: This is another type of synovial joint that allows for back and forth and side-to-side movement, but limited rotation. The classic example is the joint between the carpal and metacarpal of the thumb. Thus, D matches with III.
The correct matching is A-II, B-IV, C-I, D-III.
Step 4: Final Answer:
The correct option is (C), which reflects the matching A-II, B-IV, C-I, D-III.
Quick Tip: Associate specific, unique examples with each joint type: Saddle Joint \(\rightarrow\) Thumb; Fibrous Joint \(\rightarrow\) Skull Sutures; Cartilaginous Joint \(\rightarrow\) Vertebrae. This makes matching questions easier.
Vital capacity of lung is
View Solution
Step 1: Understanding the Question:
The question asks for the correct formula to calculate the Vital Capacity (VC) of the lungs.
Step 3: Detailed Explanation:
Let's define the terms related to lung volumes:
Tidal Volume (TV): The volume of air inspired or expired during a normal, quiet breath.
Inspiratory Reserve Volume (IRV): The additional volume of air that can be forcibly inhaled after a normal inspiration.
Expiratory Reserve Volume (ERV): The additional volume of air that can be forcibly exhaled after a normal expiration.
Residual Volume (RV): The volume of air remaining in the lungs even after a forceful expiration.
Vital Capacity (VC) is defined as the maximum amount of air a person can exhale after filling the lungs to the maximum extent (a maximal inspiration). It represents the total usable volume of the lungs for breathing.
The formula for Vital Capacity is the sum of the three volumes that can be exchanged:
\[ VC = TV + IRV + ERV \]
The total lung capacity (TLC) is the vital capacity plus the residual volume (TLC = VC + RV = TV + IRV + ERV + RV).
Step 4: Final Answer:
The correct formula for vital capacity is IRV + ERV + TV.
Quick Tip: Remember that "Vital Capacity" is the 'vital' or usable part of the lung volume. It includes everything you can possibly move in and out. The "Residual Volume" is what's left over and cannot be exhaled, so it's not part of the vital capacity.
Match List I with List II.
List I & List II
A. Ringworm & I. & Haemophilus influenzae
B. Filariasis & II. & Trichophyton
C. Malaria & III. & Wuchereria bancrofti
D. Pneumonia & IV. & Plasmodium vivax
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching the diseases in List I with their respective causative organisms in List II.
Step 3: Detailed Explanation:
A. Ringworm: Despite its name, ringworm is not caused by a worm. It is a common fungal infection of the skin, caused by fungi belonging to genera like Trichophyton, Microsporum, and Epidermophyton. Thus, A matches with II.
B. Filariasis (Elephantiasis): This is a parasitic disease caused by infection with filarial worms, specifically Wuchereria bancrofti. Thus, B matches with III.
C. Malaria: This is a protozoan disease caused by parasites of the genus Plasmodium. \textit{Plasmodium vivax is one of the species that cause malaria. Thus, C matches with IV.
D. Pneumonia: This is an infection of the lungs that can be caused by bacteria, viruses, or fungi. One of the common bacterial causes is Haemophilus influenzae (another is \textit{Streptococcus pneumoniae). Thus, D matches with I.
The correct set of matches is A-II, B-III, C-IV, D-I.
Step 4: Final Answer:
The option that corresponds to the correct matching is (B).
Quick Tip: It is crucial to know the type of pathogen for common diseases: Ringworm = Fungal; Filariasis = Helminth (worm); Malaria = Protozoan; Pneumonia = Bacterial/Viral. This helps narrow down the options quickly.
In which blood corpuscles, the HIV undergoes replication and produces progeny viruses?
View Solution
Step 1: Understanding the Question:
The question asks to identify the specific type of blood cell in which the Human Immunodeficiency Virus (HIV) replicates.
Step 3: Detailed Explanation:
HIV is a retrovirus that attacks the human immune system. Its primary target is a specific type of lymphocyte called the Helper T-cell, also known as a T\(_H\) cell or a CD4+ T-cell.
The virus uses the CD4 protein on the surface of these helper T-cells as a receptor to gain entry. Once inside the T\(_H\) cell, HIV uses its own enzyme, reverse transcriptase, to convert its RNA genome into DNA. This viral DNA is then integrated into the host cell's DNA.
The infected T\(_H\) cell is then hijacked to become a "virus factory," producing new HIV particles (progeny viruses). These new viruses bud off from the cell, ready to infect other T\(_H\) cells. The process eventually leads to the death of the host T\(_H\) cell.
The progressive destruction of T\(_H\) cells is what severely weakens the immune system, leading to Acquired Immunodeficiency Syndrome (AIDS).
Step 4: Final Answer:
HIV replicates within Helper T-cells (T\(_H\) cells).
Quick Tip: Remember that HIV attacks the "commander" of the immune system. The Helper T-cells (T\(_H\)) play a central role in coordinating the immune response, so their destruction cripples the entire system.
Match List I with List II.
List I & List II
A. Gene 'a' & I. & \(\beta\)-galactosidase
B. Gene 'y' & II. & Transacetylase
C. Gene 'i' & III. & Permease
D. Gene 'z' & IV. & Repressor protein
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching the genes of the lac operon (List I) with the proteins they encode (List II).
Step 3: Detailed Explanation:
The lac operon in E. coli consists of a regulator gene and three structural genes.
C. Gene 'i' (lacI): This is the regulator gene. It codes for the repressor protein, which binds to the operator region and prevents transcription in the absence of lactose. Thus, C matches with IV.
D. Gene 'z' (lacZ): This is the first structural gene. It codes for the enzyme \(\beta\)-galactosidase, which hydrolyzes lactose into glucose and galactose. Thus, D matches with I.
B. Gene 'y' (lacY): This is the second structural gene. It codes for the protein permease, which increases the permeability of the cell to lactose, allowing it to enter the cell. Thus, B matches with III.
A. Gene 'a' (lacA): This is the third structural gene. It codes for the enzyme transacetylase. Thus, A matches with II.
The correct matching is A-II, B-III, C-IV, D-I.
Step 4: Final Answer:
The option that corresponds to the correct matching is (C).
Quick Tip: Remember the order of the structural genes in the lac operon: z, y, a. And their functions: 'z' for \(\beta\)-galactosidase (breaks down lactose), 'y' for permease (lets lactose in), and 'a' for transacetylase. The 'i' gene stands for "inhibitor" (repressor).
Match List I with List II with respect to human eye.
List I & List II
A. Fovea & I. & Visible coloured portion of eye that regulates
& & & diameter of pupil.
B. Iris & II. & External layer of eye formed of dense
& & & connective tissue.
C. Blind spot & III. & Point of greatest visual acuity or resolution.
D. Sclera & IV. & Point where optic nerve leaves the eyeball
& & & and photoreceptor cells are absent.
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to match parts of the human eye (List I) with their correct description or function (List II).
Step 3: Detailed Explanation:
A. Fovea: The fovea is a small depression in the retina (at the center of the macula lutea) where cone cells are most concentrated. This is the region of the most acute vision, or the point of greatest visual acuity or resolution. Thus, A matches with III.
B. Iris: The iris is the thin, circular structure in the eye responsible for controlling the diameter and size of the pupil. It is the visible coloured portion of the eye. Thus, B matches with I.
C. Blind spot: This is the point on the retina where the axons of the ganglion cells exit the eye to form the optic nerve. Because there are no photoreceptor cells (rods or cones) at this location, it is insensitive to light. Thus, it is the point where the optic nerve leaves the eyeball and photoreceptor cells are absent. Thus, C matches with IV.
D. Sclera: The sclera is the tough, fibrous, white outer layer of the eyeball. It is the external layer of the eye formed of dense connective tissue. Thus, D matches with II.
The correct set of matches is A-III, B-I, C-IV, D-II.
Step 4: Final Answer:
The option that corresponds to the correct matching is (B).
Quick Tip: Create simple associations for eye parts: Fovea = Focus (sharpest vision); Iris = Colour (controls pupil); Sclera = White part (tough outer layer); Blind Spot = No vision (optic nerve exit).
Select the correct group/set of Australian Marsupials exhibiting adaptive radiation.
View Solution
Step 1: Understanding the Question:
The question asks to identify the group that consists exclusively of Australian marsupials, which are a classic example of adaptive radiation.
Step 3: Detailed Explanation:
Adaptive radiation is the evolutionary process where organisms diversify rapidly from an ancestral species into a multitude of new forms, particularly when a change in the environment makes new resources available, creates new challenges, or opens new environmental niches. Australian marsupials are a prime example, having evolved from a common ancestor to fill diverse ecological roles.
We need to find the option where all listed animals are Australian marsupials.
(A) Lemur (primate), Anteater (placental mammal), Wolf (placental mammal). This group is incorrect.
(B) Tasmanian wolf (marsupial), Bobcat (placental mammal), Marsupial mole (marsupial). The presence of a placental mammal makes this group incorrect.
(C) Numbat (marsupial anteater), Spotted cuscus (marsupial), and Flying phalanger (marsupial sugar glider) are all Australian marsupials. This group is correct.
(D) Mole (placental mammal), Flying squirrel (placental mammal), Tasmanian tiger cat (marsupial). The presence of placental mammals makes this group incorrect.
The animals in options A, B, and D that are not marsupials (e.g., Anteater, Wolf, Bobcat, Mole, Flying squirrel) are placental mammals that show convergent evolution with their marsupial counterparts.
Step 4: Final Answer:
The only group consisting entirely of Australian marsupials is Numbat, Spotted cuscus, and Flying phalanger.
Quick Tip: To answer questions about adaptive radiation in Australian marsupials, you must be able to distinguish them from their placental counterparts. For example: Marsupial Mole vs. Placental Mole; Tasmanian Wolf vs. Placental Wolf; Flying Phalanger vs. Flying Squirrel. The question is testing this distinction.
Which of the following statements are correct regarding female reproductive cycle?
A. In non-primate mammals cyclical changes during reproduction are called oestrus cycle.
B. First menstrual cycle begins at puberty and is called menopause.
C. Lack of menstruation may be indicative of pregnancy.
D. Cyclic menstruation extends between menarche and menopause.
Choose the most appropriate answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements about the female reproductive cycle from a given list.
Step 3: Detailed Explanation:
Let's evaluate each statement:
A. In non-primate mammals cyclical changes during reproduction are called oestrus cycle. This is correct. Primates (monkeys, apes, humans) have a menstrual cycle, while non-primate mammals (like cows, dogs, cats) have an oestrus cycle (or "heat" cycle).
B. First menstrual cycle begins at puberty and is called menopause. This is incorrect. The first menstrual cycle is called menarche. Menopause is the permanent cessation of the menstrual cycle.
C. Lack of menstruation may be indicative of pregnancy. This is correct. While missed periods can be due to stress, poor health, etc., it is a primary indicator of pregnancy.
D. Cyclic menstruation extends between menarche and menopause. This is correct. The reproductive life of a female, characterized by cyclic menstruation, starts at menarche and ends at menopause.
Therefore, statements A, C, and D are correct.
Step 4: Final Answer:
The correct option is (A), which includes statements A, C, and D.
Quick Tip: Remember the key terms for the start and end of the menstrual cycle: Menarche = Start (at puberty); Menopause = Pause/End (in later life). Don't confuse the two.
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Endometrium is necessary for implantation of blastocyst.
Reason R: In the absence of fertilization, the corpus luteum degenerates that causes disintegration of endometrium.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question presents an Assertion about the role of the endometrium in implantation and a Reason that describes the fate of the endometrium in the absence of fertilization. We need to evaluate their truth and relationship.
Step 3: Detailed Explanation:
Assertion A Analysis:
The endometrium is the inner lining of the uterus. It becomes thick, vascular, and rich in glands under the influence of progesterone to prepare for the implantation of the blastocyst. Implantation involves the embedding of the blastocyst into this receptive uterine wall. Therefore, the endometrium is absolutely necessary for implantation. Assertion A is true.
Reason R Analysis:
After ovulation, the remnant of the Graafian follicle develops into the corpus luteum, which secretes progesterone. Progesterone maintains the endometrium. If fertilization does not occur, the corpus luteum degenerates, leading to a fall in progesterone levels. This progesterone withdrawal causes the disintegration of the endometrium, resulting in menstruation. This statement accurately describes the events of the late luteal phase of the menstrual cycle. Reason R is true.
Explanation Analysis:
While both statements are true and related to the endometrium, Reason R explains why menstruation occurs in the absence of fertilization. It does not explain \textit{why the endometrium is necessary for implantation (i.e., its role in providing nourishment, anchorage, and forming the maternal part of the placenta). The reason for the necessity of the endometrium is its structural and physiological preparedness to receive the embryo. Therefore, R is a true statement but not the direct correct explanation for A.
Step 4: Final Answer:
Both Assertion A and Reason R are true, but R is not the correct explanation of A.
Quick Tip: When evaluating Assertion-Reason questions, always ask "Does R explain A?" In this case, R explains the menstrual cycle, while A states the function of the endometrium in pregnancy. They are two different aspects of the same structure.
Match List I with List II.
List I & List II
A. Heroin & I. & Effect on cardiovascular system
B. Marijuana & II. & Slow down body function
C. Cocaine & III. & Painkiller
D. Morphine & IV. & Interfere with transport of dopamine
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching different psychoactive drugs (List I) with their primary effects or mechanisms of action (List II).
Step 3: Detailed Explanation:
A. Heroin (Smack): Heroin (diacetylmorphine) is an opioid and a powerful central nervous system depressant. It binds to opioid receptors and is known to slow down body functions. Thus, A matches with II.
B. Marijuana (Cannabinoids): Cannabinoids interact with cannabinoid receptors in the brain. They are known to have a significant effect on the cardiovascular system, such as increasing heart rate. Thus, B matches with I.
C. Cocaine (Coke): Cocaine is a potent central nervous system stimulant. It interferes with the transport of the neurotransmitter dopamine by blocking its reuptake, leading to a buildup of dopamine in the synapse and feelings of euphoria. Thus, C matches with IV.
D. Morphine: Morphine is a very effective sedative and painkiller (analgesic). It is extracted from the poppy plant and is a primary opioid. Thus, D matches with III.
The correct matching is A-II, B-I, C-IV, D-III.
Step 4: Final Answer:
The option that corresponds to the correct matching is (B).
Quick Tip: Categorize drugs by their main effect: Depressants (Heroin, Morphine), Stimulants (Cocaine), and Hallucinogens (Marijuana has hallucinogenic properties). Knowing the category helps deduce the general effect.
Which of the following is not a cloning vector?
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given options is not a cloning vector.
Step 3: Detailed Explanation:
A cloning vector is a small piece of DNA that can be stably maintained in an organism, and into which a foreign DNA fragment can be inserted for cloning purposes. It acts as a vehicle to carry foreign DNA into a host cell.
Let's analyze the options:
pBR322: This is a well-known, artificially constructed plasmid vector used for cloning in E. coli. It is a cloning vector.
BAC (Bacterial Artificial Chromosome): This is a cloning vector based on the F-plasmid of \textit{E. coli, used to clone large DNA fragments (100-300 kb). It is a cloning vector.
YAC (Yeast Artificial Chromosome): This is a cloning vector that can accommodate very large DNA fragments (up to a million base pairs) and is replicated in yeast cells. It is a cloning vector.
Probe: A DNA probe is a short, single-stranded fragment of DNA (or RNA) that is labeled with a radioactive or fluorescent marker. It is used to detect the presence of a specific complementary nucleotide sequence in a DNA sample through hybridization. It is a detection tool, not a vehicle for carrying and replicating DNA.
Step 4: Final Answer:
A probe is a tool for detection, not a cloning vector.
Quick Tip: Remember the key difference: a \textbf{vector is like a truck that carries cargo (DNA) into a factory (host cell) to be copied. A \textbf{probe} is like a scanner used to find a specific item (a DNA sequence) in a warehouse.
Which of the following statements is correct?
View Solution
Step 1: Understanding the Question:
The question asks to identify the one correct statement among the four options related to ecological concepts of water pollution.
Step 3: Detailed Explanation:
(A) Algal Bloom decreases fish mortality: This is incorrect. Algal blooms cause a severe depletion of dissolved oxygen in the water when the algae die and are decomposed by bacteria. This leads to hypoxia or anoxia, which increases fish mortality.
(B) Eutrophication refers to increase in domestic sewage and waste water in lakes.: This is an imprecise definition. Eutrophication is the natural aging of a lake by nutrient enrichment of its water. Cultural or accelerated eutrophication is caused by pollutants like domestic sewage and agricultural fertilizers, which add excess nutrients (like nitrates and phosphates). The statement confuses the cause with the process itself.
\titem (C) Biomagnification refers to increase in concentration of the toxicant at successive trophic levels.: This is the correct definition of biomagnification. Toxic substances that are not metabolized or excreted (like DDT or mercury) accumulate in an organism's tissues and become more concentrated in organisms at higher trophic levels.
(D) Presence of large amount of nutrients in water restricts 'Algal Bloom': This is incorrect. The presence of large amounts of nutrients (eutrophication) is the primary cause that promotes or causes massive algal blooms.
Step 4: Final Answer:
The only correct statement is (C), which accurately defines biomagnification.
Quick Tip: Memorize the definitions of key environmental terms: \textbf{Eutrophication:} Nutrient enrichment. \textbf{Algal Bloom:} Consequence of eutrophication. \textbf{Biomagnification:} Toxicant concentration increases up the food chain. \textbf{BOD (Biological Oxygen Demand):} Measure of organic pollution.
Which one of the following techniques does not serve the purpose of early diagnosis of a disease for its early treatment?
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the listed techniques is generally not suitable for the \textit{early diagnosis of a disease, where the pathogen or marker concentration is very low.
Step 3: Detailed Explanation:
Early diagnosis is crucial for effective treatment. It relies on techniques that are highly sensitive and can detect pathogens or biomarkers even at very low concentrations.
(A) ELISA: This is a highly sensitive immunological assay that can detect the presence of specific antigens (from the pathogen) or antibodies (produced by the host in response to infection). It is widely used for early diagnosis (e.g., for HIV).
(B) Recombinant DNA Technology: This technology allows for the creation of DNA probes that can hybridize with the nucleic acid of a pathogen, enabling its detection even in minute quantities. It is a basis for many early diagnostic tools.
(D) PCR: The polymerase chain reaction is a technique used to amplify a specific DNA sequence. It can detect a pathogen's DNA or RNA from a very small sample by making millions of copies, making it an extremely sensitive tool for early diagnosis.
(C) Serum and Urine analysis: Conventional analysis of serum and urine typically relies on detecting the physiological symptoms of a disease, such as the presence of certain metabolites or a high concentration of pathogens. These signs are often only apparent after the infection has progressed significantly. Therefore, while useful, it is generally not considered a technique for \textit{early diagnosis compared to the molecular methods listed.
Step 4: Final Answer:
Conventional serum and urine analysis is not typically used for the very early diagnosis of diseases before symptoms are well-established.
Quick Tip: For questions on "early diagnosis," think about molecular techniques that can amplify or detect very small amounts of a substance. PCR, ELISA, and DNA probes are classic examples of such sensitive methods. Conventional methods are usually less sensitive.
Given below are two statements:
Statement I: RNA mutates at a faster rate.
Statement II: Viruses having RNA genome and shorter life span mutate and evolve faster.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to evaluate two statements concerning the mutation rate of RNA and the evolutionary rate of RNA viruses.
Step 3: Detailed Explanation:
Statement I Analysis:
Compared to DNA, RNA is inherently less stable. RNA molecules are typically single-stranded and the sugar (ribose) has a hydroxyl group at the 2' position, making it more susceptible to hydrolysis. Furthermore, the enzymes that replicate RNA genomes (RNA-dependent RNA polymerases) lack the proofreading mechanisms that DNA polymerases have. This lack of proofreading means that errors made during replication are not corrected, leading to a much higher mutation rate. Thus, Statement I is true.
Statement II Analysis:
This statement is a direct consequence of the principle described in Statement I. Viruses with RNA genomes (like influenza virus, HIV, and coronaviruses) benefit from the high mutation rate of RNA. This, combined with their short generation time (short life span), allows them to generate a vast amount of genetic variation in a short period. This rapid generation of variants enables them to evolve quickly, for example, to evade the host immune system or develop drug resistance. Thus, Statement II is also true.
Step 4: Final Answer:
Both statements are correct facts in molecular biology and virology.
Quick Tip: Remember the key reasons for RNA's high mutation rate: it is chemically less stable than DNA, and its replication machinery lacks a "spell-checker" (proofreading ability). This is why we need a new flu vaccine every year - the RNA virus mutates and evolves rapidly.
Match List I with List II.
List I & List II
A. CCK & I. & Kidney
B. GIP & II. & Heart
C. ANF & III. & Gastric gland
D. ADH & IV. & Pancreas
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching hormones/factors in List I with their target organ or source in List II.
Step 3: Detailed Explanation:
A. CCK (Cholecystokinin): This is a gastrointestinal hormone secreted by the duodenum. It acts on the pancreas to stimulate the secretion of pancreatic enzymes and on the gall bladder to cause its contraction. Thus, A matches with IV.
B. GIP (Gastric Inhibitory Peptide): This is another gastrointestinal hormone. As its name suggests, it inhibits gastric secretion and motility, meaning it acts on the gastric gland. Thus, B matches with III.
C. ANF (Atrial Natriuretic Factor): This peptide hormone is secreted by the atrial walls of the heart in response to high blood pressure. It acts on the kidneys to promote the excretion of sodium and water, thereby lowering blood pressure. Thus, C matches with II.
D. ADH (Antidiuretic Hormone or Vasopressin): This hormone is released from the posterior pituitary but acts on the collecting ducts and distal convoluted tubules of the kidney to increase water reabsorption. Thus, D matches with I.
The correct set of matches is A-IV, B-III, C-II, D-I.
Step 4: Final Answer:
The option that corresponds to the correct matching is (B).
Quick Tip: Break down the hormone names for clues: \textbf{C}hole\textbf{C}ysto\textbf{K}inin: Cholecysto- refers to the gall bladder, and -kinin refers to movement. \textbf{G}astric \textbf{I}nhibitory \textbf{P}eptide: It inhibits gastric functions. \textbf{A}trial \textbf{N}atriuretic \textbf{F}actor: Atrial refers to the heart atria. \textbf{A}nti\textbf{D}iuretic \textbf{H}ormone: Anti-diuresis means preventing water loss, a function of the kidney.
Match List I with List II.
List I (Cells) & List II (Secretion)
A. Peptic cells & I. & Mucus
B. Goblet cells & II. & Bile juice
C. Oxyntic cells & III. & Proenzyme pepsinogen
D. Hepatic cells & IV. & HCl and intrinsic factor for absorption of vitamin B\(_{12}\)
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching different types of cells (List I) with their respective secretions (List II).
Step 3: Detailed Explanation:
A. Peptic cells (or Chief cells): These cells are found in the gastric glands of the stomach and are responsible for secreting the inactive proenzyme pepsinogen. Thus, A matches with III.
B. Goblet cells: These are specialized mucus-secreting cells found in the epithelial lining of various organs, including the stomach, intestines, and respiratory tract. Their primary secretion is mucus, which serves a protective function. Thus, B matches with I.
C. Oxyntic cells (or Parietal cells): These cells are also found in the gastric glands. They secrete hydrochloric acid (HCl) and intrinsic factor, which is essential for the absorption of vitamin B\(_{12}\). Thus, C matches with IV.
D. Hepatic cells (Hepatocytes): These are the main cells of the liver. They have numerous functions, including the production and secretion of bile juice. Thus, D matches with II.
The correct set of matches is A-III, B-I, C-IV, D-II.
Step 4: Final Answer:
The option that corresponds to the correct matching is (D).
Quick Tip: Memorize the cells of the gastric gland: Mucous neck cells / Goblet cells \(\rightarrow\) Mucus Peptic/Chief cells \(\rightarrow\) Pepsinogen (Proenzyme) Oxyntic/Parietal cells \(\rightarrow\) HCl + Intrinsic factor This covers three of the four items in this question.
Given below are two statements:
Statement I: A protein is imagined as a line, the left end represented by first amino acid (C-terminal) and the right end represented by last amino acid (N-terminal)
Statement II: Adult human haemoglobin, consists of 4 subunits (two subunits of \(\alpha\) type and two subunits of \(\beta\) type.)
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to evaluate two statements. Statement I describes the convention for representing the terminals of a polypeptide chain. Statement II describes the subunit structure of adult human hemoglobin.
Step 3: Detailed Explanation:
Statement I Analysis:
A protein or polypeptide chain is a polymer of amino acids linked by peptide bonds. By convention, the sequence is written starting from the amino acid with the free amino group (-NH\(_2\)) and ending with the amino acid with the free carboxyl group (-COOH).
The beginning of the chain (the first amino acid) is called the N-terminal (amino-terminal).
The end of the chain (the last amino acid) is called the C-terminal (carboxyl-terminal).
The statement incorrectly describes the left end as C-terminal and the right end as N-terminal. It's the other way around. Therefore, Statement I is false.
Statement II Analysis:
Adult human hemoglobin (HbA) is a classic example of a protein with a quaternary structure. It is a tetramer, meaning it is composed of four polypeptide subunits. It consists of two identical \(\alpha\)-chains and two identical \(\beta\)-chains (\(\alpha_2\beta_2\)). Each subunit contains a heme group that binds oxygen. This statement is correct. Therefore, Statement II is true.
Step 4: Final Answer:
Statement I is false, and Statement II is true.
Quick Tip: Remember the alphabetical order for protein terminals: the chain starts with the \textbf{A}mino (N) terminal and ends with the \textbf{C}arboxyl (C) terminal. N comes before C in the alphabet.
Match List I with List II.
List I (Interacting species) & List II (Name of Interaction)
A. A Leopard and a Lion in a forest/grassland & I. & Competition
B. A Cuckoo laying egg in a Crow's nest & II. & Brood parasitism
C. Fungi and root of a higher plant in Mycorrhizae & III. & Mutualism
D. A cattle egret and a Cattle in a field & IV. & Commensalism
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching specific examples of species interactions (List I) with the correct ecological term for that interaction (List II).
Step 3: Detailed Explanation:
A. A Leopard and a Lion in a forest/grassland: Leopards and lions are large predators that often share the same habitat and prey on similar animals. Since they both require the same limited resources (food, territory), they are in competition with each other. Thus, A matches with I.
B. A Cuckoo laying egg in a Crow's nest: The cuckoo lays its eggs in the nest of another bird species (the host, like a crow), which then unknowingly raises the cuckoo chick, often at the expense of its own offspring. This is a classic example of brood parasitism. Thus, B matches with II.
C. Fungi and root of a higher plant in Mycorrhizae: Mycorrhiza is a symbiotic association between a fungus and the roots of a plant. The fungus helps the plant absorb nutrients and water from the soil, and the plant provides the fungus with carbohydrates. Since both partners benefit, this is an example of mutualism. Thus, C matches with III.
D. A cattle egret and a Cattle in a field: Cattle egrets are birds that follow grazing cattle. As the cattle move and graze, they stir up insects from the vegetation, which the egrets then easily catch and eat. The egret benefits, while the cattle are largely unaffected. This is a textbook example of commensalism (+/0). Thus, D matches with IV.
The correct set of matches is A-I, B-II, C-III, D-IV.
Step 4: Final Answer:
The option that corresponds to the correct matching is (B).
Quick Tip: These four are the most frequently cited examples of their respective interactions. Committing them to memory is a high-yield strategy for ecology questions. Competition \(\rightarrow\) Lion/Leopard Brood Parasitism \(\rightarrow\) Cuckoo/Crow Mutualism \(\rightarrow\) Mycorrhizae Commensalism \(\rightarrow\) Cattle Egret/Cattle
Once the undigested and unabsorbed substances enter the caecum, their backflow is prevented by-
View Solution
Step 1: Understanding the Question:
The question asks to identify the anatomical structure that prevents the backflow of contents from the caecum (part of the large intestine) into the ileum (part of the small intestine).
Step 3: Detailed Explanation:
Let's analyze the locations and functions of the given sphincters/valves:
Gastro-oesophageal sphincter: Located between the esophagus and the stomach. It prevents the backflow of acidic stomach contents into the esophagus.
Pyloric sphincter: Located between the stomach and the duodenum (the first part of the small intestine). It controls the passage of chyme from the stomach into the small intestine.
Sphincter of Oddi: Located where the common bile duct and pancreatic duct enter the duodenum. It controls the flow of bile and pancreatic juice into the small intestine.
Ileo-caecal valve: Located at the junction of the ileum (the last part of the small intestine) and the caecum (the first part of the large intestine). Its primary function is to allow the passage of digested food from the small intestine to the large intestine and to prevent the backflow of the contents of the large intestine (which has a high bacterial load) into the small intestine.
Step 4: Final Answer:
The backflow of substances from the caecum is prevented by the ileo-caecal valve.
Quick Tip: The names of the valves and sphincters often give away their location. "Ileo-caecal" is at the junction of the \textbf{ileum} and the \textbf{caecum}. "Gastro-oesophageal" is at the junction of the stomach (\textbf{gastro}) and the \textbf{esophagus}.
Match List I with List II.
List I & List II
A. P-wave & I. & Beginning of systole
B. Q-wave & II. & Repolarisation of ventricles
C. QRS complex & III. & Depolarisation of atria
D. T-wave & IV. & Depolarisation of ventricles
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching the different waves and complexes of a standard electrocardiogram (ECG) in List I with the cardiac event they represent in List II.
Step 3: Detailed Explanation:
A. P-wave: This represents the electrical excitation, or depolarisation of the atria, which leads to the contraction of both atria. Thus, A matches with III.
C. QRS complex: This complex represents the depolarisation of the ventricles, which initiates ventricular contraction (systole). Thus, C matches with IV.
B. Q-wave: The QRS complex marks the onset of ventricular systole. The Q wave is the first downward deflection and marks the beginning of ventricular systole. Thus, B matches with I.
D. T-wave: This wave represents the return of the ventricles from an excited to a normal state, which is called repolarisation of the ventricles. The end of the T-wave marks the end of systole. Thus, D matches with II.
The correct set of matches is A-III, B-I, C-IV, D-II.
Step 4: Final Answer:
The option that corresponds to the correct matching is (B).
Quick Tip: Remember the ECG sequence: \textbf{P} wave \(\rightarrow\) \textbf{A}trial depolarization (\textbf{P}recedes \textbf{A}trial contraction). \textbf{QRS} complex \(\rightarrow\) \textbf{V}entricular depolarization (\textbf{R}eally \textbf{S}trong contraction of ventricles). \textbf{T} wave \(\rightarrow\) Ven\textbf{t}ricular \textbf{r}epolarization (\textbf{T}ime to \textbf{R}elax).
Radial symmetry is NOT found in adults of phylum __________.
View Solution
Step 1: Understanding the Question:
The question asks to identify the phylum among the given options whose adult members do not exhibit radial symmetry.
Step 3: Detailed Explanation:
Let's analyze the symmetry of the adult forms in each phylum:
(A) Echinodermata: A unique feature of echinoderms (like starfish and sea urchins) is that their larvae are bilaterally symmetrical, but the adults exhibit penta-radial symmetry. So they have radial symmetry.
(B) Ctenophora (Comb jellies): These animals exhibit biradial symmetry, which is a type of radial symmetry.
(C) Hemichordata (e.g., Balanoglossus): These are worm-like marine animals. They are exclusively bilaterally symmetrical throughout their lives. They do not have radial symmetry.
(D) Coelenterata (Cnidaria): This phylum, which includes jellyfish and sea anemones, is characterized by radial symmetry.
Step 4: Final Answer:
Adults of the phylum Hemichordata are bilaterally symmetrical, not radially symmetrical.
Quick Tip: Remember the "big three" phyla with radial symmetry: Coelenterata (Cnidaria), Ctenophora, and adult Echinodermata. All other major animal phyla are primarily bilaterally symmetrical.
Match List I with List II.
List I & List II
A. Vasectomy & I. & Oral method
B. Coitus interruptus & II. & Barrier method
C. Cervical caps & III. & Surgical method
D. Saheli & IV. & Natural method
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching different contraceptive methods (List I) with their correct category (List II).
Step 3: Detailed Explanation:
A. Vasectomy: This is a permanent method of contraception for males where the vas deferens is cut and tied to prevent the transport of sperm. This is a surgical method (sterilization). Thus, A matches with III.
B. Coitus interruptus (withdrawal method): This involves withdrawing the penis from the vagina just before ejaculation. It is a traditional method that relies on timing and is considered a natural method. Thus, B matches with IV.
C. Cervical caps: These are devices made of rubber that are inserted into the vagina to cover the cervix, physically preventing sperm from entering the uterus. This is a type of barrier method. Thus, C matches with II.
D. Saheli: This is a non-steroidal contraceptive pill taken once a week. As it is a pill, it is an oral method. Thus, D matches with I.
The correct set of matches is A-III, B-IV, C-II, D-I.
Step 4: Final Answer:
The option that corresponds to the correct matching is (C).
Quick Tip: Categorize contraceptive methods into broad groups: \textbf{Natural:} Rhythm method, Coitus interruptus. \textbf{Barrier:} Condoms, Diaphragms, Cervical caps. \textbf{Chemical/Oral:} Pills (like Saheli), IUDs (hormonal). \textbf{Surgical/Terminal:} Vasectomy, Tubectomy. This framework helps in quickly classifying any given method.
Which of the following statements are correct?
A. Basophils are most abundant cells of the total WBCs
B. Basophils secrete histamine, serotonin and heparin
C. Basophils are involved in inflammatory response
D. Basophils have kidney shaped nucleus
E. Basophils are agranulocytes
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements about basophils from the given list.
Step 3: Detailed Explanation:
Let's evaluate each statement:
A. Basophils are most abundant cells of the total WBCs: This is incorrect. The most abundant WBCs are neutrophils (60-65%). Basophils are the least abundant (0.5-1%).
B. Basophils secrete histamine, serotonin and heparin: This is correct. The granules of basophils contain these substances, which are mediators of inflammation.
C. Basophils are involved in inflammatory response: This is correct. By releasing histamine, serotonin, and heparin, basophils play a key role in initiating inflammatory reactions.
D. Basophils have kidney shaped nucleus: This is incorrect. The nucleus of a basophil is typically S-shaped or bilobed, and it is often obscured by the large, dark granules. Monocytes have a kidney-shaped nucleus.
E. Basophils are agranulocytes: This is incorrect. Basophils are classified as granulocytes, along with neutrophils and eosinophils, because of the prominent granules in their cytoplasm.
Therefore, the only correct statements are B and C.
Step 4: Final Answer:
The correct option is (D), which includes statements B and C.
Quick Tip: Remember the abundance of WBCs with the mnemonic: "\textbf{N}ever \textbf{L}et \textbf{M}onkeys \textbf{E}at \textbf{B}ananas" (Neutrophils > Lymphocytes > Monocytes > Eosinophils > Basophils). This tells you basophils are the least abundant. Also, associate basophils with histamine and inflammation.
Select the correct statements.
A. Tetrad formation is seen during Leptotene.
B. During Anaphase, the centromeres split and chromatids separate.
C. Terminalization takes place during Pachytene.
D. Nucleolus, Golgi complex and ER are reformed during Telophase.
E. Crossing over takes place between sister chromatids of homologous chromosome.
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements about the events of cell division (mitosis/meiosis).
Step 3: Detailed Explanation:
Let's evaluate each statement:
A. Tetrad formation is seen during Leptotene. Incorrect. Tetrads (bivalents with four chromatids) are formed during Zygotene when homologous chromosomes pair up (synapsis) and become clearly visible in Pachytene.
B. During Anaphase, the centromeres split and chromatids separate. Correct. This statement accurately describes the key event of mitotic Anaphase and meiotic Anaphase II.
C. Terminalization takes place during Pachytene. Incorrect. Terminalization of chiasmata (movement of chiasmata towards the ends of the chromatids) begins in late Diplotene and is completed in Diakinesis.
D. Nucleolus, Golgi complex and ER are reformed during Telophase. Correct. During Telophase, the cell reverses the events of Prophase. The nuclear envelope, nucleolus, Golgi complex, and ER, which had disassembled, reform around the two sets of chromosomes.
E. Crossing over takes place between sister chromatids of homologous chromosome. Incorrect. Crossing over is the exchange of genetic material between non-sister chromatids of homologous chromosomes.
Therefore, the only correct statements are B and D.
Step 4: Final Answer:
The correct option is (C), which includes statements B and D.
Quick Tip: Pay close attention to the specific stages of Prophase I: Synapsis (Zygotene), Crossing Over (Pachytene), Chiasmata visible (Diplotene), Terminalization (Diakinesis). Also, a critical distinction is crossing over between \textbf{non-sister} chromatids.
Select the correct statements with reference to chordates.
A. Presence of a mid-dorsal, solid and double nerve cord.
B. Presence of closed circulatory system.
C. Presence of paired pharyngeal gillslits.
D. Presence of dorsal heart
E. Triploblastic pseudocoelomate animals.
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements that describe the characteristics of the phylum Chordata.
Step 3: Detailed Explanation:
Let's evaluate each statement based on the defining features of chordates:
A. Presence of a mid-dorsal, solid and double nerve cord. Incorrect. Chordates have a dorsal, hollow, and single nerve cord. A ventral, solid, and double nerve cord is characteristic of non-chordates like annelids and arthropods.
B. Presence of closed circulatory system. Correct. Chordates, especially vertebrates, have a closed circulatory system where blood is confined within vessels.
C. Presence of paired pharyngeal gill slits. Correct. All chordates possess paired pharyngeal gill slits at some stage of their life cycle. In terrestrial vertebrates, these are embryonic and not functional in adults.
D. Presence of dorsal heart. Incorrect. Chordates have a ventral heart. A dorsal heart is found in non-chordates.
E. Triploblastic pseudocoelomate animals. Incorrect. Chordates are triploblastic, but they are coelomates (possessing a true coelom), not pseudocoelomates.
The fundamental features of chordates are: (1) a notochord, (2) a dorsal hollow nerve cord, and (3) paired pharyngeal gill slits. Of the given options, B and C are correct characteristics.
Step 4: Final Answer:
The correct statements are B and C. Therefore, option (C) is the right choice.
Quick Tip: Remember the key differences between chordates and non-chordates: \begin{tabular}{|l|l|} \textbf{Chordates} & \textbf{Non-Chordates}
Dorsal, hollow, single nerve cord & Ventral, solid, double nerve cord
Ventral heart & Dorsal heart
Pharyngeal gill slits present & Pharyngeal gill slits absent
Which of the following statements are correct regarding skeletal muscle?
A. Muscle bundles are held together by collagenous connective tissue layer called fascicle.
B. Sarcoplasmic reticulum of muscle fibre is a store house of calcium ions.
C. Striated appearance of skeletal muscle fibre is due to distribution pattern of actin and myosin proteins.
D. M line is considered as functional unit of contraction called sarcomere.
Choose the most appropriate answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements about the structure and function of skeletal muscle.
Step 3: Detailed Explanation:
Let's evaluate each statement:
A. Muscle bundles are held together by collagenous connective tissue layer called fascicle. Incorrect. A muscle bundle itself is called a fascicle. The connective tissue layer that surrounds a fascicle is called the perimysium.
B. Sarcoplasmic reticulum of muscle fibre is a store house of calcium ions. Correct. The sarcoplasmic reticulum is a specialized endoplasmic reticulum that sequesters and releases calcium ions (\(Ca^{2+}\)), which are essential for initiating muscle contraction.
C. Striated appearance of skeletal muscle fibre is due to distribution pattern of actin and myosin proteins. Correct. The alternating light (I-bands, containing primarily actin) and dark (A-bands, containing myosin and overlapping actin) bands, which result from the regular arrangement of myofilaments, give skeletal muscle its characteristic striated or striped appearance.
D. M line is considered as functional unit of contraction called sarcomere. Incorrect. The functional unit of contraction is the sarcomere, which is the region of a myofibril between two successive Z-lines. The M-line is a line in the center of the A-band.
Thus, the correct statements are B and C.
Step 4: Final Answer:
The correct option is (C), which includes statements B and C only.
Quick Tip: Remember the hierarchy of muscle structure: Muscle \(\rightarrow\) Fascicle (bundle) \(\rightarrow\) Muscle Fibre (cell) \(\rightarrow\) Myofibril \(\rightarrow\) Sarcomere. Also, remember that the Sarcomere (Z-line to Z-line) is the fundamental contractile unit.
Given below are two statements:
Statement I: During G\(_0\) phase of cell cycle, the cell is metabolically inactive.
Statement II: The centrosome undergoes duplication during S phase of interphase.
In the light of the above statements, choose the most appropriate answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to evaluate two statements related to events in the cell cycle.
Step 3: Detailed Explanation:
Statement I Analysis:
The G\(_0\) phase, or quiescent stage, is a non-dividing state that cells can enter from the G\(_1\) phase. Cells in G\(_0\) exit the cell cycle and do not proliferate. However, they are not metabolically inactive. They are metabolically active and carry out their specialized functions (e.g., a neuron in G\(_0\) is actively conducting nerve impulses). They just do not divide. Therefore, Statement I is incorrect.
Statement II Analysis:
The centrosome is the primary microtubule-organizing center in animal cells and is crucial for forming the mitotic spindle. For a cell to divide correctly, the centrosome must duplicate. This duplication process begins in the late G\(_1\) phase and is completed during the S phase, concurrent with DNA replication. Therefore, Statement II is correct.
Step 4: Final Answer:
Statement I is incorrect, while Statement II is correct.
Quick Tip: Do not confuse "quiescent" (not dividing) with "inactive". Cells in the G\(_0\) phase are very much alive and active, they just aren't on the path to division. Remember that S phase is the "synthesis" phase, where both DNA and the centrosome are synthesized/duplicated.
Which one of the following is NOT an advantage of inbreeding?
View Solution
Step 1: Understanding the Question:
The question asks to identify which statement does not describe an advantage of inbreeding. This could be a disadvantage, or it could be a statement that is factually incorrect about the effects of inbreeding.
Step 3: Detailed Explanation:
Let's analyze the effects of inbreeding:
Inbreeding's primary effect is to increase homozygosity. This means the frequency of individuals with two identical alleles for a gene increases.
(A) It decreases the productivity... This phenomenon is known as inbreeding depression. It is a major disadvantage of continuous inbreeding, and therefore, not an advantage.
(B) It decreases homozygosity. This statement is factually incorrect. Inbreeding is defined by its effect of increasing homozygosity. Since it's a false statement about the effects of inbreeding, it cannot be an advantage.
(C) It exposes harmful recessive genes... By increasing homozygosity, inbreeding brings recessive alleles together, allowing harmful traits to be expressed. This is an advantage in selective breeding programs because these individuals can be identified and removed, thus purging the population of harmful alleles.
(D) Elimination of less desirable genes and accumulation of superior genes... This is a direct consequence of statement (C). By selecting for superior homozygous individuals, breeders can create pure lines. This is an advantage.
The question asks what is NOT an advantage. Statement (A) describes a disadvantage. Statement (B) is a fundamentally incorrect statement about what inbreeding does. In multiple-choice questions of this type, the factually incorrect statement is often the intended answer over a statement describing a disadvantage. Decreasing homozygosity is not an outcome of inbreeding at all, so it can't be an advantage.
Step 4: Final Answer:
The statement "It decreases homozygosity" is false and therefore cannot be an advantage of inbreeding.
Quick Tip: Remember the core principle of inbreeding: it \textbf{increases homozygosity}. Any statement that contradicts this is fundamentally incorrect. The main advantage is creating pure lines and purging bad alleles; the main disadvantage is inbreeding depression.
Which of the following statements are correct?
A. An excessive loss of body fluid from the body switches off osmoreceptors.
B. ADH facilitates water reabsorption to prevent diuresis.
C. ANF causes vasodilation.
D. ADH causes increase in blood pressure.
E. ADH is responsible for decrease in GFR.
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct statements regarding the regulation of kidney function and blood pressure.
Step 3: Detailed Explanation:
A. An excessive loss of body fluid...switches off osmoreceptors. Incorrect. Excessive fluid loss increases blood osmolarity, which activates osmoreceptors in the hypothalamus, triggering thirst and ADH release.
B. ADH facilitates water reabsorption to prevent diuresis. Correct. This is the primary function of Anti-Diuretic Hormone (ADH). It increases the permeability of the DCT and collecting ducts to water.
C. ANF causes vasodilation. Correct. Atrial Natriuretic Factor (ANF) is released by the heart atria in response to high blood pressure. It acts as a vasodilator, widening blood vessels to help lower blood pressure.
D. ADH causes increase in blood pressure. Correct. Besides its antidiuretic effect, ADH (also called vasopressin) causes constriction of blood vessels (vasoconstriction) at higher concentrations, which leads to an increase in blood pressure.
E. ADH is responsible for decrease in GFR. Incorrect. ADH's primary role is on water permeability. The vasoconstrictive effect of ADH could potentially affect GFR, but its main and consistent role is not to decrease GFR. In contrast, ANF can increase GFR.
The correct statements are B, C, and D.
Step 4: Final Answer:
The correct option is (C), which includes statements B, C, and D.
Quick Tip: Remember the opposing roles of ADH/RAAS and ANF. ADH and RAAS work to increase blood pressure and conserve water. ANF works to decrease blood pressure by promoting water and salt loss.
Which of the following are NOT under the control of thyroid hormone?
A. Maintenance of water and electrolyte balance
B. Regulation of basal metabolic rate
C. Normal rhythm of sleep-wake cycle
D. Development of immune system
E. Support the process of R.B.Cs formation
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify which functions are not primarily controlled by thyroid hormones (thyroxine).
Step 3: Detailed Explanation:
Let's analyze the functions of thyroid hormone:
A. Maintenance of water and electrolyte balance: Thyroid hormones can influence this, but it is primarily under the control of hormones like aldosterone and ADH. However, thyroid hormones do have some influence.
B. Regulation of basal metabolic rate (BMR): This is the main and most well-known function of thyroid hormone. It regulates the metabolism of carbohydrates, proteins, and fats.
C. Normal rhythm of sleep-wake cycle: This circadian rhythm is primarily regulated by the pineal gland, which secretes melatonin, and the hypothalamus. While thyroid disorders can disrupt sleep, it is not the primary controller.
D. Development of immune system: The primary organs for the development and maturation of the immune system are the bone marrow and the thymus gland (especially for T-cells). Thyroid hormone is not a primary regulator of this process.
E. Support the process of R.B.Cs formation: Thyroid hormones are essential for erythropoiesis (RBC formation).
Based on this, the functions most directly under thyroid control are B and E. Thyroid hormones also influence A. The functions that are LEAST under the direct control of thyroid hormone are C (sleep-wake cycle) and D (immune system development).
Step 4: Final Answer:
The normal rhythm of the sleep-wake cycle and the development of the immune system are not primary functions of the thyroid hormone. Therefore, the correct option is (D).
Quick Tip: The three cardinal functions of thyroid hormone to remember are: regulation of \textbf{B}asal \textbf{M}etabolic \textbf{R}ate (BMR), supporting physical and mental \textbf{D}evelopment, and promoting \textbf{E}rythropoiesis (RBC formation).
The parts of human brain that helps in regulation of sexual behaviour, expression of excitement, pleasure, rage, fear etc. are :
View Solution
Step 1: Understanding the Question:
The question asks to identify the parts of the human brain responsible for regulating emotions, motivations, and basic drives like fear and sexual behavior.
Step 3: Detailed Explanation:
These functions are primarily controlled by the limbic system and the hypothalamus.
The limbic system, often called the "emotional brain," is a group of interconnected structures including the amygdala, hippocampus, and others. It is deeply involved in motivation, emotion, learning, and memory. The amygdala is particularly associated with fear and rage, while other areas are linked to pleasure.
The hypothalamus lies just below the thalamus and is a major control center for the autonomic nervous system and the endocrine system. It regulates body temperature, thirst, hunger, and is also involved in aspects of sexual behavior and rage.
Together, the limbic system and hypothalamus form a functional unit that governs these complex behaviors. The other options are incorrect as they list structures with different primary functions.
Step 4: Final Answer:
The limbic system and hypothalamus are the parts of the brain that regulate sexual behavior and emotional expressions.
Quick Tip: For questions about emotion, motivation, memory, and basic drives (fear, rage, pleasure, sex), the answer is almost always related to the limbic system and/or the hypothalamus.
Match List I with List II.
List I & List II
A. Logistic growth & I. & Unlimited resource availability condition
B. Exponential growth & II. & Limited resource availability condition
C. Expanding age pyramid & III. & The percent individuals of pre-reproductive
& & & age is largest followed by reproductive and
& & & post reproductive age groups
D. Stable age pyramid & IV. & The percent individuals of pre-reproductives
& & & and reproductive age group are same
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires matching concepts from population ecology (List I) with their correct descriptions (List II).
Step 3: Detailed Explanation:
A. Logistic growth: This describes population growth in an environment with a carrying capacity (K), where resources are limited. The growth curve is S-shaped. Thus, A matches with II.
B. Exponential growth: This describes population growth under ideal conditions with unlimited resources. The growth curve is J-shaped. Thus, B matches with I.
C. Expanding age pyramid: This is a pyramid with a broad base, indicating that the percentage of young, pre-reproductive individuals is the largest. This signifies a growing population. Thus, C matches with III.
D. Stable age pyramid: This is a bell-shaped pyramid where the number of pre-reproductive and reproductive individuals are roughly the same. This indicates a stable or zero-growth population. Thus, D matches with IV.
The correct set of matches is A-II, B-I, C-III, D-IV.
Step 4: Final Answer:
The option that corresponds to the correct matching is (B).
Quick Tip: Associate keywords: \textbf{E}xponential \(\rightarrow\) \textbf{U}nlimited resources (\textbf{J}-shape); \textbf{L}ogistic \(\rightarrow\) \textbf{L}imited resources (\textbf{S}-shape). For age pyramids, the shape tells the story: broad base = expanding; straight sides = stable; narrow base = declining.
Which one of the following is the sequence on corresponding coding strand, if the sequence on mRNA formed is as follows
5' AUCGAUCGAUCGAUCGAUCGAUCG AUCG 3'?
View Solution
Step 1: Understanding the Question:
The question provides an mRNA sequence and asks for the sequence of the corresponding coding strand of the DNA.
Step 3: Detailed Explanation:
During transcription, the enzyme RNA polymerase reads the template strand (also called the non-coding or antisense strand) of the DNA to synthesize a complementary mRNA molecule.
The other strand of the DNA is the coding strand (or sense strand). The sequence of the coding strand is identical to the sequence of the mRNA, with two key differences:
It has the same 5' to 3' polarity.
The base Thymine (T) is present in DNA instead of Uracil (U) in RNA.
Given mRNA sequence:
5' AUCG AUCG AUCG AUCG AUCG AUCG AUCG 3'
To find the coding strand sequence, we simply replace every Uracil (U) with a Thymine (T), keeping the polarity the same:
5' ATCG ATCG ATCG ATCG ATCG ATCG ATCG 3'
Step 4: Final Answer:
The correct sequence for the coding strand is 5' ATCGATCGATCGATCGATCGATCGATCG 3', which corresponds to option (D).
Quick Tip: Remember the relationship: \textbf{Coding Strand (DNA)}: Same sequence and polarity as mRNA (just T instead of U). \textbf{Template Strand (DNA)}: Complementary and antiparallel to mRNA. For this question, just swap U for T in the given mRNA sequence.
Which of the following is characteristic feature of cockroach regarding sexual dimorphism ?
View Solution
Step 1: Understanding the Question:
The question asks to identify a feature that is present in one sex of the cockroach but not the other, thus serving as a basis for sexual dimorphism.
Step 3: Detailed Explanation:
Sexual dimorphism refers to the distinct differences in size or appearance between the sexes of an animal. In cockroaches:
Anal cerci: A pair of jointed filamentous structures that arise from the 10th abdominal segment. They are present in both males and females. They are sensory in function.
Anal styles: A pair of short, unjointed, thread-like structures that are present only in males. They arise from the 9th abdominal sternite.
Therefore, the presence of anal styles is a distinguishing feature of male cockroaches.
The other options are incorrect because both sexes have a dark brown body, anal cerci, and sclerites (the hardened plates of the exoskeleton).
Step 4: Final Answer:
The presence of anal styles is the characteristic feature of sexual dimorphism in cockroaches, as they are found only in males.
Quick Tip: Remember: \textbf{C}erci = \textbf{C}ommon (in both sexes). \textbf{S}tyles = \textbf{S}pecific to males. This simple mnemonic can help you recall the key difference.
Match List I with List II.
List I & List II
A. Mast cells & I. & Ciliated epithelium
B. Inner surface of bronchiole & II. & Areolar connective tissue
C. Blood & III. & Cuboidal epithelium
D. Tubular parts of nephron & IV. & Specialised connective tissue
Choose the correct answer from the options give below:
View Solution
Step 1: Understanding the Question:
The question requires matching the cell types or structures in List I with their correct tissue classification or location in List II.
Step 3: Detailed Explanation:
A. Mast cells: These are immune cells that release histamine and other mediators. They are found in connective tissue, particularly abundant in areolar connective tissue. Thus, A matches with II.
B. Inner surface of bronchiole: The bronchioles are part of the respiratory tract, and their inner surfaces are lined with ciliated epithelium to help move mucus and trapped particles out of the lungs. Thus, B matches with I.
C. Blood: Blood is considered a fluid specialised connective tissue because it consists of cells (RBCs, WBCs, platelets) suspended in an extracellular matrix (plasma) and has a common embryonic origin with other connective tissues. Thus, C matches with IV.
D. Tubular parts of nephron: The different segments of the kidney tubule, such as the Proximal Convoluted Tubule (PCT), are composed of cuboidal epithelium, which is specialized for secretion and absorption. Thus, D matches with III.
The correct set of matches is A-II, B-I, C-IV, D-III.
Step 4: Final Answer:
The option that corresponds to the correct matching is (D).
Quick Tip: When matching tissues, think about function. Respiration/movement of mucus \(\rightarrow\) Cilia. Secretion/absorption in tubes \(\rightarrow\) Cuboidal/Columnar. Blood is the unique fluid connective tissue. Mast cells are key components of loose/areolar tissue.
The unique mammalian characteristics are:
View Solution
Step 1: Understanding the Question:
The question asks to identify the set of characteristics that are unique to the class Mammalia.
Step 3: Detailed Explanation:
Let's analyze the characteristics given in each option:
(A) pinna, monocondylic skull and mammary glands: This is incorrect. Mammals have a dicondylic skull (two occipital condyles), while reptiles and birds have a monocondylic skull.
(B) hairs, tympanic membrane and mammary glands: This is incorrect. The tympanic membrane (eardrum) is also found in other tetrapods like frogs, reptiles, and birds.
(C) hairs, pinna and mammary glands: This is correct. All three features are characteristic of mammals:
Hairs (or fur): The presence of hair is a defining feature of mammals.
Pinna (external ear): Most mammals possess external ears for sound collection.
Mammary glands: The presence of milk-producing glands to nourish the young is the most unique mammalian trait.
(D) hairs, pinna and indirect development: This is incorrect. Most mammals exhibit direct development, where the young are born as miniature versions of the adult, without a larval stage.
Step 4: Final Answer:
The set of unique mammalian characteristics is hairs, pinna, and mammary glands.
Quick Tip: Remember the "big three" defining traits of mammals: Hairs, Mammary Glands, and a Dicondylic Skull. While the pinna is also a very common feature, the three just mentioned are the most fundamental.
In cockroach, excretion is brought about by-
A. Phallic gland \hspace{1cm} B. Urecose gland
C. Nephrocytes \hspace{1cm} D. Fat body
E. Collaterial glands
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the listed structures in a cockroach are involved in the process of excretion.
Step 3: Detailed Explanation:
The excretory system of the cockroach is adapted for water conservation, primarily excreting uric acid (uricotelism). Several structures contribute to this process:
Malpighian tubules (not listed): These are the primary excretory organs.
B. Urecose glands: These are accessory reproductive glands in some male cockroaches that also function in storing and excreting uric acid.
C. Nephrocytes: These are specialized cells in the body cavity that absorb nitrogenous wastes from the hemolymph.
D. Fat body: The cells of the fat body also play a role in storing and metabolizing nitrogenous waste products.
The other listed structures are not excretory:
A. Phallic gland: This is an accessory reproductive gland in the male cockroach.
E. Collaterial glands: These are accessory reproductive glands in the female cockroach that secrete the protective egg case (ootheca).
Therefore, the structures involved in excretion from the given list are the urecose gland, nephrocytes, and the fat body.
Step 4: Final Answer:
The correct combination of excretory structures is B, C, and D.
Quick Tip: In cockroaches, remember that excretion is not just done by one organ. It's a team effort involving Malpighian tubules (the main players), and supported by the Fat Body, Nephrocytes, and Urecose glands. Phallic and Collaterial glands are strictly for reproduction.


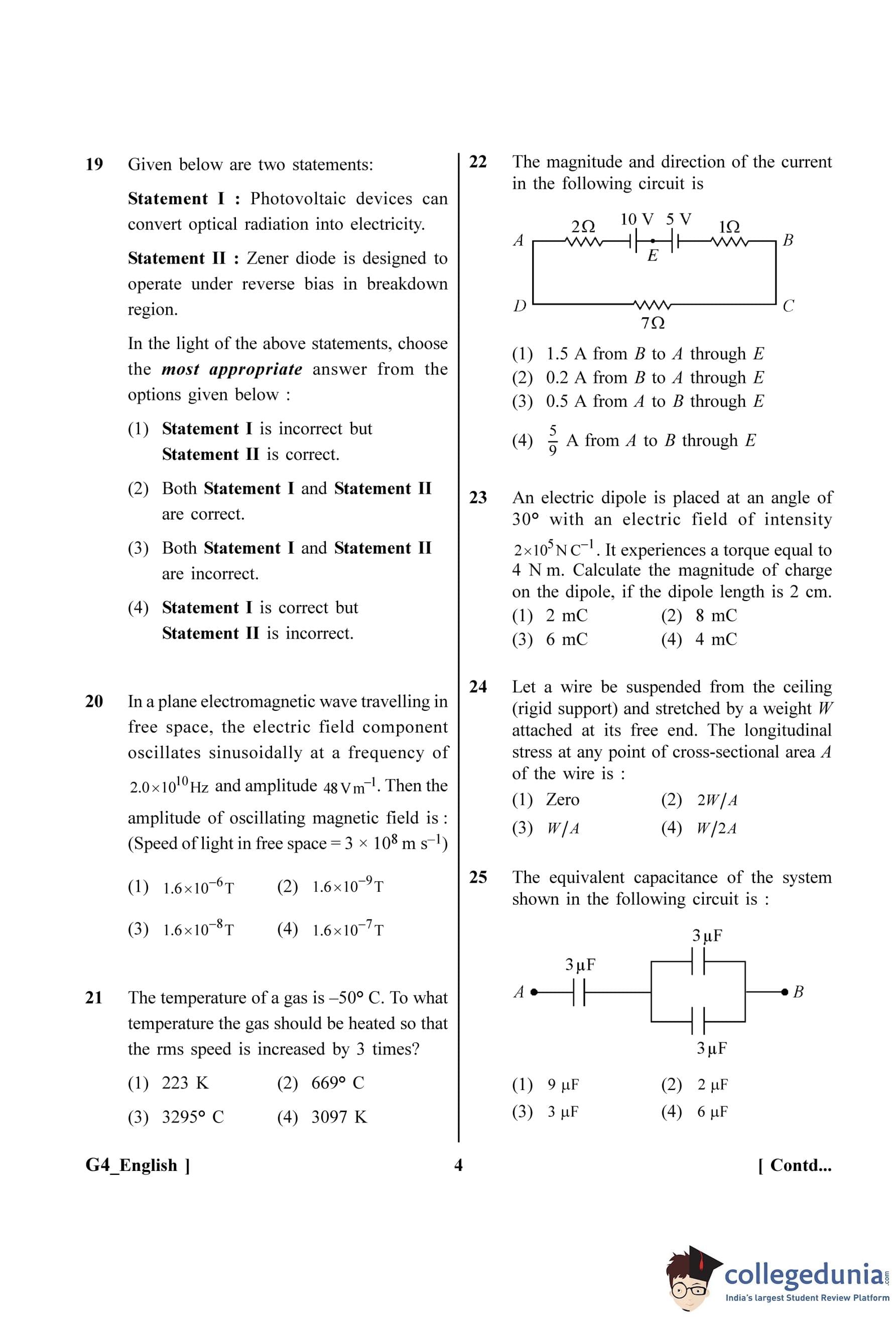
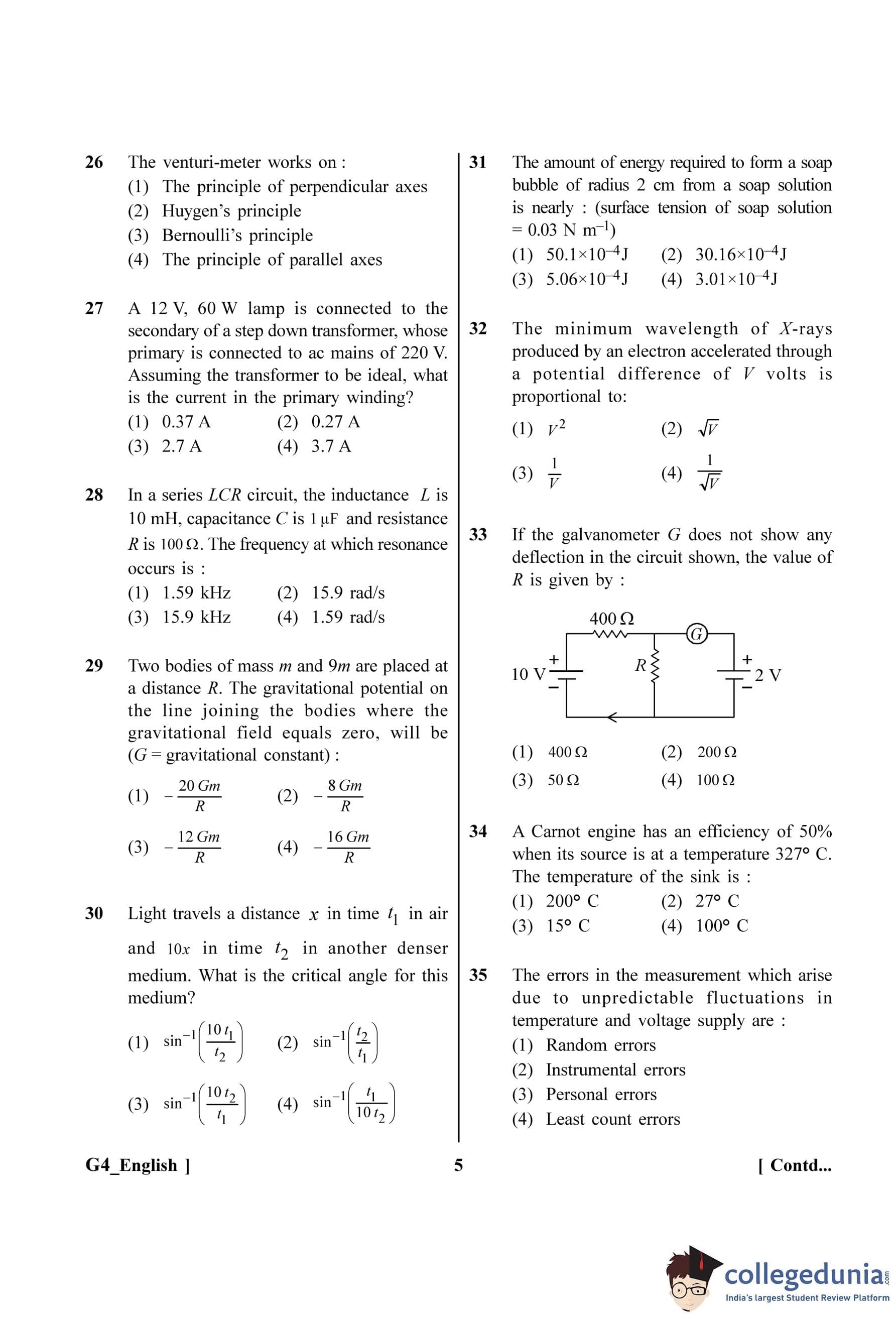
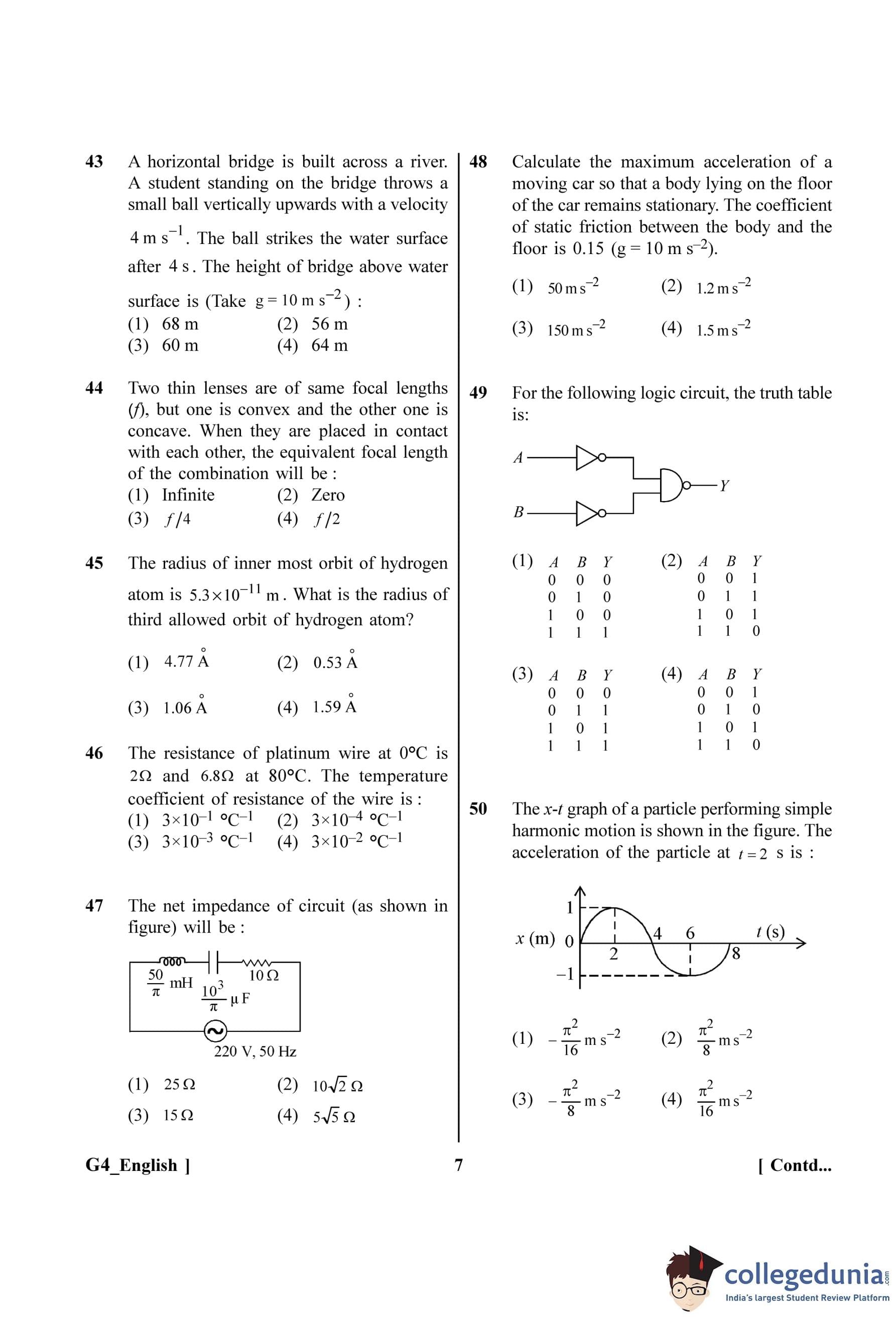

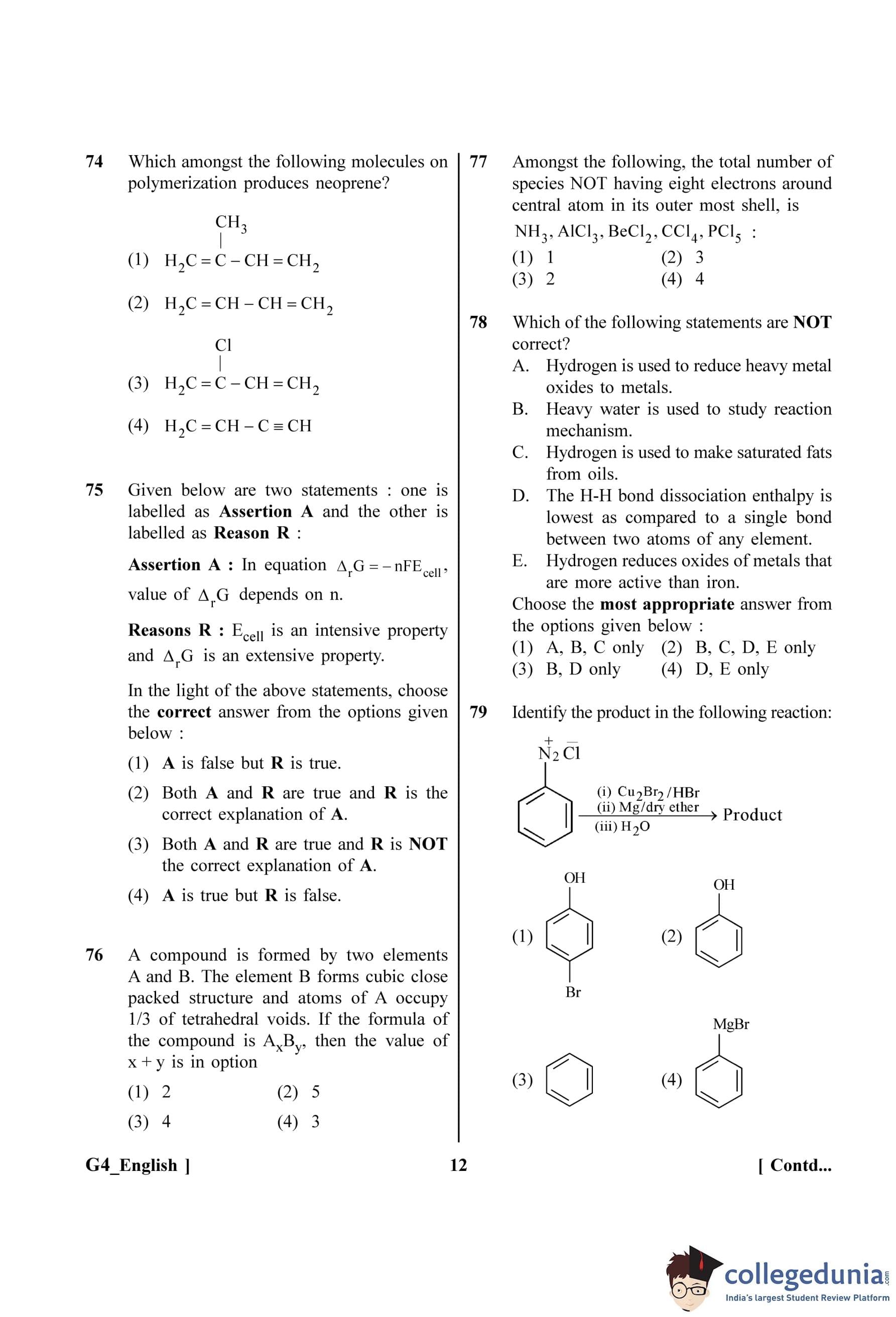
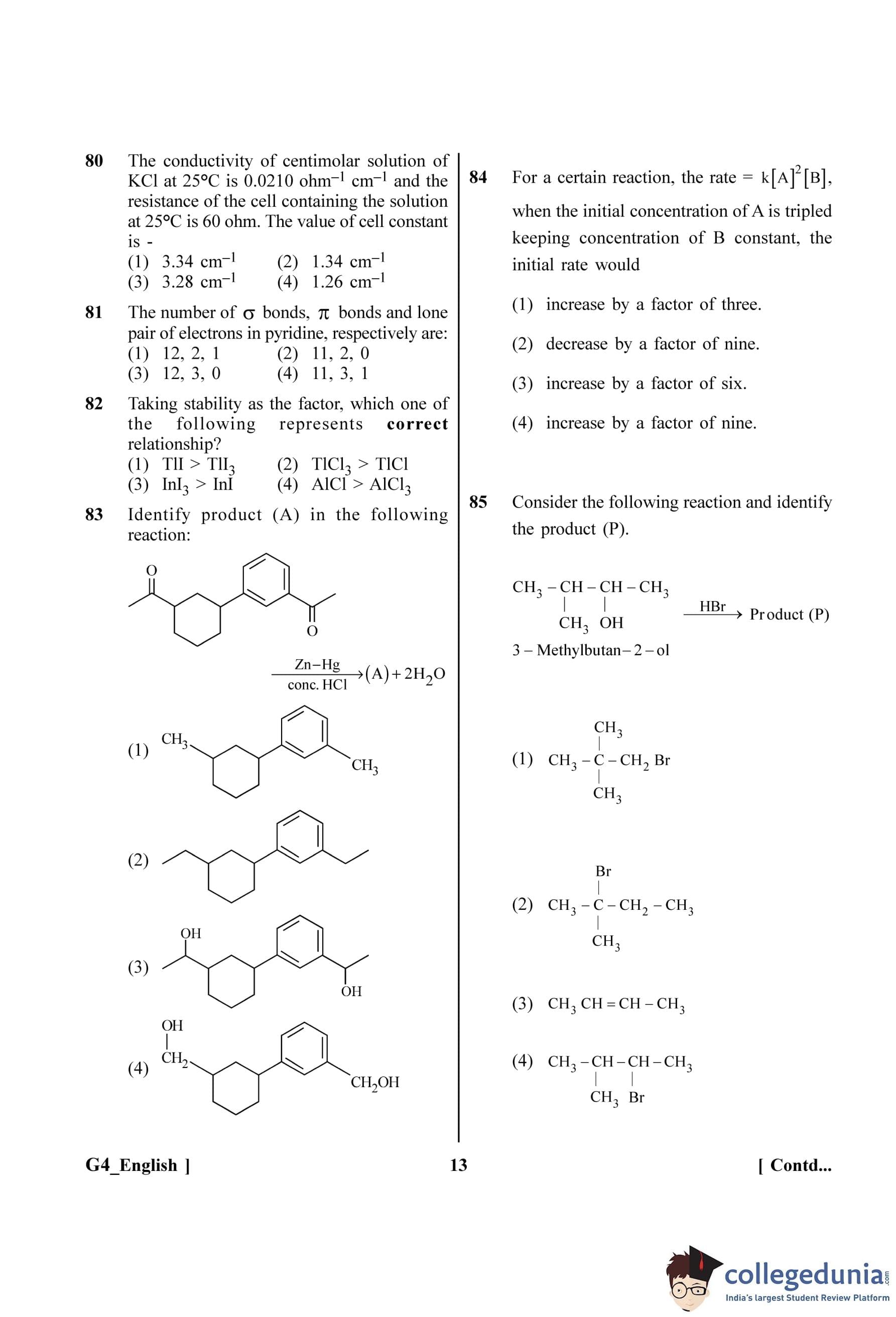
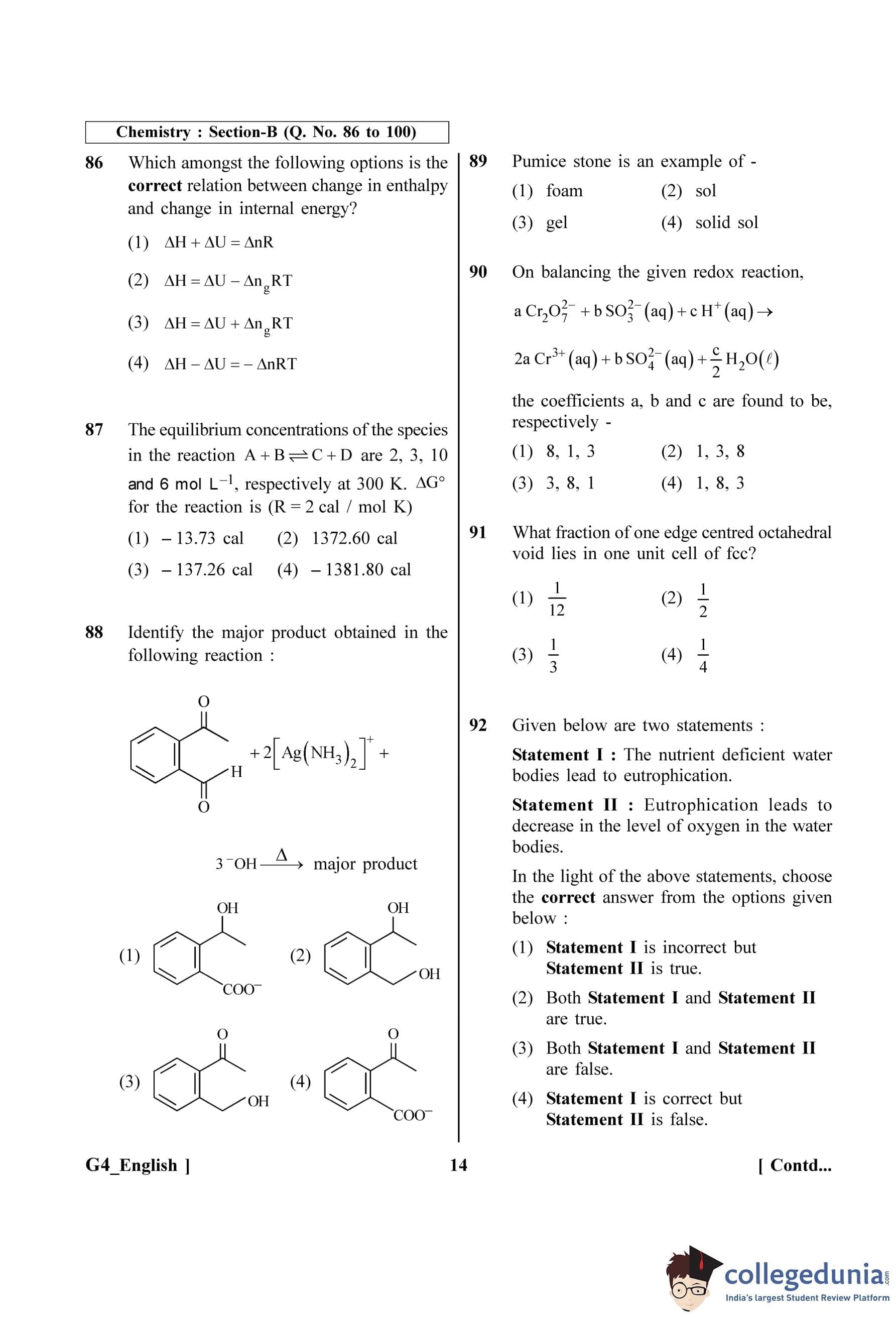
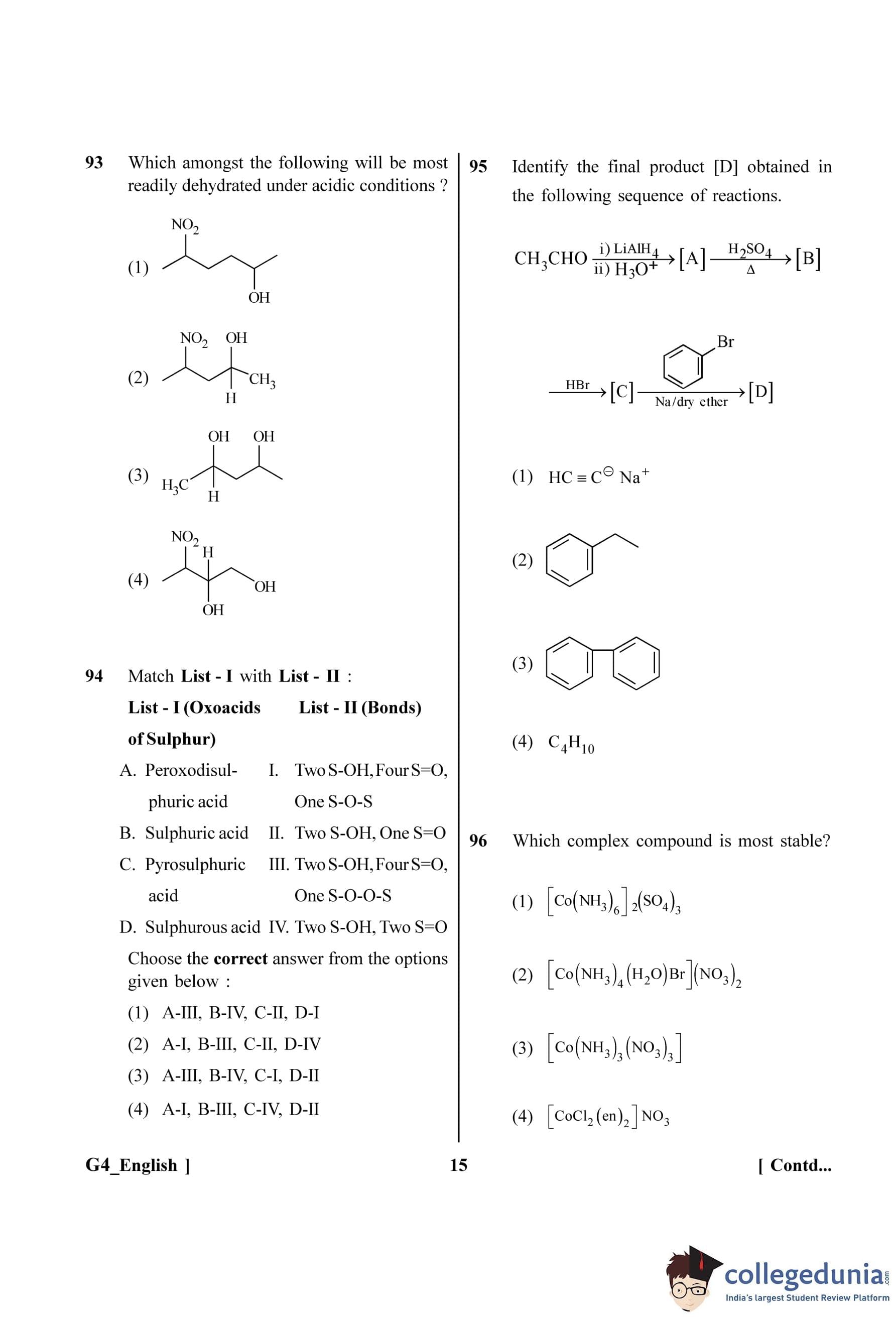
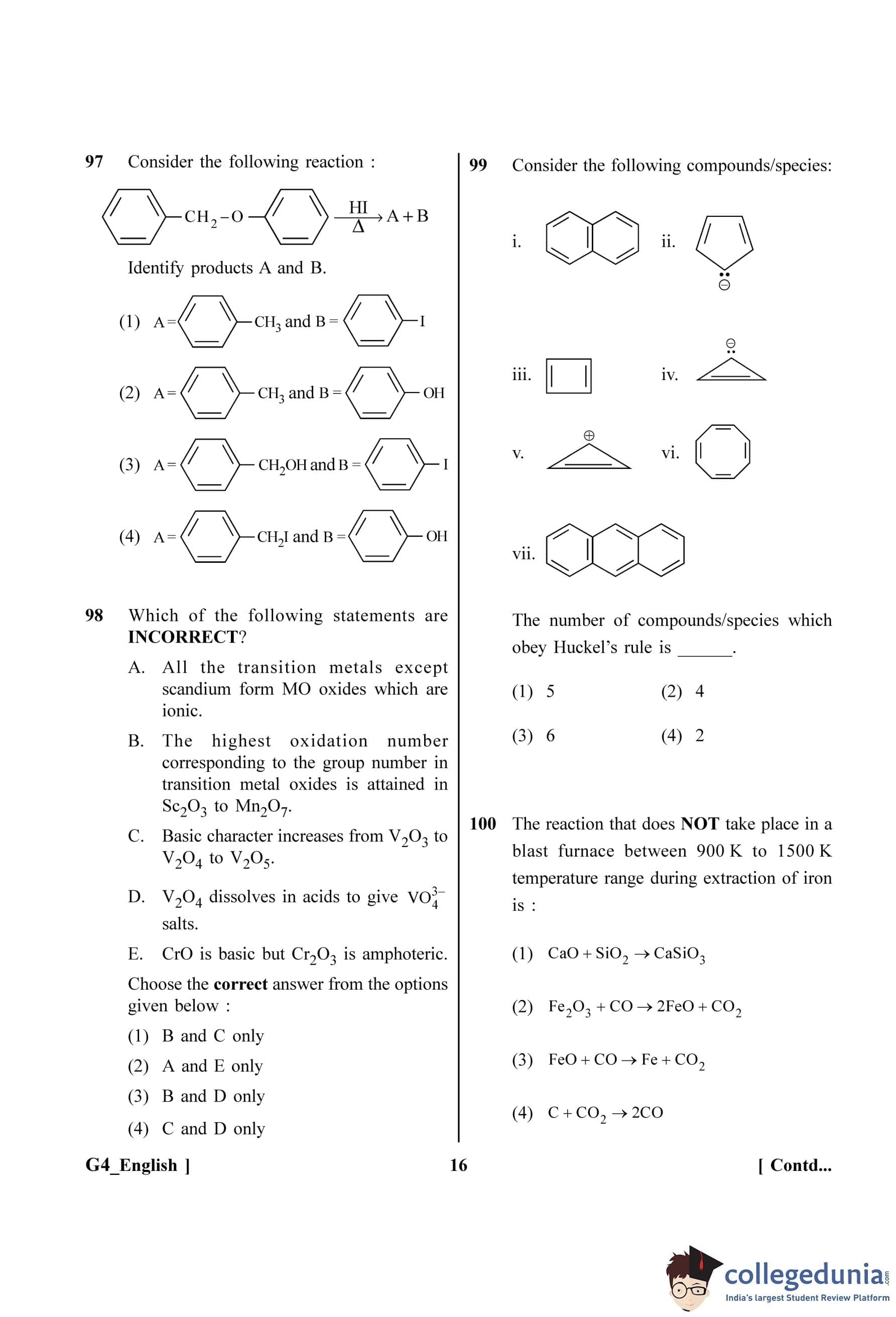















NEET Previous Year Question Papers with Answer Keys
| NEET 2022 Question Papers | NEET 2021 Question Papers | NEET 2020 Question Papers |
| NEET 2019 Question Papers | NEET 2018 Question Papers | NEET 2017 Question Papers |



Comments