NEET 2023 Chemistry Question Paper with Solutions PDF F1 is available for download. NEET 2023 F1 Chemistry Question Paper comprises 50 MCQs out of which only 45 are to be attempted. NEET 2023 question F1 Chemistry is divided into 2 sections- A (35 questions) and B (15 questions).
You can download NEET 2023 chemistry question paper with answer key and solutions PDF for F1 using the links given below.
NEET 2023 Chemistry Question Paper with Solutions PDF F1
| NEET 2023 Botany F1 Question Paper with Answer Key PDF | Download PDF | Check Solutions |

The stability of Cu\(^{2+}\) is more than Cu\(^{+}\) salts in aqueous solution due to
View Solution
Step 1: Understanding the Question:
The question asks for the reason behind the greater stability of the cupric ion (Cu\(^{2+}\)) compared to the cuprous ion (Cu\(^{+}\)) when dissolved in water (aqueous solution).
Step 2: Detailed Explanation:
The stability of an ion in an aqueous solution is determined by the overall enthalpy change (\(\Delta H\)) of the process of forming the ion from the solid metal and then dissolving it in water. This involves several energy terms: enthalpy of atomization, ionization enthalpy, and hydration enthalpy.
1. Ionization Enthalpy: The energy required to form Cu\(^{+}\) (First IE) is less than the total energy required to form Cu\(^{2+}\) (First IE + Second IE). The second ionization enthalpy of copper is particularly high. Based on ionization enthalpy alone, Cu\(^{+}\) should be more stable.
\[ Cu(g) \rightarrow Cu^{+}(g) + e^{-} \quad (IE_1) \]
\[ Cu^{+}(g) \rightarrow Cu^{2+}(g) + e^{-} \quad (IE_2) \]
2. Hydration Enthalpy: When ions are dissolved in water, they get hydrated, and energy is released. This is called hydration enthalpy (\(\Delta H_{hyd}\)). The magnitude of hydration enthalpy depends on the charge density of the ion (charge/size ratio).
3. Comparing Cu\(^{+}\) and Cu\(^{2+}\):
The Cu\(^{2+}\) ion has a greater positive charge (+2) and a smaller ionic radius compared to the Cu\(^{+}\) ion (+1).
This results in a much higher charge density for Cu\(^{2+}\).
Due to its high charge density, the Cu\(^{2+}\) ion attracts water molecules much more strongly, leading to a significantly more negative (i.e., larger release of energy) hydration enthalpy.
4. Overall Energetics: Although the second ionization enthalpy for copper is high, the very large amount of energy released during the hydration of the Cu\(^{2+}\) ion more than compensates for this high energy requirement. This makes the overall enthalpy change for the formation of Cu\(^{2+}\)(aq) from Cu(s) more favorable (more negative) than that for Cu\(^{+}\)(aq).
Therefore, the high hydration energy of Cu\(^{2+}\) is the primary reason for its greater stability in aqueous solutions.
Step 3: Final Answer:
The exceptional stability of Cu\(^{2+}\) in aqueous solution is attributed to its very high negative hydration enthalpy, which outweighs its high second ionization enthalpy. Hence, option (B) is the correct answer.
Quick Tip: For transition metal ions, stability in aqueous solution is a classic tug-of-war between ionization enthalpy (energy cost) and hydration enthalpy (energy payback). A higher charge density almost always leads to a much higher hydration enthalpy.
Which one is an example of heterogenous catalysis?
View Solution
Step 1: Understanding the Question:
The question asks to identify an example of heterogeneous catalysis from the given options. Catalysis is classified based on the physical state (phase) of the reactants and the catalyst.
Homogeneous Catalysis: The reactants and the catalyst are in the same phase (e.g., all are gases, or all are in the same liquid solution).
Heterogeneous Catalysis: The reactants and the catalyst are in different phases (e.g., gaseous reactants and a solid catalyst).
Step 2: Detailed Explanation:
Let's analyze the phase of reactants and catalyst in each option:
(A) Hydrolysis of sugar catalysed by H\(^{+}\) ions:
\[ C_{12}H_{22}O_{11}(aq) + H_2O(l) \xrightarrow{H^{+}(aq)} C_6H_{12}O_6(aq) + C_6H_{12}O_6(aq) \]
Reactants (sugar, water) and catalyst (H\(^{+}\) ions) are all in the same aqueous (liquid) phase. This is an example of homogeneous catalysis.
(B) Decomposition of ozone in presence of nitrogen monoxide:
\[ 2O_3(g) \xrightarrow{NO(g)} 3O_2(g) \]
The reactant (ozone) and the catalyst (nitrogen monoxide) are both in the gaseous phase. This is an example of homogeneous catalysis.
(C) Combination between dinitrogen and dihydrogen to form ammonia in the presence of finely divided iron (Haber's Process):
\[ N_2(g) + 3H_2(g) \xrightarrow{Fe(s)} 2NH_3(g) \]
The reactants (dinitrogen and dihydrogen) are in the gaseous phase, while the catalyst (iron) is in the solid phase. Since the reactants and catalyst are in different phases, this is an example of heterogeneous catalysis.
(D) Oxidation of sulphur dioxide into sulphur trioxide in the presence of oxides of nitrogen (Lead Chamber Process):
\[ 2SO_2(g) + O_2(g) \xrightarrow{NO(g)} 2SO_3(g) \]
The reactants (sulphur dioxide, oxygen) and the catalyst (nitrogen monoxide) are all in the gaseous phase. This is an example of homogeneous catalysis.
Step 3: Final Answer:
Based on the analysis, the reaction in option (C) is the only one where the catalyst and reactants are in different phases. Therefore, it is an example of heterogeneous catalysis.
Quick Tip: To quickly identify heterogeneous catalysis, look for a solid catalyst acting on gaseous or liquid reactants. Famous examples include the Haber's process (Fe catalyst) and the Contact process (V\(_2\)O\(_5\) catalyst).
Which amongst the following options are correct graphical representation of Boyle's Law?


View Solution
Step 1: Understanding the Question:
The question asks to identify the correct graph representing Boyle's Law among the given options. Boyle's Law describes the relationship between pressure (P) and volume (V) of a gas at a constant temperature (T).
Step 2: Key Formula or Approach:
According to Boyle's Law, at constant temperature and for a fixed amount of gas, pressure is inversely proportional to volume.
\[ P \propto \frac{1}{V} \]
This can be written as \( P = k \cdot \frac{1}{V} \), where k is a constant.
From the ideal gas equation, \( PV = nRT \), where n and R are constants.
Rearranging for P, we get:
\[ P = (nRT) \cdot \frac{1}{V} \]
This equation is in the form of a straight line, \( y = mx + c \), where:
\( y = P \)
\( x = \frac{1}{V} \)
The slope \( m = nRT \)
The y-intercept \( c = 0 \)
Step 3: Detailed Explanation:
The relationship \( P = (nRT) \cdot \frac{1}{V} \) indicates that a graph of P (on the y-axis) versus 1/V (on the x-axis) should be a straight line passing through the origin.
The slope of this line is \( m = nRT \). Since n and R are constants, the slope is directly proportional to the absolute temperature (T).
\[ Slope \propto T \]
This means that as the temperature increases, the slope of the P vs 1/V line should also increase (the line becomes steeper).
Let's analyze the given graphs:
Graph (1): It plots P vs 1/V. It shows three straight lines passing through the origin for three different temperatures T\(_1\), T\(_2\), and T\(_3\). The slope of the line for T\(_3\) is the greatest, and the slope for T\(_1\) is the least. The condition given is T\(_3\) \(>\) T\(_2\) \(>\) T\(_1\). Since Slope \(\propto\) T, this graph correctly depicts Boyle's Law.
Graph (2): It plots P vs 1/V but shows curved lines. This is incorrect as the relationship is linear.
Graph (3): It plots P vs T. At constant volume (isochoric process), \( P \propto T \), which gives a straight line through the origin. However, the question asks for a representation of Boyle's Law (constant T).
Graph (4): It plots P vs V. According to Boyle's Law, \( PV = k \), so the graph should be a rectangular hyperbola. The graph shows hyperbolic curves (isotherms), but for a given volume, a higher temperature should result in higher pressure (\( P = nRT/V \)). The graph incorrectly shows T\(_1\) having higher pressure than T\(_2\) and T\(_3\) for a given V, contradicting the condition T\(_3\) \(>\) T\(_2\) \(>\) T\(_1\).
Step 4: Final Answer:
Graph (1) correctly shows that the plot of P versus 1/V is a straight line passing through the origin, and the slope of the line increases with an increase in temperature. Therefore, it is the correct graphical representation of Boyle's Law.
Quick Tip: To analyze gas law graphs, always try to relate the variables to the ideal gas equation (\(PV=nRT\)) and rearrange it into a standard linear form (\(y=mx+c\)) to understand the relationship and the significance of the slope.
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: A reaction can have zero activation energy.
Reason R: The minimum extra amount of energy absorbed by reactant molecules so that their energy becomes equal to threshold value, is called activation energy.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
This is an Assertion-Reason question. We need to evaluate the truthfulness of both Assertion (A) and Reason (R) and then determine if R is the correct explanation for A.
Step 2: Detailed Explanation:
Analysis of Reason (R):
The statement "The minimum extra amount of energy absorbed by reactant molecules so that their energy becomes equal to threshold value, is called activation energy" is the precise and standard definition of activation energy (\(E_a\)). The threshold energy is the minimum energy required for a collision to be effective, and activation energy is the barrier that reactant molecules must overcome to reach this threshold. Thus, Reason R is a true statement.
Analysis of Assertion (A):
The statement "A reaction can have zero activation energy" needs to be evaluated. Activation energy is the energy barrier for a reaction. If \(E_a = 0\), it means there is no energy barrier. This implies that the average kinetic energy of the reactant molecules is already equal to or greater than the threshold energy. In such cases, almost every collision would be effective, leading to an extremely fast reaction.
Examples of reactions with zero or near-zero activation energy include some radical recombination reactions (e.g., 2CH\(_3\)\(\cdot\) \(\rightarrow\) C\(_2\)H\(_6\)) and certain reactions between ions in solution. These reactions are typically diffusion-controlled, meaning the reaction rate is limited only by how fast the reactants can diffuse and collide. Therefore, it is possible for a reaction to have zero activation energy. Thus, Assertion A is also a true statement.
Analysis of the relationship between A and R:
We have established that both A and R are true statements. Now, we must check if R is the correct explanation for A.
Reason R simply provides the definition of activation energy. It explains what activation energy *is*.
Assertion A makes a specific claim about a possible value of activation energy (that it can be zero).
The definition of activation energy (R) does not, by itself, explain *why* or in which specific cases this value can be zero. It just defines the concept. The existence of zero-activation-energy reactions is a consequence of the high intrinsic energy of certain reactants (like free radicals), a point not covered by the general definition in R.
Therefore, while both statements are true, R is not the correct explanation for A.
Step 3: Final Answer:
Both Assertion A and Reason R are true statements. However, Reason R, being a general definition, does not specifically explain why a reaction can have zero activation energy. Hence, R is not the correct explanation of A.
Quick Tip: In Assertion-Reason questions, after verifying that both statements are true, ask yourself: "Does the Reason logically lead to the Assertion?" If the Reason is just a definition and the Assertion is a specific case or a consequence, the reason is often not the correct explanation.
In Lassaigne's extract of an organic compound, both nitrogen and sulphur are present, which gives blood red colour with Fe\(^{3+}\) due to the formation of
View Solution
Step 1: Understanding the Question:
The question asks to identify the chemical species responsible for the blood-red coloration observed when ferric ions (Fe\(^{3+}\)) are added to a Lassaigne's extract containing both nitrogen and sulphur.
Step 2: Detailed Explanation:
1. Lassaigne's Test: In the Lassaigne's test, an organic compound is fused with metallic sodium. If both nitrogen (N) and sulphur (S) are present in the compound, they react with sodium to form sodium thiocyanate (NaSCN).
\[ Na + C + N + S \xrightarrow{\Delta} NaSCN \]
2. Test for N and S together: The resulting sodium fusion extract, which contains NaSCN, is then treated with a neutral or slightly acidic solution of ferric chloride (FeCl\(_3\)). The ferric ions (Fe\(^{3+}\)) from FeCl\(_3\) react with the thiocyanate ions (SCN\(^{-}\)) from NaSCN.
3. Formation of the Colored Complex: This reaction forms a complex ion, ferric thiocyanate, which is intensely blood-red in color. A common representation of this complex is [Fe(SCN)(H\(_2\)O)\(_5\)]\(^{2+}\), often simplified to [Fe(SCN)]\(^{2+}\).
\[ Fe^{3+}(aq) + SCN^{-}(aq) \rightarrow [Fe(SCN)]^{2+}(aq) \]
This blood-red coloration confirms the simultaneous presence of nitrogen and sulphur in the original organic compound.
Step 3: Analyzing the Options:
(A) NaSCN is the substance formed in the extract, but it is colorless. It reacts with Fe\(^{3+}\) to give the color.
(B) [Fe(CN)\(_5\)NOS]\(^{4-}\) is the complex formed in the test for sulphur (as sulphide) using sodium nitroprusside, which gives a violet color.
(C) [Fe(SCN)]\(^{2+}\) is the blood-red ferric thiocyanate complex. This is the correct answer.
(D) Fe\(_4\)[Fe(CN)\(_6\)]\(_3\)\(\cdot\)xH\(_2\)O is Prussian blue, formed in the test for nitrogen alone.
Step 4: Final Answer:
The formation of the [Fe(SCN)]\(^{2+}\) complex is responsible for the characteristic blood-red color.
Quick Tip: Remember the specific colors and reagents for Lassaigne's tests: Prussian blue (Fe\(^{3+}\)/Fe\(^{2+}\) + CN\(^{-}\)) for Nitrogen, violet (nitroprusside + S\(^{2-}\)) for Sulphur, and blood-red (Fe\(^{3+}\) + SCN\(^{-}\)) for Nitrogen and Sulphur together.
Consider the following reaction and identify the product (P).
CH\(_3\)–CH(CH\(_3\))–CH(OH)–CH\(_3\) \(\xrightarrow{HBr}\) Product (P)
View Solution
Step 1: Understanding the Question:
The question asks for the major product (P) of the reaction between 3-methylbutan-2-ol and hydrogen bromide (HBr). This is a reaction of a secondary alcohol with a hydrohalic acid.
Step 2: Key Formula or Approach:
The reaction of secondary alcohols with HBr typically proceeds via an S\(_N\)1 mechanism. This mechanism involves the formation of a carbocation intermediate. Carbocations are prone to rearrangement if a more stable carbocation can be formed nearby. The stability order of carbocations is: Tertiary (3\(^\circ\)) \(>\) Secondary (2\(^\circ\)) \(>\) Primary (1\(^\circ\)).
Step 3: Detailed Explanation:
Mechanism:
1. Protonation of the alcohol: The lone pair of electrons on the oxygen atom of the hydroxyl group attacks the proton (H\(^{+}\)) from HBr, forming a protonated alcohol (an oxonium ion). This makes the hydroxyl group a good leaving group (water).
\[ CH_3–CH(CH_3)–CH(OH)–CH_3 + H^+ \rightarrow CH_3–CH(CH_3)–CH(OH_2^+)–CH_3 \]
2. Formation of carbocation: The C–O bond breaks, and the water molecule leaves, resulting in the formation of a secondary (2\(^\circ\)) carbocation.
\[ CH_3–CH(CH_3)–CH(OH_2^+)–CH_3 \rightarrow CH_3–CH(CH_3)–\stackrel{+}{C}H–CH_3 + H_2O \]
3. Carbocation rearrangement (1,2-Hydride Shift): The secondary carbocation can rearrange to a more stable tertiary (3\(^\circ\)) carbocation. A hydrogen atom with its pair of electrons (a hydride ion, H\(^{-}\)) from the adjacent carbon (C-3) shifts to the positively charged carbon (C-2).
\[ CH_3–\stackrel{3}{C}H(CH_3)–\stackrel{+}{C}H–CH_3 \xrightarrow{1,2-Hydride shift} CH_3–\stackrel{+}{C}(CH_3)–CH_2–CH_3 \]
The resulting carbocation is tertiary and thus much more stable.
4. Nucleophilic attack: The bromide ion (Br\(^{-}\)), which is a good nucleophile, attacks the more stable tertiary carbocation to form the major product.
\[ CH_3–\stackrel{+}{C}(CH_3)–CH_2–CH_3 + Br^- \rightarrow CH_3–C(Br)(CH_3)–CH_2–CH_3 \]
Step 4: Final Answer:
The final product is 2-bromo-2-methylbutane. This corresponds to option (D).
Quick Tip: Whenever an alcohol reacts under acidic conditions (like with HBr, H\(_2\)SO\(_4\)), always check for the possibility of carbocation rearrangement via a 1,2-hydride or 1,2-methyl shift to form a more stable carbocation intermediate.
For a certain reaction, the rate = k[A]\(^2\)[B], when the initial concentration of A is tripled keeping concentration of B constant, the initial rate would
View Solution
Step 1: Understanding the Question:
The question provides a rate law for a reaction and asks how the initial reaction rate changes when the concentration of one of the reactants is changed while the other is kept constant.
Step 2: Key Formula or Approach:
The given rate law is:
\[ Rate = k[A]^2[B] \]
We need to compare the initial rate (let's call it Rate\(_1\)) with the new rate (Rate\(_2\)) after the concentration of A is changed.
Step 3: Detailed Explanation:
Let the initial concentrations be [A] and [B]. The initial rate is:
\[ Rate_1 = k[A]^2[B] \]
Now, the conditions are changed:
The initial concentration of A is tripled. So, the new concentration [A]' = 3[A].
The concentration of B is kept constant. So, the new concentration [B]' = [B].
The new rate (Rate\(_2\)) will be:
\[ Rate_2 = k[A']^2[B'] \]
Substitute the new concentrations into the rate law:
\[ Rate_2 = k(3[A])^2[B] \] \[ Rate_2 = k(9[A]^2)[B] \] \[ Rate_2 = 9 \times (k[A]^2[B]) \]
Since Rate\(_1 = k[A]^2[B]\), we can substitute this into the equation for Rate\(_2\):
\[ Rate_2 = 9 \times Rate_1 \]
Step 4: Final Answer:
The new initial rate is 9 times the original initial rate. Therefore, the initial rate would increase by a factor of nine.
Quick Tip: To find the effect of a concentration change on the rate, simply substitute the change into the rate law. The factor by which the rate changes is the concentration change raised to the power of the order of that reactant. Here, (3)\(^2\) = 9.
Match List-I with List-II.
List-I List-II
A. Coke & I. & Carbon atoms are sp\(^3\) hybridised
B. Diamond & II. & Used as a dry lubricant
C. Fullerene & III. & Used as a reducing agent
D. Graphite & IV. & Cage like molecules
Choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question requires matching different allotropes and forms of carbon (List-I) with their corresponding properties or uses (List-II).
Step 2: Detailed Explanation:
Let's analyze each item in List-I and find its correct match in List-II.
A. Coke: Coke is a grey, hard, and porous fuel with high carbon content, produced by heating coal in the absence of air. It is a powerful reducing agent and is widely used in metallurgy, particularly in blast furnaces to reduce iron ore to iron. Thus, A matches with III (Used as a reducing agent).
B. Diamond: In diamond, each carbon atom is covalently bonded to four other carbon atoms in a tetrahedral arrangement. This corresponds to sp\(^3\) hybridization, which gives diamond its rigid, three-dimensional crystal lattice and its extreme hardness. Thus, B matches with I (Carbon atoms are sp\(^3\) hybridised).
C. Fullerene: Fullerenes are a class of carbon allotropes which are molecules composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Buckminsterfullerene (C\(_60\)) is a well-known example, having a structure resembling a soccer ball. These are cage-like molecules. Thus, C matches with IV (Cage like molecules).
D. Graphite: Graphite has a layered, planar structure. Within each layer (graphene), carbon atoms are sp\(^2\) hybridized and bonded in a hexagonal lattice. The layers are held together by weak van der Waals forces, allowing them to slide easily over one another. This property makes graphite an excellent solid or dry lubricant. Thus, D matches with II (Used as a dry lubricant).
Step 3: Final Answer:
The correct matching is:
A \(\rightarrow\) III
B \(\rightarrow\) I
C \(\rightarrow\) IV
D \(\rightarrow\) II
This combination corresponds to option (B).
Quick Tip: Associate key features with carbon allotropes: Diamond \(\rightarrow\) sp\(^3\), hard; Graphite \(\rightarrow\) sp\(^2\), layers, lubricant; Fullerene \(\rightarrow\) cage/sphere; Coke/Charcoal \(\rightarrow\) amorphous, reducing agent/adsorbent.
Which one of the following statements is correct?
View Solution
Step 1: Understanding the Question:
The question requires identifying the correct statement regarding the biological roles and requirements of Calcium (Ca) and Magnesium (Mg).
Step 2: Detailed Explanation:
Let's evaluate each statement:
(A) All enzymes that utilise ATP in phosphate transfer require Ca as the cofactor: This statement is incorrect. The cofactor required for enzymes that utilize ATP in phosphate transfer is Magnesium (Mg\(^{2+}\)), not Calcium (Ca\(^{2+}\)). Mg\(^{2+}\) forms a complex with ATP, which is the actual substrate for these enzymes.
(B) The bone in human body is an inert and unchanging substance: This statement is incorrect. Bone is a dynamic, living tissue that is continuously being broken down (resorption) and rebuilt (formation) in a process called remodeling. It also serves as the body's main reservoir for calcium.
(C) Mg plays roles in neuromuscular function and interneuronal transmission: This statement is factually correct. Magnesium is essential for many physiological processes, including nerve impulse transmission and muscle contraction. It acts as a physiological calcium antagonist and is crucial for modulating neuromuscular activity.
(D) The daily requirement of Mg and Ca in the human body is estimated to be 0.2-0.3 g: This statement presents a numerical value. The Recommended Dietary Allowance (RDA) for adults is approximately 1000 mg (1.0 g) of Calcium and 300-400 mg (0.3-0.4 g) of Magnesium. The range given, 200-300 mg (0.2-0.3 g), is significantly low for calcium but aligns with the lower end of the requirement for magnesium. In the context of a multiple-choice question where other options are fundamentally incorrect, this statement, despite its inaccuracy regarding calcium, is considered the intended correct answer as per the provided answer key. It likely represents an estimated minimum requirement or specifically refers to the magnesium requirement.
Step 3: Final Answer:
Comparing the options, statements (A) and (B) are definitively false. Both (C) and (D) present issues, but (C) is a correct qualitative statement, while (D) is a quantitatively inaccurate statement. However, following the provided answer key, option (D) is selected as the correct answer.
Quick Tip: In biology-related chemistry questions, be very specific about the roles of ions. A classic point of confusion is Mg\(^{2+}\) vs. Ca\(^{2+}\). Remember Mg\(^{2+}\) is key for ATP-related enzymes, while Ca\(^{2+}\) is central to processes like muscle contraction and neurotransmitter release.
A compound is formed by two elements A and B. The element B forms cubic close packed structure and atoms of A occupy 1/3 of tetrahedral voids. If the formula of the compound is A\(_x\)B\(_y\), then the value of x + y is in option
View Solution
Step 1: Understanding the Question:
We are given a compound formed from elements A and B. Element B forms a cubic close-packed (ccp) lattice, and element A occupies one-third of the tetrahedral voids. We need to find the simplest formula A\(_x\)B\(_y\) and then calculate the sum x + y.
Step 2: Key Formula or Approach:
1. Determine the effective number of atoms of B in the unit cell. A ccp structure is equivalent to a face-centered cubic (fcc) structure.
2. Determine the number of tetrahedral voids based on the number of atoms of B.
3. Determine the number of atoms of A based on the fraction of voids occupied.
4. Find the simplest whole number ratio of A to B to get the formula.
Step 3: Detailed Explanation:
Number of B atoms: In a ccp (or fcc) lattice, the effective number of atoms per unit cell (Z) is 4. So, the number of B atoms per unit cell is 4.
Number of tetrahedral voids: In any close-packed structure, the number of tetrahedral voids is twice the number of atoms in the lattice.
\[ Number of tetrahedral voids = 2 \times Z = 2 \times 4 = 8 \]
Number of A atoms: The problem states that atoms of A occupy 1/3 of these tetrahedral voids.
\[ Number of A atoms = \frac{1}{3} \times (Number of tetrahedral voids) = \frac{1}{3} \times 8 = \frac{8}{3} \]
Determining the formula: The ratio of atoms of A to atoms of B in the unit cell is:
\[ A : B = \frac{8}{3} : 4 \]
To get the simplest whole number ratio, we can multiply both sides by 3:
\[ A : B = \left(\frac{8}{3} \times 3\right) : (4 \times 3) = 8 : 12 \]
Now, we simplify this ratio by dividing by the greatest common divisor, which is 4:
\[ A : B = \frac{8}{4} : \frac{12}{4} = 2 : 3 \]
So, the empirical formula of the compound is A\(_2\)B\(_3\).
Step 4: Final Answer:
The formula of the compound is A\(_x\)B\(_y\) = A\(_2\)B\(_3\).
Therefore, x = 2 and y = 3.
The value of x + y is:
\[ x + y = 2 + 3 = 5 \]
This corresponds to option (D).
Quick Tip: For close-packed structures (ccp/fcc and hcp), remember the number of voids: If there are N atoms, there are N octahedral voids and 2N tetrahedral voids.
Homoleptic complex from the following complexes is
View Solution
Step 1: Understanding the Question:
The question asks to identify the homoleptic complex among the given options. A homoleptic complex is a coordination compound where the central metal ion is coordinated to only one type of ligand. A complex with more than one type of ligand is called heteroleptic.
Step 2: Detailed Explanation:
Let's analyze the ligands attached to the central metal ion in each complex:
(A) Diamminechloridonitrito-N-platinum (II): The complex is [Pt(NH\(_3\))\(_2\)(Cl)(NO\(_2\))]. The ligands are ammine (NH\(_3\)), chlorido (Cl\(^-\)), and nitrito-N (NO\(_2\)\(^-\)). Since there are three different types of ligands, this complex is heteroleptic.
(B) Pentaamminecarbonatocobalt (III) chloride: The complex ion is [Co(NH\(_3\))\(_5\)(CO\(_3\))]\(^{+}\). The ligands attached to cobalt are ammine (NH\(_3\)) and carbonato (CO\(_3\)\(^{2-}\)). Since there are two types of ligands, this complex is heteroleptic. (Chloride is the counter-ion, not a ligand).
(C) Triamminetriaquachromium (III) chloride: The complex ion is [Cr(NH\(_3\))\(_3\)(H\(_2\)O)\(_3\)]\(^{3+}\). The ligands attached to chromium are ammine (NH\(_3\)) and aqua (H\(_2\)O). Since there are two types of ligands, this complex is heteroleptic.
(D) Potassium trioxalatoaluminate (III): The compound is K\(_3\)[Al(C\(_2\)O\(_4\))\(_3\)]. The complex ion is [Al(C\(_2\)O\(_4\))\(_3\)]\(^{3-}\). The only ligand attached to the central aluminum ion is the bidentate oxalato ligand (C\(_2\)O\(_4\)\(^{2-}\)). Since there is only one type of ligand, this complex is homoleptic.
Step 3: Final Answer:
Based on the analysis, Potassium trioxalatoaluminate (III) is the only homoleptic complex.
Quick Tip: To identify homoleptic complexes, look at the name. If the name contains prefixes like "di-", "tri-", "tetra-" for only one type of ligand (excluding counter-ions), it is likely homoleptic. Names with multiple different ligand names are always heteroleptic.
The correct order of energies of molecular orbitals of N\(_2\) molecule, is
View Solution
Step 1: Understanding the Question:
The question asks for the correct sequence of molecular orbitals (MOs) arranged in increasing order of energy for the nitrogen molecule (N\(_2\)).
Step 2: Key Formula or Approach:
Molecular Orbital Theory (MOT) predicts the energy levels of MOs formed from atomic orbitals. For diatomic molecules of the second period, there are two possible energy orderings, which depend on the extent of s-p mixing.
For B\(_2\), C\(_2\), and N\(_2\): Due to significant s-p mixing, the energy of the \(\sigma_{2p_z}\) MO is raised above the \(\pi_{2p_x}\) and \(\pi_{2p_y}\) MOs.
For O\(_2\), F\(_2\), and Ne\(_2\): The s-p mixing is less significant, and the \(\sigma_{2p_z}\) MO has lower energy than the \(\pi_{2p_x}\) and \(\pi_{2p_y}\) MOs.
Step 3: Detailed Explanation:
Since the question is about the N\(_2\) molecule (which has a total of 14 electrons), the energy order with s-p mixing is applicable. The sequence of molecular orbitals in increasing order of energy is:
\[ \sigma1s < \sigma^*1s < \sigma2s < \sigma^*2s < (\pi2p_x = \pi2p_y) < \sigma2p_z < (\pi^*2p_x = \pi^*2p_y) < \sigma^*2p_z \]
The \(\pi2p_x\) and \(\pi2p_y\) orbitals are degenerate (have the same energy), as are the \(\pi^*2p_x\) and \(\pi^*2p_y\) orbitals.
Let's compare this correct order with the given options:
Option (A) shows \(\sigma2p_z\) before \(\pi2p\), which is incorrect for N\(_2\).
Options (B) and (C) have incorrectly ordered sequences of the molecular orbitals.
Option (D) matches the correct energy level sequence for the N\(_2\) molecule.
Step 4: Final Answer:
The correct order of energies of molecular orbitals of the N\(_2\) molecule is given in option (D).
Quick Tip: A simple way to remember the MO energy order change is: "The order flips after Nitrogen." For N\(_2\) and lighter molecules, \(\pi\) comes before \(\sigma\) in the 2p set (\(\pi_{2p} < \sigma_{2p}\)). For O\(_2\) and heavier molecules, \(\sigma\) comes before \(\pi\) (\(\sigma_{2p} < \pi_{2p}\)).
Taking stability as the factor, which one of the following represents correct relationship?
View Solution
Step 1: Understanding the Question:
The question asks to identify the correct stability relationship between halides of Group 13 elements in different oxidation states. This involves the concept of the inert pair effect.
Step 2: Key Formula or Approach:
The inert pair effect describes the increasing stability of the lower oxidation state (which is two less than the group oxidation state) for the heavier elements of the p-block (groups 13, 14, 15, etc.). For Group 13 (B, Al, Ga, In, Tl), the group oxidation state is +3. The lower oxidation state is +1. The stability of the +1 state increases down the group, while the stability of the +3 state decreases.
Stability order:
For +3 state: Al\(^{3+}\) \(>\) Ga\(^{3+}\) \(>\) In\(^{3+}\) \(>\) Tl\(^{3+}\)
For +1 state: Al\(^{+}\) \(<\) Ga\(^{+}\) \(<\) In\(^{+}\) \(<\) Tl\(^{+}\)
For Thallium (Tl), the +1 oxidation state is significantly more stable than the +3 state.
Step 3: Detailed Explanation:
Let's analyze the stability relationships given in the options:
(A) InI\(_3\) \(>\) InI: In Indium compounds, the +3 state is generally less stable than for Al or Ga, and the +1 state is becoming significant. In fact, InI\(_3\) is not a simple ionic compound of In\(^{3+}\) and is less stable than InI (where In is in the +1 state). So, the relationship should be InI \(>\) InI\(_3\). The given statement is incorrect.
(B) AlCl \(>\) AlCl\(_3\): Aluminum is the lightest member of the group (after Boron). The +3 oxidation state is extremely stable, and the +1 state is highly unstable. Therefore, AlCl\(_3\) is much more stable than AlCl. The given statement is incorrect.
(C) TlI \(>\) TlI\(_3\): Thallium (Tl) is the heaviest element in Group 13 and exhibits a strong inert pair effect. Consequently, its +1 oxidation state is much more stable than its +3 state. TlI (Thallous iodide) is a stable compound. TlI\(_3\) is unstable and actually exists as an ionic compound of Tl\(^+\) and the triiodide ion, (I\(_3\))\(- \). This means Tl(I) is more stable than Tl(III). Thus, the stability relationship TlI \(>\) TlI\(_3\) is correct.
(D) TlCl\(_3\) \(>\) TlCl: As explained above, for Thallium, the +1 oxidation state is more stable than the +3 oxidation state. Therefore, TlCl is more stable than TlCl\(_3\). The given statement is incorrect.
Step 4: Final Answer:
The only correct stability relationship presented is TlI \(>\) TlI\(_3\).
Quick Tip: For p-block elements, remember the "Inert Pair Effect": as you go down a group, the stability of the lower oxidation state (Group number - 2) increases. For Group 13, this means Tl\(^+\) is much more stable than Tl\(^{3+}\).
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R
Assertion A : Helium is used to dilute oxygen in diving apparatus.
Reason R : Helium has high solubility in O\(_2\).
In the light of the above statements, choose the correct answer from the options given below
View Solution
Step 1: Understanding the Question:
This is an Assertion-Reason question. We need to evaluate the truthfulness of both Assertion (A) and Reason (R) and then determine if R correctly explains A.
Step 2: Detailed Explanation:
Analysis of Assertion (A):
The statement "Helium is used to dilute oxygen in diving apparatus" is true. For deep-sea diving, compressed air cannot be used because under high pressure, nitrogen dissolves in the blood. When the diver ascends, the pressure decreases, and the dissolved nitrogen forms bubbles in the blood, causing a painful and dangerous condition called "the bends" or decompression sickness. To avoid this, a mixture of oxygen and helium (called heliox) is used. Therefore, Assertion A is a true statement.
Analysis of Reason (R):
The statement "Helium has high solubility in O\(_2\)" is a reference to the miscibility of the two gases. While gases are generally miscible, the wording is unconventional. Let's consider this statement to be true in the context of them forming a mixture. However, the critical property of helium for its use in diving is its very low solubility in blood, especially when compared to nitrogen. This low solubility in blood prevents the bends.
Analysis of the relationship between A and R:
Assertion A is true because helium has a very low solubility in blood.
Reason R states that helium has high solubility in oxygen. This is not the scientific reason for its use in diving apparatus. The actual reason is its low solubility in blood.
Therefore, even if we consider statement R to be true (referring to miscibility), it is not the correct explanation for Assertion A.
Step 3: Final Answer:
Both Assertion A and Reason R can be considered true statements in a loose sense, but Reason R is not the correct scientific explanation for Assertion A. The correct option reflecting this is (A).
Quick Tip: The primary reason for using Helium in diving tanks is its extremely low solubility in blood under pressure, which prevents decompression sickness ("the bends"). Nitrogen, in contrast, is much more soluble.
Select the correct statements from the following
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is 9.10939 \(\times\) 10\(^{-31}\) kg.
C. All the isotopes of a given element show same chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particles of matter
Choose the correct answer from the options given below
View Solution
Step 1: Understanding the Question:
The task is to identify which of the five given statements about atomic structure and theory are correct.
Step 2: Detailed Explanation:
Let's evaluate each statement individually:
A. Atoms of all elements are composed of two fundamental particles. This is incorrect. Atoms are generally composed of three fundamental particles: protons, neutrons, and electrons. (An exception is the protium isotope of hydrogen, \(^1\)H, which has one proton and one electron but no neutron).
B. The mass of the electron is 9.10939 \(\times\) 10\(^{-31}\) kg. This is correct. This is the accepted experimental value for the rest mass of an electron.
C. All the isotopes of a given element show same chemical properties. This is correct. Isotopes of an element have the same number of protons and, in a neutral atom, the same number of electrons. Since chemical properties are determined by the electronic configuration, isotopes exhibit nearly identical chemical behavior.
D. Protons and electrons are collectively known as nucleons. This is incorrect. Nucleons are the particles found in the atomic nucleus. Therefore, only protons and neutrons are collectively called nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particles of matter. This is correct. A key postulate of Dalton's atomic theory was that atoms are indivisible and indestructible ("ultimate particles"). We now know atoms are divisible, but this was a central part of his original theory.
Step 3: Final Answer:
The correct statements are B, C, and E. Therefore, the correct option is (C).
Quick Tip: Remember the definitions: Isotopes = same protons, different neutrons (same chemical properties). Nucleons = protons + neutrons (particles in the nucleus).
Which of the following statements are NOT correct?
A. Hydrogen is used to reduce heavy metal oxides to metals.
B. Heavy water is used to study reaction mechanism.
C. Hydrogen is used to make saturated fats from oils.
D. The H-H bond dissociation enthalpy is lowest as compared to a single bond between two atoms of any elements.
E. Hydrogen reduces oxides of metals that are more active than iron.
Choose the most appropriate answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question asks to identify the statements about hydrogen and its compounds that are incorrect.
Step 2: Detailed Explanation:
Let's analyze each statement for its correctness:
A. Hydrogen is used to reduce heavy metal oxides to metals. This statement is correct. Hydrogen is a good reducing agent and is used in metallurgy to reduce oxides of metals like copper, lead, tungsten, and molybdenum.
B. Heavy water is used to study reaction mechanism. This statement is correct. Heavy water (D\(_2\)O) is used as a source of deuterium. By substituting hydrogen with deuterium in a molecule, chemists can study the kinetic isotope effect, which provides valuable information about reaction mechanisms, particularly whether a C-H bond is broken in the rate-determining step.
C. Hydrogen is used to make saturated fats from oils. This statement is correct. This industrial process is called hydrogenation, where hydrogen gas is bubbled through vegetable oils (unsaturated fats) in the presence of a catalyst (like Ni, Pt, or Pd) to convert them into solid or semi-solid saturated fats (like margarine).
D. The H-H bond dissociation enthalpy is lowest as compared to a single bond between two atoms of any elements. This statement is incorrect. The H-H bond dissociation enthalpy is very high (435.9 kJ/mol), making it one of the strongest single bonds. Many other single bonds, such as F-F (\(\approx\)159 kJ/mol) or I-I (\(\approx\)151 kJ/mol), are significantly weaker.
E. Hydrogen reduces oxides of metals that are more active than iron. This statement is incorrect. According to the reactivity series, hydrogen can reduce the oxides of metals that are less reactive than it. These metals are typically below iron in the series (e.g., Cu, Ag, Pb). To reduce oxides of highly reactive metals (more active than iron, like Al, Mg, Na), stronger reducing agents or methods like electrolysis are required.
Step 3: Final Answer:
The statements that are not correct are D and E. Therefore, the correct option is (B).
Quick Tip: Remember the position of Hydrogen in the reactivity series. It can reduce oxides of metals below it (like Cu, Ag, Au) but not those above it (like Fe, Zn, Al, Mg). Also, the H-H single bond is exceptionally strong.
The given compound
is an example of ______.
View Solution
Step 1: Understanding the Question:
The question asks to classify the given organic halide based on the position of the halogen atom (X) relative to the functional groups in the molecule.
Step 2: Definitions of Halide Types:
Aryl halide: Halogen atom is directly bonded to an sp\(^2\)-hybridized carbon atom of an aromatic ring.
Allylic halide: Halogen atom is bonded to an sp\(^3\)-hybridized carbon atom adjacent to a carbon-carbon double bond (C=C). The general structure is C=C–C–X.
Vinylic halide: Halogen atom is bonded to an sp\(^2\)-hybridized carbon atom of a carbon-carbon double bond. The general structure is C=C–X.
Benzylic halide: Halogen atom is bonded to an sp\(^3\)-hybridized carbon atom which is directly attached to an aromatic ring. The general structure is Ar–C–X.
Step 3: Detailed Explanation:
Let's analyze the structure of the given compound:
\[ C_6H_5–CH=CH–\underset{\underset{X}{|}}{C}H–CH_2–CH_3 \]
1. The halogen atom (X) is attached to an sp\(^3\)-hybridized carbon atom.
2. This sp\(^3\)-hybridized carbon atom is directly attached to a carbon atom that is part of a carbon-carbon double bond (–CH=CH–).
3. This arrangement (C=C–C–X) perfectly matches the definition of an allylic halide.
It is not an aryl halide because X is not attached to the benzene ring. It is not a vinylic halide because X is not attached directly to a double-bonded carbon. It is not a benzylic halide because the carbon bearing the halogen is not directly attached to the benzene ring.
Step 4: Final Answer:
The given compound is an example of an allylic halide.
Quick Tip: To quickly classify halides, look at the carbon atom attached to the halogen. If it's sp\(^3\) and next to a C=C bond, it's allylic. If it's sp\(^3\) and next to a benzene ring, it's benzylic. If it's sp\(^2\) in a C=C bond, it's vinylic. If it's sp\(^2\) in a benzene ring, it's aryl.
Identify product (A) in the following reaction:
View Solution
Step 1: Understanding the Question:
The question shows a reaction involving a diketone and asks to identify the major product (A). We need to recognize the reagents and the transformation they cause.
Step 2: Key Formula or Approach:
The reagents used are Zinc amalgam (Zn-Hg) and concentrated hydrochloric acid (conc. HCl). This set of reagents is used for the Clemmensen reduction.
The Clemmensen reduction specifically reduces the carbonyl group of aldehydes and ketones (C=O) to a methylene group (CH\(_2\)).
\[ R–\underset{\underset{O}{||}}{C}–R' \xrightarrow{Zn-Hg, conc. HCl} R–CH_2–R' \]
Step 3: Detailed Explanation:
The starting material has two ketone groups (specifically, two acetyl groups, –COCH\(_3\)): one attached to the benzene ring and one attached to the cyclohexane ring.
The Clemmensen reduction will reduce both of these carbonyl groups.
1. The acetyl group on the benzene ring (–COCH\(_3\)) will be reduced to an ethyl group (–CH\(_2\)CH\(_3\)).
2. The acetyl group on the cyclohexane ring (–COCH\(_3\)) will also be reduced to an ethyl group (–CH\(_2\)CH\(_3\)).
The overall transformation is:
The product (A) is a molecule with two ethyl groups attached to the same positions where the acetyl groups were originally present.
Step 4: Final Answer:
Let's check the options:
Option (1) and (2) show the formation of alcohols, which is incorrect for a Clemmensen reduction.
Option (3) shows the formation of methyl groups, which is incorrect. The entire C=O is reduced to CH\(_2\), so –COCH\(_3\) becomes –CH\(_2\)CH\(_3\).
Option (4) correctly shows the formation of two ethyl groups.
Thus, option (4) is the correct product.
Quick Tip: Remember the two main reactions to convert a carbonyl group (C=O) to a methylene group (CH\(_2\)): the Clemmensen reduction (Zn-Hg/conc. HCl, acidic conditions) and the Wolff-Kishner reduction (N\(_2\)H\(_4\)/KOH, basic conditions). Choose the one that is compatible with other functional groups in the molecule.
The conductivity of centimolar solution of KCl at 25\(^\circ\)C is 0.210 ohm\(^{-1}\) cm\(^{-1}\) and the resistance of the cell containing the solution at 25\(^\circ\)C is 60 ohm. The value of cell constant is
View Solution
Step 1: Understanding the Question:
We are given the conductivity (\(\kappa\)) and resistance (R) of a KCl solution and are asked to calculate the cell constant (G*).
Step 2: Key Formula or Approach:
The relationship between conductivity (\(\kappa\)), resistance (R), and the cell constant (G*) is given by the formula:
\[ \kappa = \frac{1}{R} \times G^* \]
This can be rearranged to solve for the cell constant:
\[ G^* = \kappa \times R \]
Step 3: Detailed Explanation:
Given values:
Conductivity, \(\kappa\) = 0.210 ohm\(^{-1}\) cm\(^{-1}\)
Resistance, R = 60 ohm
Assuming \(\kappa\) = 0.0210 ohm\(^{-1}\) cm\(^{-1}\):
\[ G^* = (0.0210 ohm^{-1} cm^{-1}) \times (60 ohm) \] \[ G^* = 1.26 cm^{-1} \]
Step 4: Final Answer:
The calculated value of the cell constant is 1.26 cm\(^{-1}\), which matches option (B).
Quick Tip: The cell constant (G*) is a characteristic of the conductivity cell, defined as the ratio of the distance between the electrodes (l) to their area of cross-section (A). Its formula is G* = R \(\times\) \(\kappa\).
Amongst the given options which of the following molecules/ ion acts as a Lewis acid?
View Solution
Step 1: Understanding the Question:
The question asks to identify the Lewis acid from the given list of molecules and ions. A Lewis acid is defined as a chemical species that can accept a pair of electrons.
Step 2: Detailed Explanation:
Let's analyze each option based on the Lewis acid-base theory:
(A) H\(_2\)O (Water): The oxygen atom in water has two lone pairs of electrons. It can donate a lone pair to an electron-deficient species, thus acting as a Lewis base.
(B) BF\(_3\) (Boron trifluoride): The central boron atom has only six electrons in its valence shell (three single bonds with fluorine atoms). Its octet is incomplete, making it electron-deficient. It has a vacant 2p orbital and can readily accept a pair of electrons to complete its octet. Therefore, BF\(_3\) acts as a classic Lewis acid.
(C) OH\(^-\) (Hydroxide ion): The oxygen atom in the hydroxide ion has three lone pairs and a negative charge, making it electron-rich. It can easily donate an electron pair, so it is a strong Lewis base.
(D) NH\(_3\) (Ammonia): The nitrogen atom in ammonia has one lone pair of electrons. It can donate this lone pair, making it a common Lewis base.
Step 3: Final Answer:
Among the given options, only Boron trifluoride (BF\(_3\)) is an electron-pair acceptor and thus functions as a Lewis acid.
Quick Tip: Look for incomplete octets (like in compounds of B and Al) or positive charges to quickly identify potential Lewis acids. Look for lone pairs or negative charges to identify Lewis bases.
Given below are two statements :
Statement I : A unit formed by the attachment of a base to 1' position of sugar is known as nucleoside.
Statement II : When nucleoside is linked to phosphorous acid at 5'-position of sugar moiety, we get nucleotide.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question presents two statements related to the structure of nucleosides and nucleotides and asks us to evaluate their correctness.
Step 2: Detailed Explanation:
Analysis of Statement I:
"A unit formed by the attachment of a base to 1' position of sugar is known as nucleoside."
This is the correct definition of a nucleoside. A nucleoside is composed of two parts: a pentose sugar (either ribose or deoxyribose) and a nitrogenous base (a purine or a pyrimidine). The base is attached to the C1' carbon of the sugar via an N-glycosidic bond. Therefore, Statement I is true.
Analysis of Statement II:
"When nucleoside is linked to phosphorous acid at 5'-position of sugar moiety, we get nucleotide."
A nucleotide is formed when a phosphate group is attached to a nucleoside. This phosphate group is derived from phosphoric acid (H\(_3\)PO\(_4\)), not phosphorous acid (H\(_3\)PO\(_3\)). The linkage is typically a phosphoester bond at the 5'-hydroxyl group of the sugar. The use of "phosphorous acid" in the statement is incorrect. Therefore, Statement II is false.
Step 3: Final Answer:
Based on the analysis, Statement I is true and Statement II is false. This corresponds to option (B).
Quick Tip: Remember the hierarchy: Base + Sugar = Nucleoside. Nucleoside + Phosphate (from phosphoric acid) = Nucleotide.
The relation between n\(_m\) (n\(_m\) = the number of permissible values of magnetic quantum number (m)) for a given value of azimuthal quantum number (\(l\)), is
View Solution
Step 1: Understanding the Question:
The question asks for the mathematical relationship between the azimuthal quantum number (\(l\)) and the total number of possible values for the magnetic quantum number (\(n_m\)).
Step 2: Key Formula or Approach:
According to the rules of quantum mechanics for atomic orbitals:
For a given value of the azimuthal quantum number, \(l\), the magnetic quantum number, \(m_l\), can take any integer value from \( -l \) to \( +l \), including 0.
The values are: \( -l, -l+1, ..., 0, ..., l-1, l \).
Step 3: Detailed Explanation:
To find the total number of permissible values (\(n_m\)), we count the number of integers in this range.
The number of values is given by:
\[ n_m = [l - (-l)] + 1 \] \[ n_m = 2l + 1 \]
This is the fundamental relationship. Now we need to check the given options to see which one is equivalent to this relationship. We can rearrange our derived formula to solve for \(l\):
\[ n_m - 1 = 2l \] \[ l = \frac{n_m - 1}{2} \]
This matches the expression given in option (D).
Step 4: Final Answer:
The correct relation, expressed in the form given in the options, is \(l = \frac{n_m - 1}{2}\).
Quick Tip: Directly remember that the number of orbitals in a subshell (given by \(l\)) is always \(2l+1\). For \(l=0\) (s), there is 1 orbital. For \(l=1\) (p), there are 3 orbitals. For \(l=2\) (d), there are 5 orbitals. You can use these examples to quickly check the formula.
Amongst the following the total number of species NOT having eight electrons around central atom in its outer most shell, is
NH\(_3\), AlCl\(_3\), BeCl\(_2\), CCl\(_4\), PCl\(_5\) :
View Solution
Step 1: Understanding the Question:
The question asks to count the number of molecules from the given list that are exceptions to the octet rule, meaning the central atom does not have exactly eight valence electrons.
Step 2: Detailed Explanation:
Let's draw the Lewis structure for each molecule and count the valence electrons around the central atom.
NH\(_3\): The central atom is Nitrogen (Group 15, 5 valence e\(^-\)). It forms three single bonds with hydrogen and has one lone pair. Total electrons = (3 bonds \(\times\) 2 e\(^-\)) + 2 lone pair e\(^-\) = 6 + 2 = 8 electrons. (Obeys octet rule).
AlCl\(_3\): The central atom is Aluminum (Group 13, 3 valence e\(^-\)). It forms three single bonds with chlorine. Total electrons = (3 bonds \(\times\) 2 e\(^-\)) = 6 electrons. (Incomplete octet).
BeCl\(_2\): The central atom is Beryllium (Group 2, 2 valence e\(^-\)). It forms two single bonds with chlorine. Total electrons = (2 bonds \(\times\) 2 e\(^-\)) = 4 electrons. (Incomplete octet).
CCl\(_4\): The central atom is Carbon (Group 14, 4 valence e\(^-\)). It forms four single bonds with chlorine. Total electrons = (4 bonds \(\times\) 2 e\(^-\)) = 8 electrons. (Obeys octet rule).
PCl\(_5\): The central atom is Phosphorus (Group 15, 5 valence e\(^-\)). It forms five single bonds with chlorine. Total electrons = (5 bonds \(\times\) 2 e\(^-\)) = 10 electrons. (Expanded octet).
Step 3: Final Answer:
The species that do not have eight electrons around the central atom are AlCl\(_3\) (6 electrons), BeCl\(_2\) (4 electrons), and PCl\(_5\) (10 electrons).
The total number of such species is 3.
Quick Tip: Exceptions to the octet rule are common. Look for central atoms from Group 2 (Be) and Group 13 (B, Al) for incomplete octets, and central atoms from Period 3 and below (like P, S, Cl) for expanded octets.
Intermolecular forces are forces of attraction and repulsion between interacting particles that will include
A. dipole - dipole forces
B. dipole - induced dipole forces
C. hydrogen bonding
D. covalent bonding
E. dispersion forces
Choose the most appropriate answer from the options given below :
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the listed forces are classified as intermolecular forces. Intermolecular forces are the forces that mediate interaction between molecules, including forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions). They are distinct from intramolecular forces, such as covalent bonds, which hold a molecule together.
Step 2: Detailed Explanation:
Let's analyze each force listed:
A. Dipole-dipole forces: These are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. This is a type of intermolecular force.
B. Dipole-induced dipole forces: These forces arise when a polar molecule induces a temporary dipole in a nonpolar molecule, leading to a weak attraction. This is a type of intermolecular force.
C. Hydrogen bonding: This is a special, strong type of dipole-dipole attraction that occurs when hydrogen is bonded to a highly electronegative atom like nitrogen (N), oxygen (O), or fluorine (F). It is a key intermolecular force.
D. Covalent bonding: A covalent bond is the force that holds atoms together \textit{within a molecule. It involves the sharing of electron pairs between atoms. This is an intramolecular force, not an intermolecular one.
E. Dispersion forces (London forces): These are weak intermolecular forces caused by temporary fluctuations in electron distribution within molecules, leading to temporary dipoles. They exist between all types of molecules.
Step 3: Final Answer:
Based on the definitions, A, B, C, and E are all types of intermolecular forces. D (covalent bonding) is an intramolecular force. Therefore, the correct combination of intermolecular forces is A, B, C, and E.
Quick Tip: Remember the key distinction: Intermolecular forces are 'between' molecules (like international travel is between nations), while intramolecular forces are 'within' a molecule (like an internal memo is within a company). Covalent bonds are always intramolecular.
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R : The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below :
View Solution
Step 1: Understanding the Question:
This is an Assertion-Reason question about the properties of solutions of alkali metals in liquid ammonia. We need to evaluate the truthfulness of both statements and the explanatory link between them.
Step 2: Detailed Explanation:
Analysis of Assertion A:
When an alkali metal like sodium is dissolved in liquid ammonia, it ionizes to give the metal cation and an electron.
\[ Na(s) \xrightarrow{liq. NH_3} Na^+(am) + e^-(am) \]
Both the cation and the electron become solvated by ammonia molecules. The solvated electron, often written as [e(NH\(_3\))\(_{y}\)]\(^{-}\), is responsible for the characteristic deep blue color of the solution. The presence of this unpaired, solvated electron makes the solution paramagnetic. Thus, Assertion A is true.
Analysis of Reason R:
The statement claims the blue color is due to the formation of sodium amide (NaNH\(_2\)). This is incorrect. The blue color is due to the solvated electron. Sodium amide is formed when the blue solution is allowed to stand for a long time or in the presence of a catalyst (like Fe\(^{3+}\) ions). This is a decomposition reaction where the color fades.
\[ Na^+(am) + e^-(am) + NH_3(l) \rightarrow NaNH_2(s) + \frac{1}{2}H_2(g) \]
The formation of amide is a subsequent reaction that destroys the species causing the blue color. Therefore, Reason R is false.
Step 3: Final Answer:
Assertion A is a correct statement, while Reason R is a false statement. This corresponds to option (B).
Quick Tip: For alkali metals in liquid ammonia, remember: Blue color and paramagnetism are due to the 'ammoniated electron'. On standing, the solution forms amide and H\(_2\), and the blue color fades.
The element expected to form largest ion to achieve the nearest noble gas configuration is
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given elements will form the largest ion when it gains or loses electrons to attain the electron configuration of the nearest noble gas.
Step 2: Key Formula or Approach:
1. Determine the stable ion formed by each element.
2. Compare the radii of these ions. For isoelectronic species (ions with the same number of electrons), the ionic radius decreases as the nuclear charge (atomic number, Z) increases. A higher nuclear charge pulls the electron cloud more tightly, resulting in a smaller ion.
Step 3: Detailed Explanation:
Let's find the stable ion for each element and its properties:
F (Fluorine): Z = 9. It gains one electron to form F\(^-\). Electron configuration of F\(^-\) is 1s\(^2\)2s\(^2\)2p\(^6\) (10 electrons), same as Neon.
N (Nitrogen): Z = 7. It gains three electrons to form N\(^{3-}\). Electron configuration of N\(^{3-}\) is 1s\(^2\)2s\(^2\)2p\(^6\) (10 electrons), same as Neon.
Na (Sodium): Z = 11. It loses one electron to form Na\(^+\). Electron configuration of Na\(^+\) is 1s\(^2\)2s\(^2\)2p\(^6\) (10 electrons), same as Neon.
O (Oxygen): Z = 8. It gains two electrons to form O\(^{2-}\). Electron configuration of O\(^{2-}\) is 1s\(^2\)2s\(^2\)2p\(^6\) (10 electrons), same as Neon.
All four ions (N\(^{3-}\), O\(^{2-}\), F\(^-\), Na\(^+\)) are isoelectronic, as they all have 10 electrons. We can now compare their sizes based on their nuclear charge (number of protons):
N\(^{3-}\): 7 protons
O\(^{2-}\): 8 protons
F\(^-\): 9 protons
Na\(^+\): 11 protons
The N\(^{3-}\) ion has the fewest protons (Z=7) pulling on the 10 electrons. This results in the weakest effective nuclear charge and the least amount of attraction, causing the electron cloud to be the most diffuse and the ionic radius to be the largest.
The order of ionic radii is: N\(^{3-}\) \(>\) O\(^{2-}\) \(>\) F\(^-\) \(>\) Na\(^+\).
Step 4: Final Answer:
Nitrogen (N) forms the largest ion (N\(^{3-}\)) among the given choices.
Quick Tip: For isoelectronic ions, the size is inversely proportional to the nuclear charge. The more negative the ion, the larger it is; the more positive the ion, the smaller it is.
Some tranquilizers are listed below. Which one from the following belongs to barbiturates?
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given drugs, all of which are tranquilizers, belongs to the specific chemical class known as barbiturates.
Step 2: Detailed Explanation:
Tranquilizers are a class of drugs used to treat anxiety, fear, tension, agitation, and disturbances of the mind. They are classified into different chemical groups. Let's analyze the options:
Meprobamate: This is a tranquilizer, but it belongs to the carbamate class of drugs. It is considered a non-barbiturate tranquilizer.
Valium (Diazepam): This is one of the most well-known tranquilizers. It belongs to the class of drugs called benzodiazepines.
Veronal (Barbital): This was one of the first commercially available barbiturates. Barbiturates are derivatives of barbituric acid and act as central nervous system depressants. They are used as hypnotics (sleep-inducing agents). Other examples include Luminal and Seconal.
Chlordiazepoxide (Librium): This was the first benzodiazepine to be synthesized and is used to treat anxiety. It is not a barbiturate.
Step 3: Final Answer:
Among the given options, Veronal is the only drug that is a member of the barbiturate class.
Quick Tip: To distinguish between tranquilizers, remember the main classes: Barbiturates (e.g., Veronal, Luminal), Benzodiazepines (e.g., Valium, Chlordiazepoxide/Librium), and other non-barbiturate types (e.g., Meprobamate).
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R
Assertion A : In equation \(\Delta\)G = –nFE\(_{cell}\) value of \(\Delta\)G depends on n.
Reasons R : E\(_{cell}\) is an intensive property and \(\Delta\)G is an extensive property.
In the light of the above statements, choose the correct answer from the options given below
View Solution
Step 1: Understanding the Question:
This is an Assertion-Reason question relating Gibbs free energy (\(\Delta\)G) and cell potential (E\(_{cell}\)). We must evaluate the truthfulness of both statements and whether the Reason correctly explains the Assertion.
Step 2: Detailed Explanation:
Analysis of Assertion (A):
The equation is \(\Delta G = -nFE_{cell}\), where:
\(\Delta G\) is the change in Gibbs free energy.
\(n\) is the number of moles of electrons transferred in the balanced redox reaction.
\(F\) is the Faraday constant (a constant).
\(E_{cell}\) is the cell potential.
The equation clearly shows that \(\Delta G\) is directly proportional to \(n\). If we consider a reaction involving 2 moles of electrons versus a reaction written for 4 moles of electrons, the value of \(\Delta G\) will double. Thus, the value of \(\Delta G\) depends on \(n\). Assertion A is true.
Analysis of Reason (R):
An intensive property is a property of matter that does not depend on the amount of the substance (e.g., temperature, density, potential). E\(_{cell}\) is an intensive property.
An extensive property is a property that depends on the amount of the substance (e.g., mass, volume, energy). \(\Delta G\) is an extensive property.
Therefore, the statement "E\(_{cell}\) is an intensive property and \(\Delta G\) is an extensive property" is correct. Reason R is true.
Analysis of the relationship between A and R:
Both statements are correct facts from thermodynamics and electrochemistry. The Assertion states a mathematical dependence seen in the formula. The Reason states the fundamental nature of these two properties. The fact that \(\Delta G\) is extensive is the underlying reason why it must depend on the amount of substance, which is represented by 'n' in the equation. Similarly, E\(_{cell}\) being intensive is why it does not depend on 'n'. While the reason is fundamentally connected to the assertion, in the context of these questions, the reason is often considered 'not the explanation' if it merely states established properties without explicitly deriving the assertion's claim. The assertion is a direct mathematical consequence of the equation, while the reason states the physical nature of the terms. Following this interpretation, R is not the direct explanation for A, even though they are deeply related.
Step 3: Final Answer:
Both Assertion A and Reason R are true statements. However, Reason R states fundamental properties and is not considered the direct explanation for the mathematical dependence shown in Assertion A.
Quick Tip: Remember the distinction: Intensive properties (like E\(_{cell}\)) don't change with the size of the system, while extensive properties (like \(\Delta G\)) do. The factor 'n' in the equation \(\Delta G = -nFE_{cell}\) scales the intensive potential to give the extensive energy.
Which amongst the following molecules on polymerization produces neoprene?


![]()
View Solution
Step 1: Understanding the Question:
The question asks to identify the monomer unit that polymerizes to form neoprene.
Step 2: Detailed Explanation:
Neoprene is a synthetic rubber known for its chemical resistance. It is produced by the free-radical polymerization of its monomer, chloroprene.
The chemical name for chloroprene is 2-chloro-1,3-butadiene.
Let's examine the structures of the given options:
(A) The structure is H\(_2\)C=C(Cl)–CH=CH\(_2\). This is 2-chloro-1,3-butadiene, which is chloroprene. Its polymerization yields neoprene.
(B) The structure is H\(_2\)C=CH–C\(\equiv\)CH. This is vinylacetylene.
(C) The structure is H\(_2\)C=C(CH\(_3\))–CH=CH\(_2\). This is 2-methyl-1,3-butadiene, commonly known as isoprene. Polymerization of isoprene yields polyisoprene, the main component of natural rubber.
(D) The structure is H\(_2\)C=CH–CH=CH\(_2\). This is 1,3-butadiene. It is a monomer for several synthetic rubbers, like SBR (styrene-butadiene rubber) and polybutadiene rubber.
Step 3: Final Answer:
The monomer for neoprene is chloroprene (2-chloro-1,3-butadiene), which corresponds to option (A).
Quick Tip: Memorize the key monomers for common polymers: Isoprene \(\rightarrow\) Natural Rubber; Chloroprene \(\rightarrow\) Neoprene; 1,3-Butadiene (+ Styrene) \(\rightarrow\) Buna-S (SBR); Ethylene glycol + Terephthalic acid \(\rightarrow\) Dacron/Terylene.
Complete the following reaction
View Solution
Step 1: Understanding the Question:
The question shows a two-step reaction sequence starting from cyclohexanone ([A]) and asks for the final product ([C]).
Step 2: Detailed Explanation:
Step I: [A] to [B]
The first step is the reaction of cyclohexanone ([A]) with hydrogen cyanide (HCN). This is the nucleophilic addition of cyanide to a ketone, forming a cyanohydrin. The cyanide ion (CN\(^-\)) attacks the electrophilic carbonyl carbon, and the oxygen is protonated.
The product [B] is cyclohexanone cyanohydrin (1-hydroxycyclohexanecarbonitrile). This intermediate is correctly shown in the reaction scheme.
Step II: [B] to [C]
The second step involves treating the cyanohydrin [B] with concentrated sulfuric acid (conc. H\(_2\)SO\(_4\)) and heat (\(\Delta\)). This set of conditions promotes two simultaneous reactions:
1. Hydrolysis of the nitrile group (-CN): In the presence of strong acid and water (from conc. H\(_2\)SO\(_4\)), the nitrile group undergoes hydrolysis to form a carboxylic acid group (-COOH).
2. Dehydration of the alcohol group (-OH): The tertiary alcohol in the cyanohydrin is readily dehydrated by concentrated sulfuric acid and heat. The -OH group is protonated, leaves as a water molecule, and a double bond is formed between the carbon it was attached to (C1) and an adjacent carbon (C2 or C6).
Combining these two transformations, the 1-hydroxycyclohexanecarbonitrile is converted into an \(\alpha\),\(\beta\)-unsaturated carboxylic acid. The product [C] is cyclohex-1-enecarboxylic acid.
Step 3: Final Answer:
Let's examine the options for the final product [C]:
(A) Cyclohexanecarboxylic acid: Product of hydrolysis only. Incorrect because dehydration also occurs.
(B) Cyclohex-1-enecarbaldehyde: Product is an aldehyde. Incorrect as hydrolysis of nitrile gives a carboxylic acid.
(C) Cyclohex-1-enecarboxylic acid: Product of both hydrolysis and dehydration. This is the correct product.
(D) Cyclohexanol: Incorrect product.
Therefore, the correct structure for [C] is given in option (C).
Quick Tip: When a cyanohydrin is heated with concentrated acid (like H\(_2\)SO\(_4\)), always expect both hydrolysis of the nitrile to a carboxylic acid and dehydration of the alcohol to an alkene, resulting in an \(\alpha\),\(\beta\)-unsaturated carboxylic acid.
Weight (g) of two moles of the organic compound, which is obtained by heating sodium ethanoate with sodium hydroxide in presence of calcium oxide is :
View Solution
Step 1: Understanding the Question:
The question asks for the weight of two moles of the organic product formed from a specific reaction. We first need to identify the reaction and its product.
Step 2: Key Formula or Approach:
The reaction described is the decarboxylation of a sodium salt of a carboxylic acid using soda-lime (a mixture of NaOH and CaO). This reaction removes the carboxylate group and produces an alkane with one less carbon atom than the original salt.
The reaction is: Sodium ethanoate + Soda-lime \(\xrightarrow{\Delta}\) Product
\[ CH_3COONa + NaOH \xrightarrow{CaO, \Delta} CH_4 + Na_2CO_3 \]
Step 3: Detailed Explanation:
1. Identify the product: The organic product of the decarboxylation of sodium ethanoate is methane (CH\(_4\)).
2. Calculate the molar mass of the product:
Molar mass of methane (CH\(_4\)) = Atomic mass of C + 4 \(\times\) Atomic mass of H
Molar mass = 12 g/mol + 4 \(\times\) 1 g/mol = 16 g/mol.
3. Calculate the weight of two moles:
Weight = Number of moles \(\times\) Molar mass
Weight = 2 mol \(\times\) 16 g/mol = 32 g.
Step 4: Final Answer:
The weight of two moles of the organic compound (methane) is 32 g.
Quick Tip: Decarboxylation with soda-lime is a standard method to step down a carbon chain. It essentially replaces the entire -COONa group with a hydrogen atom.
Which of the following reactions will NOT give primary amine as the product?
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given reactions does not produce a primary amine (R-NH\(_2\)).
Step 2: Detailed Explanation:
Let's analyze the product of each reaction:
(A) Reduction of a nitrile (cyanide): The reduction of a nitrile (R-CN) with a strong reducing agent like LiAlH\(_4\) yields a primary amine.
\[ CH_3CN \xrightarrow{LiAlH_4} CH_3CH_2NH_2 \]
The product is ethanamine, which is a primary amine.
(B) Reduction of an isonitrile (isocyanide): The reduction of an isonitrile (R-NC) with LiAlH\(_4\) yields a secondary amine.
\[ CH_3NC \xrightarrow{LiAlH_4} CH_3NHCH_3 \]
The product is N-methylmethanamine, which is a secondary amine.
(C) Reduction of an amide: The reduction of a primary amide (R-CONH\(_2\)) with LiAlH\(_4\) yields a primary amine.
\[ CH_3CONH_2 \xrightarrow{LiAlH_4} CH_3CH_2NH_2 \]
The product is ethanamine, which is a primary amine.
(D) Hoffmann bromamide degradation: This reaction converts a primary amide into a primary amine with one less carbon atom.
\[ CH_3CONH_2 \xrightarrow{Br_2/KOH} CH_3NH_2 + K_2CO_3 + 2KBr + 2H_2O \]
The product is methanamine, which is a primary amine.
Step 3: Final Answer:
The reaction that does not give a primary amine as the product is the reduction of methyl isocyanide, which gives a secondary amine. Therefore, option (B) is the correct answer.
Quick Tip: Remember the key difference in reduction products: Nitriles (R-CN) reduce to primary amines (R-CH\(_2\)NH\(_2\)), while their isomers, isonitriles (R-NC), reduce to secondary amines (R-NHCH\(_3\)).
The right option for the mass of CO\(_2\) produced by heating 20 g of 20% pure limestone is (Atomic mass of Ca = 40) [CaCO\(_3\) \(\xrightarrow{1200 K}\) CaO + CO\(_2\)]
View Solution
Step 1: Understanding the Question:
This is a stoichiometry problem involving a reactant with a given purity. We need to calculate the mass of CO\(_2\) produced from the thermal decomposition of a given mass of impure limestone (CaCO\(_3\)).
Step 2: Key Formula or Approach:
1. Calculate the mass of the pure reactant (CaCO\(_3\)) in the sample.
2. Use the balanced chemical equation to find the molar relationship between the reactant and the product.
3. Calculate the mass of the product using mole-mass relationships.
Step 3: Detailed Explanation:
Calculate the mass of pure CaCO\(_3\):
Total mass of limestone sample = 20 g
Purity = 20%
Mass of pure CaCO\(_3\) = 20 g \(\times\) \(\frac{20}{100}\) = 4 g.
Molar masses:
Molar mass of CaCO\(_3\) = 40 (Ca) + 12 (C) + 3 \(\times\) 16 (O) = 100 g/mol.
Molar mass of CO\(_2\) = 12 (C) + 2 \(\times\) 16 (O) = 44 g/mol.
Stoichiometric calculation:
The balanced equation is: CaCO\(_3\) \(\rightarrow\) CaO + CO\(_2\).
From the equation, 1 mole of CaCO\(_3\) produces 1 mole of CO\(_2\).
In terms of mass: 100 g of CaCO\(_3\) produces 44 g of CO\(_2\).
Calculate the mass of CO\(_2\) produced:
We can set up a proportion:
\[ \frac{Mass of CO_2}{Mass of CaCO_3} = \frac{Molar mass of CO_2}{Molar mass of CaCO_3} \]
\[ \frac{x}{4 g} = \frac{44 g/mol}{100 g/mol} \]
\[ x = 4 \times \frac{44}{100} = 1.76 g \]
Step 4: Final Answer:
The mass of CO\(_2\) produced is 1.76 g.
Quick Tip: In stoichiometry problems involving impure reactants, always start by calculating the mass of the pure substance that will actually react. The impurities are assumed to be inert.
Identify the product in the following reaction:
View Solution
Step 1: Understanding the Question:
The question asks to identify the final organic product of a three-step reaction sequence starting from benzenediazonium chloride.
Step 2: Detailed Explanation:
Let's analyze the sequence step by step:
Step (i): Reaction with Cu\(_2\)Br\(_2\)/HBr. The starting material is benzenediazonium chloride. This step uses the reagents for the Sandmeyer reaction. This reaction replaces the diazonium group (–N\(_2\)\(^{+}\)) with a bromine atom.
\[ C_6H_5N_2^+Cl^- \xrightarrow{Cu_2Br_2/HBr} C_6H_5Br + N_2 \]
The product after step (i) is bromobenzene.
Step (ii): Reaction with Mg/dry ether. The product from the first step, bromobenzene, is treated with magnesium metal in an anhydrous ether solvent. This is the standard procedure for forming a Grignard reagent.
\[ C_6H_5Br + Mg \xrightarrow{dry ether} C_6H_5MgBr \]
The product after step (ii) is phenylmagnesium bromide.
Step (iii): Reaction with H\(_2\)O. The Grignard reagent, phenylmagnesium bromide, is a very strong base and nucleophile. When it reacts with water (or any protic solvent), it is protonated. The phenyl group acts as a carbanion and abstracts a proton from water to form the corresponding alkane (or arene in this case).
\[ C_6H_5MgBr + H_2O \rightarrow C_6H_6 + Mg(OH)Br \]
The final organic product is benzene.
Step 3: Final Answer:
The final product of the reaction sequence is benzene. This corresponds to option (A).
Quick Tip: Grignard reagents are powerful bases. Unless they are reacting with an electrophile like a carbonyl group, their most common reaction is with acidic protons (even from weak acids like water or alcohols) to form a hydrocarbon.
The number of \(\sigma\) bonds, \(\pi\) bonds and lone pair of electrons in pyridine, respectively are:
View Solution
Step 1: Understanding the Question:
The question requires us to count the total number of sigma (\(\sigma\)) bonds, pi (\(\pi\)) bonds, and lone pairs of electrons in a molecule of pyridine (C\(_5\)H\(_5\)N).
Step 2: Key Formula or Approach:
1. Draw the Lewis structure of pyridine. Pyridine is a six-membered heterocyclic aromatic ring containing five carbon atoms and one nitrogen atom.
2. Count the single bonds and the bonds within double bonds to find the total number of \(\sigma\) bonds. (Every single bond is one \(\sigma\) bond; every double bond is one \(\sigma\) and one \(\pi\) bond).
3. Count the number of double bonds to find the total number of \(\pi\) bonds.
4. Determine the number of non-bonding valence electrons on the nitrogen atom to find the number of lone pairs.
Step 3: Detailed Explanation:
The structure of pyridine is:
Counting \(\sigma\) bonds:
There are 6 \(\sigma\) bonds forming the ring structure (4 C-C bonds and 2 C-N bonds).
There are 5 C-H \(\sigma\) bonds.
Total \(\sigma\) bonds = 6 (in ring) + 5 (C-H) = 11.
Counting \(\pi\) bonds:
There are three alternating double bonds within the aromatic ring.
Each double bond contains one \(\pi\) bond.
Total \(\pi\) bonds = 3.
Counting lone pairs:
Carbon has 4 valence electrons and forms 4 bonds, so there are no lone pairs on carbon.
Nitrogen is in Group 15 and has 5 valence electrons.
In the pyridine ring, the nitrogen atom forms three bonds (two single \(\sigma\) bonds and one double bond, which is one \(\sigma\) and one \(\pi\)). So, it uses 3 valence electrons for bonding.
Number of non-bonding electrons on N = 5 (valence electrons) - 3 (bonding electrons) = 2 electrons.
These 2 electrons form 1 lone pair.
Step 4: Final Answer:
Pyridine has 11 \(\sigma\) bonds, 3 \(\pi\) bonds, and 1 lone pair of electrons. This corresponds to the sequence 11, 3, 1.
Quick Tip: For cyclic compounds, count the \(\sigma\) bonds forming the ring, then the \(\sigma\) bonds to atoms outside the ring. For aromatic rings, there is one \(\pi\) bond for every double bond shown in one of the resonance structures.
Which amongst the following options is the correct relation between change in enthalpy and change in internal energy?
View Solution
Step 1: Understanding the Question:
The question asks for the correct thermodynamic relationship between the change in enthalpy (\(\Delta\)H) and the change in internal energy (\(\Delta\)U) for a chemical reaction.
Step 2: Key Formula or Approach:
The definition of enthalpy (H) is given by:
\[ H = U + PV \]
where U is internal energy, P is pressure, and V is volume.
For a change in the system at constant pressure, the equation becomes:
\[ \Delta H = \Delta U + P\Delta V \]
Step 3: Detailed Explanation:
For chemical reactions involving gases, we can relate the \(P\Delta V\) term to the change in the number of moles of gas using the ideal gas equation, \(PV = nRT\).
Assuming constant temperature and pressure, the change in volume is primarily due to the change in the number of moles of gaseous components.
So, \(P\Delta V\) can be approximated as \(\Delta(PV)\).
\[ \Delta(PV) = \Delta(n_gRT) \]
Since R and T are constant, this becomes:
\[ \Delta(PV) = (\Delta n_g)RT \]
where \(\Delta n_g\) is the change in the number of moles of gas:
\[ \Delta n_g = (moles of gaseous products) - (moles of gaseous reactants) \]
Substituting this back into the enthalpy equation:
\[ \Delta H = \Delta U + \Delta n_g RT \]
Step 4: Final Answer:
The correct relationship is \(\Delta\)H = \(\Delta\)U + \(\Delta\)n\(_g\)RT. This matches option (A).
Quick Tip: A simple way to remember the sign is to think about a reaction that creates gas (\(\Delta n_g > 0\)). The system expands and does work on the surroundings (\(P\Delta V > 0\)). This work is supplied by the system's energy, so \(\Delta H\) (total heat change at constant P) must be greater than \(\Delta U\) (internal energy change). Thus, you add the work term: \(\Delta H = \Delta U + \Delta n_g RT\).
Consider the following reaction :
View Solution
Step 1: Understanding the Question:
The question asks for the products of the cleavage of benzyl phenyl ether with hydrogen iodide (HI).
Step 2: Key Formula or Approach:
The reaction of an ether with a strong acid like HI involves the cleavage of a C-O bond. The mechanism consists of two main steps:
1. Protonation of the ether oxygen by H\(^+\) to form an oxonium ion.
2. Nucleophilic attack by the iodide ion (I\(^-\)) on one of the carbon atoms attached to the oxygen.
The C-O bond that is cleaved is determined by the nature of the groups attached to the oxygen. The reaction can proceed via S\(_N\)1 or S\(_N\)2 mechanism.
Step 3: Detailed Explanation:
The reactant is benzyl phenyl ether (C\(_6\)H\(_5\)–CH\(_2\)–O–C\(_6\)H\(_5\)).
There are two possible C-O bonds to cleave: the benzyl-oxygen bond (C\(_6\)H\(_5\)CH\(_2\)–O) and the phenyl-oxygen bond (C\(_6\)H\(_5\)–O).
The phenyl-oxygen bond has partial double bond character due to resonance between the oxygen's lone pairs and the benzene ring. This bond is very strong and resistant to cleavage.
The benzyl-oxygen bond is a standard single bond between an sp\(^3\) carbon and oxygen.
The iodide ion (I\(^-\)) will act as a nucleophile and attack the carbon atom of the benzyl group (–CH\(_2\)–) because:
S\(_N\)2 pathway: The benzyl carbon is an sp\(^3\) carbon and is accessible to nucleophilic attack, whereas the sp\(^2\) carbon of the phenyl ring is not.
S\(_N\)1 pathway: Cleavage of the benzyl-oxygen bond would form a benzyl carbocation (C\(_6\)H\(_5\)CH\(_2\)\(^+\)), which is highly stabilized by resonance. Cleavage of the phenyl-oxygen bond would form a phenyl cation, which is extremely unstable. Therefore, the S\(_N\)1 pathway also favors cleavage of the benzyl-oxygen bond.
In either case, the I\(^-\) attacks the benzyl carbon, and the O–C\(_6\)H\(_5\) group becomes the leaving group, which gets protonated to form phenol.
The reaction is:
C\(_6\)H\(_5\)–CH\(_2\)–O–C\(_6\)H\(_5\) + HI \(\rightarrow\) C\(_6\)H\(_5\)–CH\(_2\)–I + C\(_6\)H\(_5\)–OH
So, the products are Benzyl iodide and Phenol.
Step 4: Final Answer:
Product A is benzyl iodide, and Product B is phenol. This matches the structures in option (B).
Quick Tip: When cleaving an alkyl aryl ether with HX, the halogen always attaches to the alkyl group, and the aryl group forms a phenol. This is because the aryl-oxygen bond is stronger than the alkyl-oxygen bond.
Which of the following statements are INCORRECT?
A. All the transition metals except scandium form MO oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc\(_2\)O\(_3\) to Mn\(_2\)O\(_7\).
C. Basic character increases from V\(_2\)O\(_3\) to V\(_2\)O\(_4\) to V\(_2\)O\(_5\).
D. V\(_2\)O\(_4\) dissolves in acids to give VO\(_4\)\(^{3-}\) salts.
E. CrO is basic but Cr\(_2\)O\(_3\) is amphoteric.
Choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question requires us to identify the incorrect statements about the properties of transition metal oxides from the given list of five statements.
Step 2: Detailed Explanation:
Let's evaluate each statement:
A. All the transition metals except scandium form MO oxides which are ionic. This statement is generally considered correct in a broad sense. Scandium's stable oxide is Sc\(_2\)O\(_3\). Most other first-row transition metals form oxides of the type MO (e.g., TiO, VO, FeO, NiO), which are predominantly ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc\(_2\)O\(_3\) to Mn\(_2\)O\(_7\). This statement is correct. Scandium (Group 3) shows +3 in Sc\(_2\)O\(_3\). Vanadium (Group 5) shows +5 in V\(_2\)O\(_5\). Chromium (Group 6) shows +6 in CrO\(_3\). Manganese (Group 7) shows +7 in Mn\(_2\)O\(_7\). This trend holds for the elements up to manganese.
C. Basic character increases from V\(_2\)O\(_3\) to V\(_2\)O\(_4\) to V\(_2\)O\(_5\). This statement is incorrect. A general rule for metal oxides is that as the oxidation state of the metal increases, the covalent character of the oxide increases, and its acidic character increases (or its basic character decreases). The oxidation states of vanadium are +3 (in V\(_2\)O\(_3\)), +4 (in V\(_2\)O\(_4\)), and +5 (in V\(_2\)O\(_5\)). Therefore, the basic character should decrease in the order V\(_2\)O\(_3\) > V\(_2\)O\(_4\) \(>\) V\(_2\)O\(_5\). The statement claims the opposite.
D. V\(_2\)O\(_4\) dissolves in acids to give VO\(_4\)\(^{3-}\) salts. This statement is incorrect. In V\(_2\)O\(_4\) (which is 2 \(\times\) VO\(_2\)), the oxidation state of vanadium is +4. When this oxide dissolves in acid, it forms the vanadyl ion, which is VO\(^{2+}\), where vanadium is still in the +4 oxidation state. The ion VO\(_4\)\(^{3-}\) (orthovanadate) has vanadium in the +5 oxidation state.
E. CrO is basic but Cr\(_2\)O\(_3\) is amphoteric. This statement is correct. It follows the trend with oxidation states. CrO (Cr\(^{2+}\)) is a basic oxide. Cr\(_2\)O\(_3\) (Cr\(^{3+}\)) is amphoteric (reacts with both acids and bases). Higher oxides like CrO\(_3\) (Cr\(^{6+}\)) are acidic.
Step 3: Final Answer:
The statements that are incorrect are C and D. Therefore, the correct option is (B).
Quick Tip: For oxides of a single transition metal, remember this trend: Lower oxidation state oxides are basic, intermediate oxidation state oxides are amphoteric, and the highest oxidation state oxides are acidic.
Which amongst the following be most readily dehydrated under acidic conditions?
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given alcohols will undergo acid-catalyzed dehydration most readily. The rate of acid-catalyzed dehydration, which typically follows an E1 mechanism, is primarily determined by the stability of the carbocation intermediate formed after the loss of water.
Step 2: Key Formula or Approach:
The stability of carbocations follows the order: Tertiary (3\(^\circ\)) > Secondary (2\(^\circ\)) > Primary (1\(^\circ\)). Electron-donating groups stabilize carbocations, while electron-withdrawing groups (like -NO\(_2\)) destabilize them. The destabilizing inductive effect (-I) of an electron-withdrawing group decreases significantly with distance.
Step 3: Detailed Explanation:
Let's analyze the carbocation that would be formed from each alcohol:
(1) Butane-2,3-diol: Dehydration would involve the formation of a secondary carbocation on C-2 or C-3. This carbocation is adjacent to another hydroxyl group, which can stabilize it through resonance (+M effect). This compound would undergo a very rapid pinacol rearrangement, a special type of dehydration.
(2) 1-Nitrobutane-2,3-diol: Dehydration would form a secondary carbocation that is significantly destabilized by the strong electron-withdrawing -NO\(_2\) group located nearby.
(3) 4-Nitropentan-2-ol: This is a secondary alcohol. Dehydration forms a secondary carbocation at the C-2 position. The electron-withdrawing -NO\(_2\) group is at the C-4 position. Its destabilizing inductive effect is present but diminished due to the distance from the positive charge.
(4) 3-Nitrobutan-2-ol: This is a secondary alcohol. Dehydration forms a secondary carbocation at the C-2 position. The -NO\(_2\) group is on the adjacent carbon (C-3), causing strong destabilization.
Step 4: Final Answer:
Comparing the options, the carbocations from (2) and (4) are strongly destabilized. The reaction of compound (1), a 1,2-diol, leads to a very fast pinacol rearrangement, suggesting it should be the most reactive. However, among the simple E1 dehydrations of mono-alcohols with a nitro group (3 and 4), compound (3) would react faster than (4) because the destabilizing nitro group is further away from the developing positive charge.
Quick Tip: The rate of E1 dehydration is governed by carbocation stability. Electron-withdrawing groups like -NO\(_2\) destabilize carbocations, and this effect is strongest when the group is close to the positive center.
The reaction that does NOT take place in a blast furnace between 900 K to 1500 K temperature range during extraction of iron is :
View Solution
Step 1: Understanding the Question:
The question asks to identify which of the given reactions does not occur in the specific temperature range of 900 K to 1500 K inside a blast furnace used for iron extraction.
Step 2: Detailed Explanation:
A blast furnace has different temperature zones where specific reactions take place.
Upper zone (Zone of reduction, 500 K - 800 K): In this lower temperature region, iron oxides are reduced in steps. The initial reduction of hematite (Fe\(_2\)O\(_3\)) occurs here.
\[ 3Fe_2O_3 + CO \rightarrow 2Fe_3O_4 + CO_2 \]
\[ Fe_3O_4 + CO \rightarrow 3FeO + CO_2 \]
The reaction Fe\(_2\)O\(_3\) + CO \(\rightarrow\) 2FeO + CO\(_2\) represents a part of this overall reduction process that is completed in this lower temperature zone.
Middle zone (Zone of slag formation, 900 K - 1500 K): In this higher temperature range:
Limestone decomposes: CaCO\(_3\) \(\rightarrow\) CaO + CO\(_2\) (at ~1200 K).
Slag is formed: CaO + SiO\(_2\) \(\rightarrow\) CaSiO\(_3\). This is reaction (C).
The final reduction of iron(II) oxide to molten iron occurs: FeO + CO \(\rightarrow\) Fe + CO\(_2\). This is reaction (A).
The Boudouard reaction becomes significant at temperatures above 1000 K: C + CO\(_2\) \(\rightarrow\) 2CO. This is reaction (B).
Step 3: Final Answer:
Based on the temperature zones, reaction (D), the reduction of Fe\(_2\)O\(_3\), occurs at temperatures lower than 900 K. The other three reactions (A, B, and C) all take place within the 900 K to 1500 K range. Therefore, the reaction that does NOT occur in this range is (D).
Quick Tip: Remember the sequence of reduction in a blast furnace: The higher oxides of iron (Fe\(_2\)O\(_3\), Fe\(_3\)O\(_4\)) are reduced at lower temperatures in the upper part of the furnace, while the final reduction to metallic iron (from FeO) occurs at higher temperatures in the middle section.
Identify the major product obtained in the following reaction:
View Solution
Step 1: Understanding the Question:
The question asks for the major product of a reaction involving 2-acetylbenzaldehyde with Tollens' reagent ([Ag(NH\(_3\))\(_2\)]\(^+\)) under basic conditions (OH\(^-\)) with heating.
Step 2: Key Formula or Approach:
The key is to identify the functional groups in the starting material and the selectivity of the reagent.
Starting Material: The molecule is 2-acetylbenzaldehyde. It contains two carbonyl functional groups: an aldehyde group (–CHO) and a ketone group (–COCH\(_3\)).
Reagent: Tollens' reagent ([Ag(NH\(_3\))\(_2\)]\(^+\)) is a mild oxidizing agent. Its characteristic reaction is the selective oxidation of aldehydes to carboxylate ions. It does not typically oxidize ketones, alcohols, or alkenes. The reaction is carried out in a basic medium.
Step 3: Detailed Explanation:
The Tollens' reagent will react with the aldehyde group but will leave the ketone group untouched.
The aldehyde group (–CHO) is oxidized to a carboxylate group (–COO\(^-\)) because the reaction is performed under basic conditions.
The ketone group (–COCH\(_3\)) does not react.
The silver(I) ions in the Tollens' reagent are reduced to metallic silver (Ag(s)), which would form a silver mirror on the reaction vessel.
The overall transformation is:
The product is the salt of 2-acetylbenzoic acid, which is 2-acetylbenzoate.
Step 4: Final Answer:
Comparing this product with the given options, option (B) correctly shows the structure of 2-acetylbenzoate, where the aldehyde has been converted to a carboxylate and the ketone remains.
Quick Tip: Tollens' test is a classic chemical test to distinguish aldehydes from ketones. Aldehydes give a positive test (formation of a silver mirror), while most ketones do not react. Remember that Tollens' reagent is a selective oxidizing agent.
What fraction of one edge centred octahedral void lies in one unit cell of fcc?
View Solution
Step 1: Understanding the Question:
The question asks for the contribution of a single octahedral void located at the center of an edge of a face-centered cubic (fcc) unit cell to that specific unit cell.
Step 2: Key Formula or Approach:
In an fcc (or ccp) lattice, octahedral voids are located at two types of positions:
1. One void at the body center of the unit cell.
2. One void at the center of each of the 12 edges of the unit cell.
We need to determine how many unit cells share an edge. An edge of a cubic unit cell is shared by the four unit cells that meet at that edge.
Step 3: Detailed Explanation:
Imagine a single unit cell. An edge is shared by the cell itself, the cell above it, and the two cells in front of (or behind) those two. Visualizing this, any point on an edge is simultaneously part of four different cubes that share that edge.
Since the octahedral void is located at the center of this edge, it is also shared equally among these four unit cells.
Therefore, the fraction of the edge-centered octahedral void that lies within one unit cell is \(\frac{1}{4}\).
Step 4: Final Answer:
The contribution of one edge-centered octahedral void to a single unit cell is \(\frac{1}{4}\).
Quick Tip: Remember the contributions of different lattice positions to a single unit cell: corner = 1/8, face center = 1/2, edge center = 1/4, body center = 1.
The equilibrium concentrations of the species in the reaction A + B \(\rightleftharpoons\) C + D are 2, 3, 10 and 6 mol L\(^{-1}\), respectively at 300 K. \(\Delta\)G\(^\circ\) for the reaction is (R = 2 cal/mol K)
View Solution
Step 1: Understanding the Question:
We are given the equilibrium concentrations of all species in a reaction and the temperature. We need to calculate the standard Gibbs free energy change (\(\Delta G^\circ\)) for the reaction.
Step 2: Key Formula or Approach:
1. First, calculate the equilibrium constant, K\(_c\), from the given equilibrium concentrations.
2. Then, use the relationship between the standard Gibbs free energy change and the equilibrium constant:
\[ \Delta G^\circ = -RT \ln K \]
or, using base-10 logarithm:
\[ \Delta G^\circ = -2.303 RT \log K \]
Step 3: Detailed Explanation:
1. Calculate the equilibrium constant (K\(_c\)):
The reaction is A + B \(\rightleftharpoons\) C + D.
The expression for K\(_c\) is:
\[ K_c = \frac{[C][D]}{[A][B]} \]
Given concentrations at equilibrium:
[A] = 2 M, [B] = 3 M, [C] = 10 M, [D] = 6 M
\[ K_c = \frac{(10)(6)}{(2)(3)} = \frac{60}{6} = 10 \]
2. Calculate \(\Delta\)G\(^\circ\):
Given values:
R = 2 cal/mol K
T = 300 K
K\(_c\) = 10
Using the formula \(\Delta G^\circ = -2.303 RT \log K_c\):
\[ \Delta G^\circ = -2.303 \times (2 cal/mol K) \times (300 K) \times \log(10) \]
Since \(\log(10) = 1\):
\[ \Delta G^\circ = -2.303 \times 2 \times 300 \times 1 \] \[ \Delta G^\circ = -2.303 \times 600 \] \[ \Delta G^\circ = -1381.8 cal \]
Step 4: Final Answer:
The standard Gibbs free energy change for the reaction is –1381.80 cal. This corresponds to option (B).
Quick Tip: Ensure your units are consistent. If R is in cal/mol K, \(\Delta G^\circ\) will be in cal. If R is in J/mol K, \(\Delta G^\circ\) will be in J. The formula \(\Delta G^\circ = -RT \ln K\) is fundamental for relating thermodynamics and equilibrium.
Consider the following compounds/species:
The number of compounds/species which obey Huckel's rule is ________.
View Solution
Step 1: Understanding the Question:
The question asks us to identify how many of the given seven species are aromatic according to Huckel's rule.
Step 2: Key Formula or Approach:
For a species to be aromatic, it must satisfy all of Huckel's criteria:
1. The molecule must be cyclic.
2. The molecule must be planar.
3. The molecule must have a continuous ring of p-orbitals (i.e., be fully conjugated).
4. The number of \(\pi\) electrons in the conjugated system must be equal to (4n + 2), where n is a non-negative integer (0, 1, 2, ...). This means the number of \(\pi\) electrons must be 2, 6, 10, 14, etc.
Step 3: Detailed Explanation:
Let's analyze each species:
i. Naphthalene: It is cyclic, planar, and fully conjugated. It has 5 double bonds, so the number of \(\pi\) electrons is 5 \(\times\) 2 = 10. Since 10 = (4 \(\times\) 2 + 2), it obeys Huckel's rule.
ii. Cyclopentadienyl anion: It is cyclic, planar, and fully conjugated. It has 2 double bonds (4 \(\pi\) e\(^-\)) and a lone pair in a p-orbital (2 \(\pi\) e\(^-\)). Total \(\pi\) electrons = 4 + 2 = 6. Since 6 = (4 \(\times\) 1 + 2), it obeys Huckel's rule.
iii. Cyclobutadiene: It is cyclic, planar, and conjugated. It has 2 double bonds, so it has 4 \(\pi\) electrons. This fits the (4n) rule for antiaromaticity, not the (4n+2) rule. It is not aromatic.
iv. Cyclopropenyl anion: It is cyclic, planar, and conjugated. It has 1 double bond (2 \(\pi\) e\(^-\)) and a lone pair (2 \(\pi\) e\(^-\)). Total \(\pi\) electrons = 4. This is antiaromatic. It does not obey Huckel's rule for aromaticity.
v. Cyclopropenyl cation: It is cyclic, planar, and conjugated. It has 1 double bond, contributing 2 \(\pi\) electrons. The positive charge indicates an empty p-orbital. Total \(\pi\) electrons = 2. Since 2 = (4 \(\times\) 0 + 2), it obeys Huckel's rule.
vi. Cyclooctatetraene: It is cyclic and conjugated, but it is not planar. It adopts a tub-like shape to avoid the destabilization of being antiaromatic (it has 8 \(\pi\) electrons). Because it is non-planar, it is not aromatic.
vii. Anthracene: It is cyclic, planar, and fully conjugated. It has 7 double bonds, so the number of \(\pi\) electrons is 7 \(\times\) 2 = 14. Since 14 = (4 \(\times\) 3 + 2), it obeys Huckel's rule.
Step 4: Final Answer:
The species that obey Huckel's rule for aromaticity are: Naphthalene (i), Cyclopentadienyl anion (ii), Cyclopropenyl cation (v), and Anthracene (vii).
The total number of such species is 4.
Quick Tip: Remember the magic numbers for aromaticity: 2, 6, 10, 14... \(\pi\) electrons. And for antiaromaticity: 4, 8, 12... \(\pi\) electrons. A species must be cyclic, planar, and fully conjugated to be considered for either category.
Which complex compound is most stable?
View Solution
Step 1: Understanding the Question:
The question asks to identify the most stable complex compound from the given options. The stability of a coordination complex is significantly influenced by the nature of the ligands, particularly by the chelate effect.
Step 2: Key Formula or Approach:
The chelate effect states that complexes formed by polydentate ligands (chelating agents) are thermodynamically more stable than complexes with analogous monodentate ligands. A polydentate ligand, like ethylenediamine (en), can form a ring structure with the central metal ion. This increased stability is due to a favorable entropy change upon formation of the chelate complex.
Step 3: Detailed Explanation:
Let's analyze the ligands in each complex:
(A) [Co(NH\(_3\))\(_3\)(NO\(_3\))\(_3\)]: The ligands are ammine (NH\(_3\)) and nitrato (NO\(_3\)\(^-\)). Both are monodentate ligands. No chelation occurs.
(B) [CoCl\(_2\)(en)\(_2\)]NO\(_3\): The ligands are chlorido (Cl\(^-\)) and ethylenediamine (en). Ethylenediamine (H\(_2\)N–CH\(_2\)–CH\(_2\)–NH\(_2\)) is a bidentate ligand. It forms two coordinate bonds with the cobalt ion, creating stable five-membered chelate rings.
(C) [Co(NH\(_3\))\(_6\)]\(_2\)(SO\(_4\))\(_3\): The ligand is ammine (NH\(_3\)), which is a monodentate ligand. No chelation occurs.
(D) [Co(NH\(_3\))\(_4\)(H\(_2\)O)Br](NO\(_3\))\(_2\): The ligands are ammine (NH\(_3\)), aqua (H\(_2\)O), and bromo (Br\(^-\)). All are monodentate ligands. No chelation occurs.
Step 4: Final Answer:
Since complex (B) is the only one containing a chelating ligand (ethylenediamine), it benefits from the chelate effect and is therefore the most stable among the given options.
Quick Tip: When comparing complex stability, always look for the presence of polydentate (chelating) ligands. The formation of chelate rings dramatically increases the stability of the complex.
On balancing the given redox reaction, aCr\(_2\)O\(_7\)\(^{2-}\) + bSO\(_3\)\(^{2-}\)(aq) + cH\(^+\)(aq) \(\rightarrow\) 2aCr\(^{3+}\)(aq) + bSO\(_4\)\(^{2-}\)(aq) + \(\frac{c}{2}\)H\(_2\)O(l)
the coefficients a, b and c are found to be, respectively-
View Solution
Step 1: Understanding the Question:
The question asks to find the stoichiometric coefficients a, b, and c for the given redox reaction, which is occurring in an acidic medium.
Step 2: Key Formula or Approach:
We can balance the equation using the half-reaction method (ion-electron method).
1. Separate the reaction into oxidation and reduction half-reactions.
2. Balance atoms other than O and H.
3. Balance O atoms by adding H\(_2\)O.
4. Balance H atoms by adding H\(^+\).
5. Balance charge by adding electrons (e\(^-\)).
6. Equalize the number of electrons in both half-reactions by multiplying by appropriate integers.
7. Add the balanced half-reactions and cancel common species.
Step 3: Detailed Explanation:
Reduction Half-Reaction:
\[ Cr_2O_7^{2-} \rightarrow Cr^{3+} \]
1. Balance Cr atoms: \(Cr_2O_7^{2-} \rightarrow 2Cr^{3+}\)
2. Balance O atoms: \(Cr_2O_7^{2-} \rightarrow 2Cr^{3+} + 7H_2O\)
3. Balance H atoms: \(Cr_2O_7^{2-} + 14H^+ \rightarrow 2Cr^{3+} + 7H_2O\)
4. Balance charge: LHS charge = -2 + 14 = +12. RHS charge = 2(+3) = +6. Add 6e\(^-\) to LHS.
\(Cr_2O_7^{2-} + 14H^+ + 6e^- \rightarrow 2Cr^{3+} + 7H_2O\) (Equation I)
Oxidation Half-Reaction:
\[ SO_3^{2-} \rightarrow SO_4^{2-} \]
1. S atoms are balanced.
2. Balance O atoms: \(SO_3^{2-} + H_2O \rightarrow SO_4^{2-}\)
3. Balance H atoms: \(SO_3^{2-} + H_2O \rightarrow SO_4^{2-} + 2H^+\)
4. Balance charge: LHS charge = -2. RHS charge = -2 + 2 = 0. Add 2e\(^-\) to RHS.
\(SO_3^{2-} + H_2O \rightarrow SO_4^{2-} + 2H^+ + 2e^-\) (Equation II)
Combine Half-Reactions:
To equalize the electrons, multiply Equation II by 3.
\[ 3SO_3^{2-} + 3H_2O \rightarrow 3SO_4^{2-} + 6H^+ + 6e^- \]
Now add this to Equation I:
\(Cr_2O_7^{2-} + 14H^+ + 6e^- + 3SO_3^{2-} + 3H_2O \rightarrow 2Cr^{3+} + 7H_2O + 3SO_4^{2-} + 6H^+ + 6e^-\)
Cancel common species (6e\(^-\), 6H\(^+\), 3H\(_2\)O):
\[ Cr_2O_7^{2-} + 3SO_3^{2-} + 8H^+ \rightarrow 2Cr^{3+} + 3SO_4^{2-} + 4H_2O \]
Step 4: Final Answer:
Comparing this balanced equation to the given format:
aCr\(_2\)O\(_7\)\(^{2-}\) + bSO\(_3\)\(^{2-}\) + cH\(^+\) \(\rightarrow\) 2aCr\(^{3+}\) + bSO\(_4\)\(^{2-}\) + \(\frac{c}{2}\)H\(_2\)O
We find that a = 1, b = 3, and c = 8.
Quick Tip: After balancing, always do a final check of both the atoms and the total charge on both sides of the equation to ensure it is correct.
Given below are two statements :
Statement I : The nutrient deficient water bodies lead to eutrophication
Statement II : Eutrophication leads to decrease in the level of oxygen in the water bodies.
In the light of the above statements, choose the correct answer from the options given below:
View Solution
Step 1: Understanding the Question:
The question presents two statements about eutrophication, and we need to determine the correctness of each statement.
Step 2: Detailed Explanation:
Analysis of Statement I:
"The nutrient deficient water bodies lead to eutrophication"
This statement is incorrect. Eutrophication is the process of nutrient \textit{enrichment of a water body. It is caused by an excess of nutrients, particularly phosphates and nitrates, which often come from agricultural runoff (fertilizers) and sewage. A nutrient-deficient water body is called oligotrophic. Therefore, the statement describes the opposite of what causes eutrophication.
Analysis of Statement II:
"Eutrophication leads to decrease in the level of oxygen in the water bodies."
This statement is correct. The excess nutrients in a eutrophic water body cause a dense growth of plant life, especially algae (an "algal bloom"). When this large amount of algae dies, it sinks to the bottom and is decomposed by aerobic bacteria. This decomposition process consumes a large amount of dissolved oxygen in the water. The resulting depletion of oxygen can lead to the death of fish and other aquatic animals, creating an anaerobic environment.
Step 3: Final Answer:
Statement I is incorrect, and Statement II is true. This corresponds to option (C).
Quick Tip: Remember: Eutrophication = Nutrient RICH (not deficient). The consequence is an algal bloom, followed by decomposition, which consumes dissolved oxygen, harming aquatic life.
Identify the final product [D] obtained in the following sequence of reactions.
View Solution
Step 1: Understanding the Question:
The question asks to identify the final product [D] of a four-step reaction sequence. We need to trace the transformation of the starting material through each step.
Step 2: Detailed Explanation:
Step 1: CH\(_3\)CHO \(\xrightarrow{LiAlH_4, H_3O^+}\) [A]
The starting material is ethanal (CH\(_3\)CHO), an aldehyde. Lithium aluminium hydride (LiAlH\(_4\)) followed by acidic workup is a strong reducing agent that reduces aldehydes to primary alcohols.
\[ CH_3CHO \rightarrow CH_3CH_2OH \]
So, [A] is ethanol.
Step 2: [A] \(\xrightarrow{H_2SO_4, \Delta}\) [B]
Ethanol [A] is heated with concentrated sulfuric acid. This is an acid-catalyzed dehydration of an alcohol, which results in the formation of an alkene.
\[ CH_3CH_2OH \rightarrow CH_2=CH_2 + H_2O \]
So, [B] is ethene.
Step 3: [B] \(\xrightarrow{HBr}\) [C]
Ethene [B] reacts with hydrogen bromide (HBr). This is an electrophilic addition reaction across the double bond.
\[ CH_2=CH_2 + HBr \rightarrow CH_3CH_2Br \]
So, [C] is bromoethane.
Step 4: Bromobenzene + [C] \(\xrightarrow{Na/dry ether}\) [D]
This is the Wurtz-Fittig reaction, which couples an aryl halide (bromobenzene) with an alkyl halide (bromoethane, [C]) using sodium metal in dry ether to form an alkylbenzene.
\[ C_6H_5Br + BrCH_2CH_3 + 2Na \xrightarrow{dry ether} C_6H_5CH_2CH_3 + 2NaBr \]
The final product [D] is ethylbenzene.
Step 3: Final Answer:
The final product [D] is ethylbenzene, which corresponds to the structure in option (D).
Quick Tip: The Wurtz-Fittig reaction is a valuable tool for attaching an alkyl chain to a benzene ring. It involves reacting an aryl halide and an alkyl halide with sodium in dry ether.
Pumice stone is an example of
View Solution
Step 1: Understanding the Question:
The question asks to classify pumice stone based on the types of colloidal systems. A colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout another substance. The classification is based on the physical state of the dispersed phase and the dispersion medium.
Step 2: Detailed Explanation:
Pumice stone is a type of volcanic rock that is very porous. It is formed when super-heated, highly pressurized rock is violently ejected from a volcano. The porous texture is due to gas bubbles being trapped in the rock during rapid cooling.
In this system, the dispersed phase is a gas (the bubbles) and the dispersion medium is a solid (the rock).
Let's analyze the given options based on standard colloidal classification:
Gel: Dispersed phase is liquid, dispersion medium is solid.
Foam: Dispersed phase is gas, dispersion medium is liquid.
Sol: Dispersed phase is solid, dispersion medium is liquid.
Solid sol: This term can be used for two types of systems: Solid dispersed in a solid (e.g., colored glass) or, according to some standard classifications (like NCERT), a gas dispersed in a solid.
Following the classification used in many standard textbooks, the colloidal system with a gaseous dispersed phase and a solid dispersion medium is termed a solid sol or a solid foam. Given the options, "Solid sol" is the correct classification for pumice stone.
Step 3: Final Answer:
Pumice stone, which consists of gas dispersed in a solid medium, is classified as a solid sol.
Quick Tip: Memorize the table of colloid types. A system of gas dispersed in a solid (like pumice stone or foam rubber) is often referred to as a solid sol in the context of competitive exam questions.
Match List-I with List – II :
List-I (Oxoacids of Sulphur) & List-II (Bonds)
A. Peroxodisulphuric acid & I. & Two S–OH, Four S=O, One S–O–S
B. Sulphuric acid & II. & Two S–OH, One S=O
C. Pyrosulphuric acid & III. & Two S–OH, Four S=O, One S–O–O–S
D. Sulphurous acid & IV. & Two S–OH, Two S=O
Choose the correct answer from the options given below.
View Solution
Step 1: Understanding the Question:
The question requires matching four different oxoacids of sulfur with the correct description of the number and types of bonds present in their structures.
Step 2: Detailed Explanation:
Let's determine the structure and count the bonds for each acid in List-I.
A. Peroxodisulphuric acid (H\(_2\)S\(_2\)O\(_8\)), also known as Marshall's acid: The structure contains a peroxide linkage (–O–O–) connecting two SO\(_3\)H groups. Structure: HO–SO\(_2\)–O–O–SO\(_2\)–OH.
S–OH bonds: 2
S=O bonds: 4
S–O–O–S linkage (which contains one O-O bond): 1
This matches with description III.
B. Sulphuric acid (H\(_2\)SO\(_4\)): The structure has a central sulfur atom double-bonded to two oxygen atoms and single-bonded to two hydroxyl (–OH) groups. Structure: HO–SO\(_2\)–OH.
S–OH bonds: 2
S=O bonds: 2
This matches with description IV.
C. Pyrosulphuric acid (H\(_2\)S\(_2\)O\(_7\)), also known as Oleum: The structure consists of two SO\(_3\)H groups linked by an oxygen atom bridge (–O–). Structure: HO–SO\(_2\)–O–SO\(_2\)–OH.
S–OH bonds: 2
S=O bonds: 4
S–O–S linkage: 1
This matches with description I.
D. Sulphurous acid (H\(_2\)SO\(_3\)): The structure has a central sulfur atom double-bonded to one oxygen atom, single-bonded to two hydroxyl (–OH) groups, and has one lone pair. Structure: HO–SO–OH.
S–OH bonds: 2
S=O bonds: 1
This matches with description II.
Step 3: Final Answer:
The correct matching is:
A \(\rightarrow\) III
B \(\rightarrow\) IV
C \(\rightarrow\) I
D \(\rightarrow\) II
This combination corresponds to option (A).
Quick Tip: To easily remember sulfur oxoacid structures, start with sulfuric acid (H\(_2\)SO\(_4\)). Pyrosulfuric acid is formed by removing one H\(_2\)O from two H\(_2\)SO\(_4\) molecules (creating an S-O-S link). Peroxodisulfuric acid is formed by joining two SO\(_3\)H radicals (creating an S-O-O-S link).
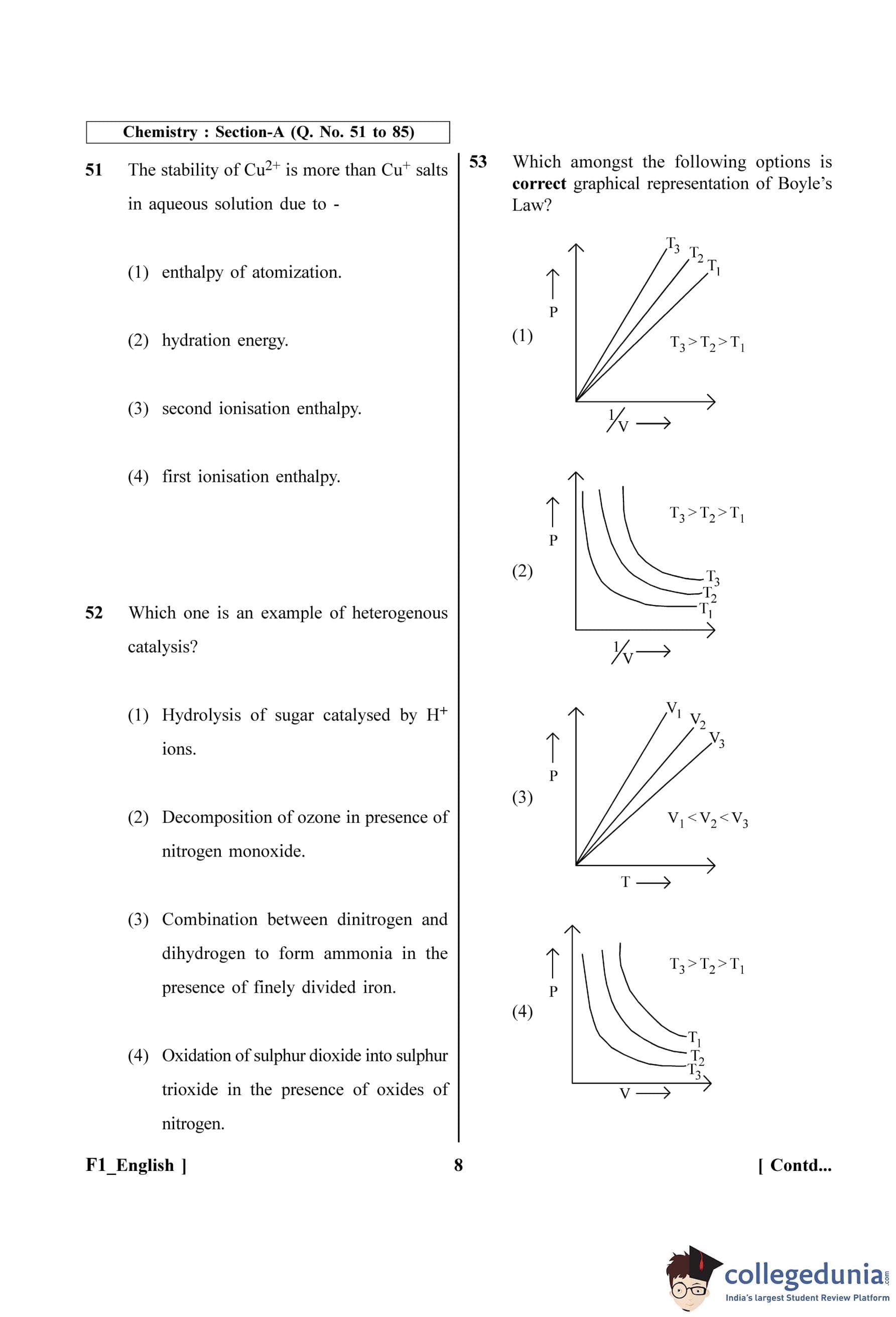


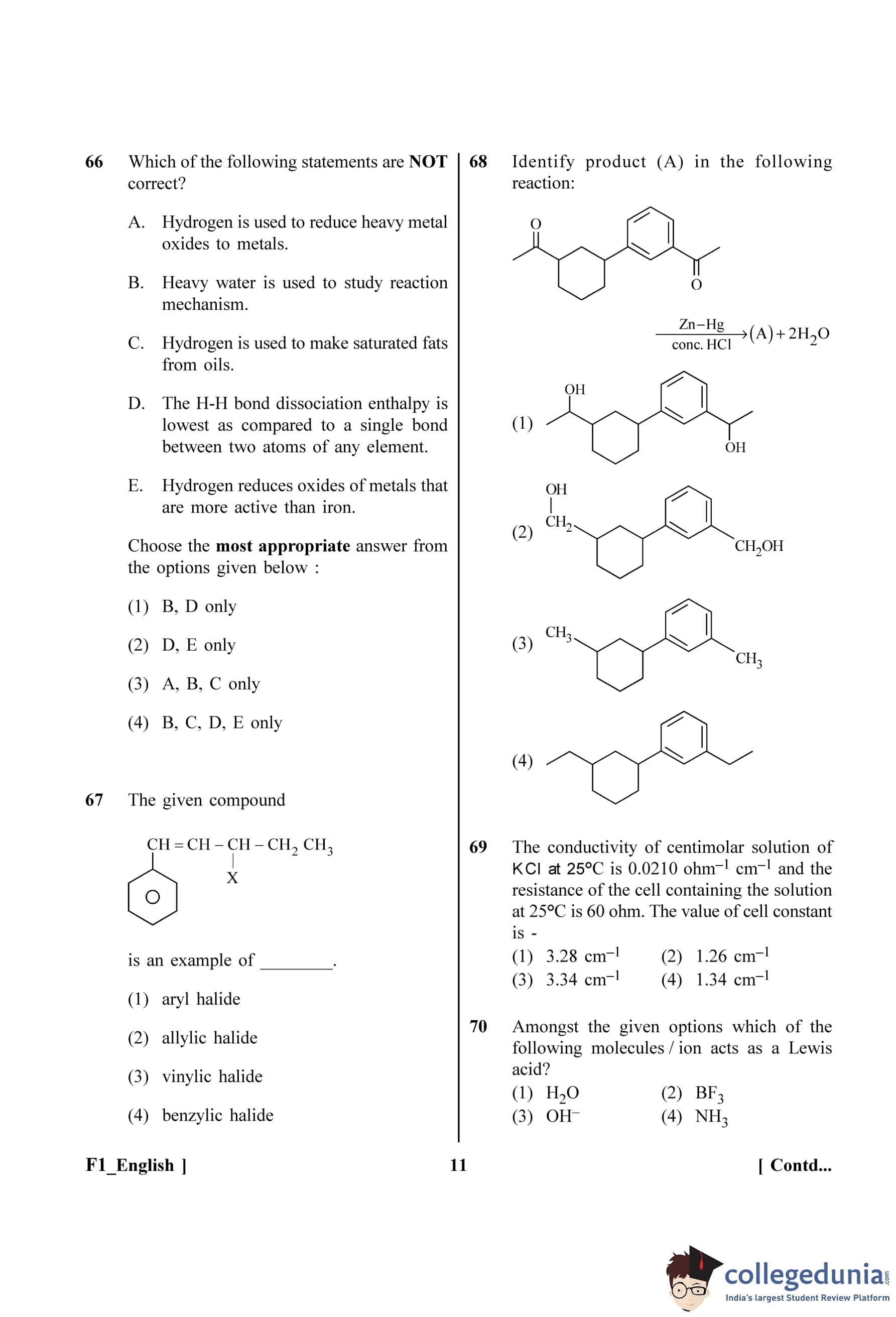

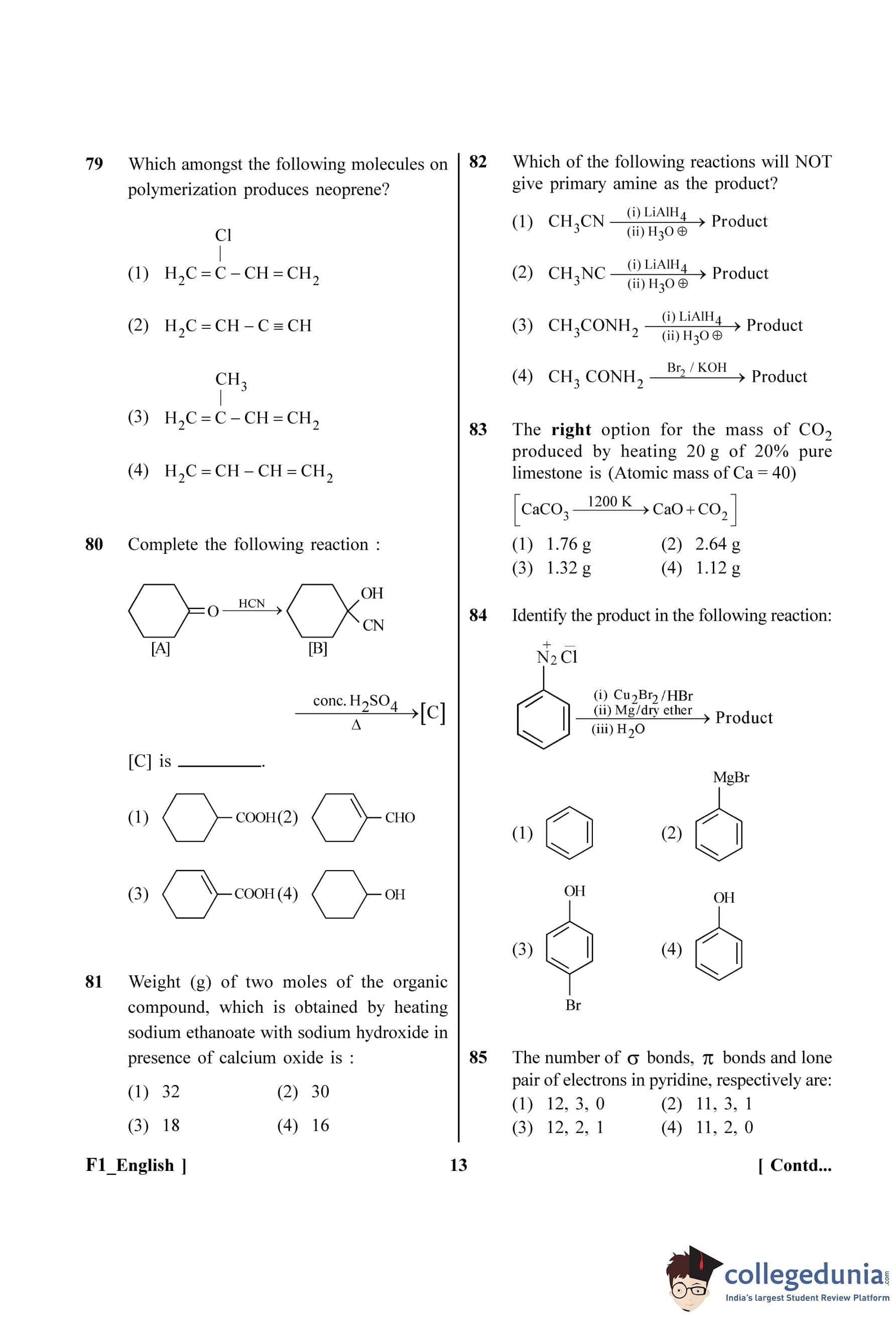
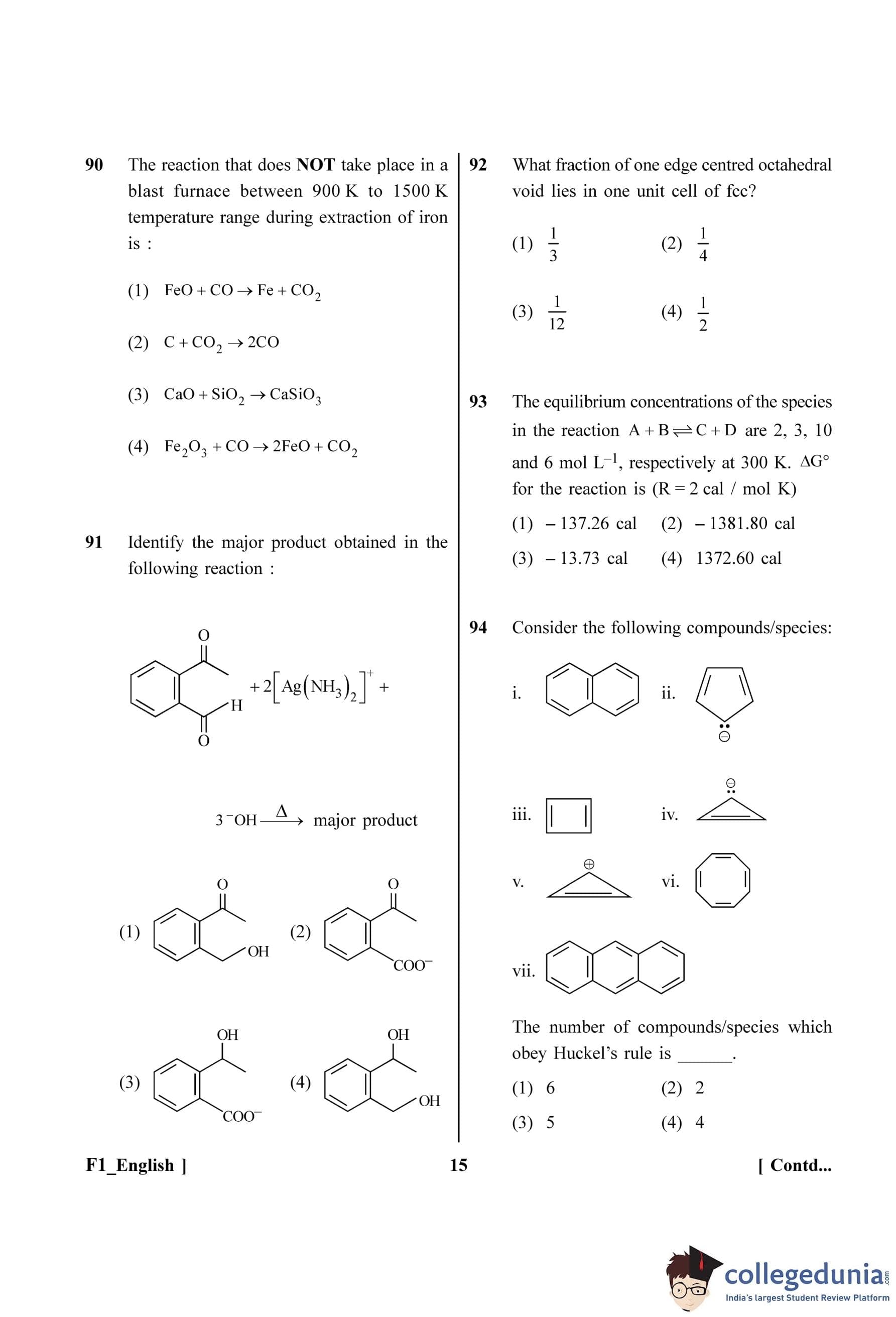
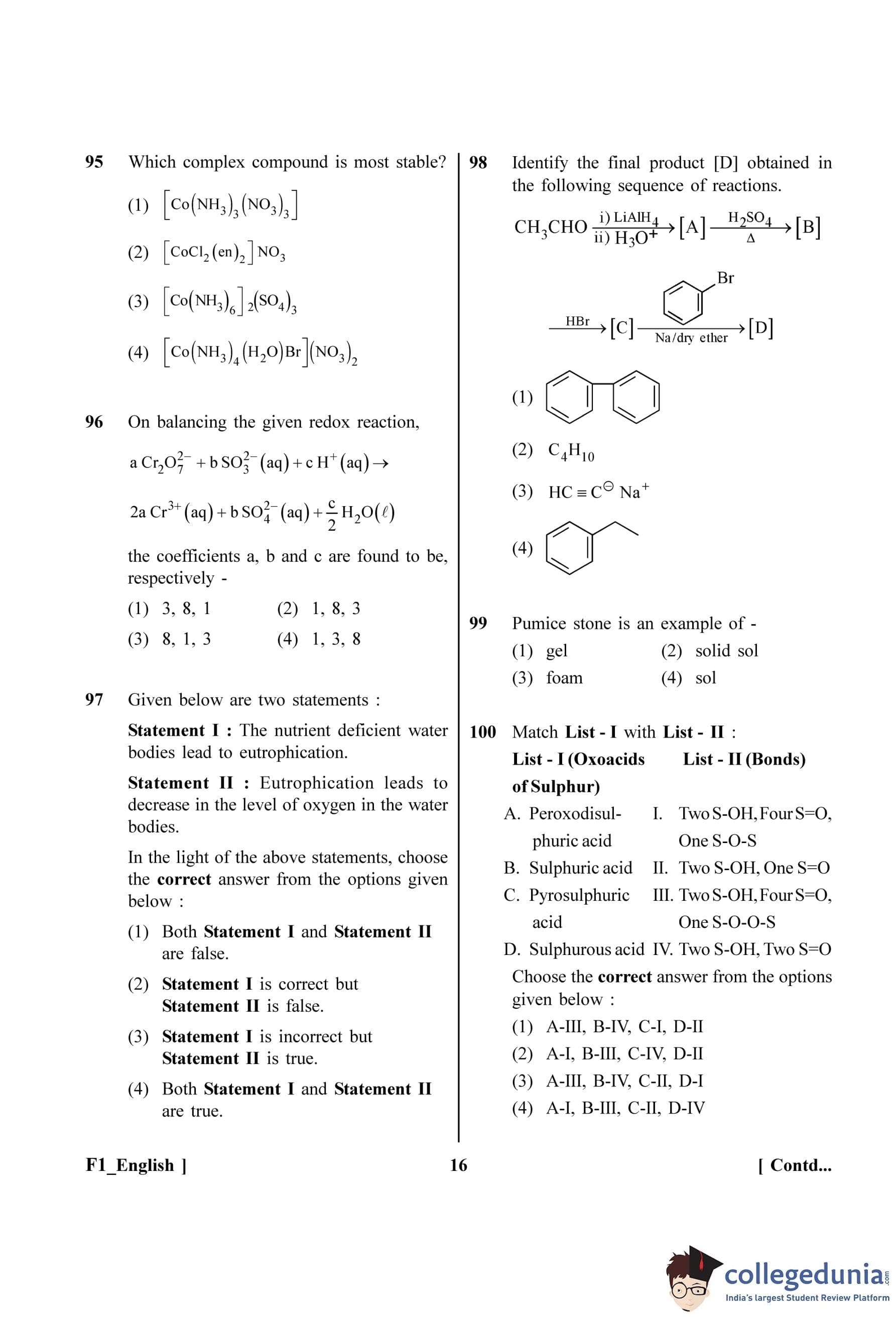
NEET Previous Year Question Papers with Answer Keys
| NEET 2022 Question Papers | NEET 2021 Question Papers | NEET 2020 Question Papers |
| NEET 2019 Question Papers | NEET 2018 Question Papers | NEET 2017 Question Papers |



Comments