Gaurav Goplani Content Writer
Content Writer
The bonding nature of Carbon is a Covalent bond. A covalent bond involves the sharing of two electrons between two atoms. These two electrons are known as a sharing pair or bonding pair. When the electrons are shared, the constant balance of attractive and repulsive forces between atoms is called covalent bonding.
Carbons usually exist in two-state, as a free state and as a combined state. Graphite, Diamond and Fullerene are examples of the free state of carbon and Carbon-dioxide, Glucose and Sugar are examples of the combined state of carbon.
| Table of Content |
Keyterms: Carbon, Covalent bond, Electron, Atoms, Carbon-dioxide, Glucose, Sugar, covalent bonding
Covalent Bond in Carbon
[Click Here for Sample Questions]
The atomic number of carbon is 6 and hence its electronic configuration is 2,4. In order to achieve the inert gas electronic configuration, carbon needs 4 electrons. But carbon cannot form an ionic bond. Hence it could either gain four electrons forming C4- cation or lose four electrons forming C4+ cations. But to gain electrons, it would be difficult for the nucleus with 6 protons to hold on to ten electrons and to lose electrons, it would require a large amount of energy to remove four electrons.
Hence in order to solve this problem, carbon shares its valence electrons with other carbon atoms or with atoms of other elements. A covalent bond is a bond formed as the result of the mutual sharing of electron pairs between two atoms in a molecule. There are three types of covalent bond, namely Single Covalent Bond, Double Covalent Bond and Triple Covalent Bond.
- Single Covalent Bond is formed when two electrons are shared between two atoms in a molecule. (Example: F2, Cl2, H2, etc)
- Double Covalent Bond is formed when two pairs of electrons are shared between two atoms in a molecule. (Example: O2, CO2, etc)
- Triple Covalent Bond is formed when three pairs of electrons are shared between two atoms in a molecule. (Example: N2)
Allotropes of Carbon
[Click Here for Sample Questions]
The existence of different forms of an element that has different physical properties but the same chemical properties is called allotropes. Carbon has three crystalline allotropes namely Diamond, Graphite and Fullerene. The Amorphous allotropes of carbon are Coal, Coke, Charcoal, Lamp Black and Gas Carbon.
Diamond:
- Diamond has a regular tetrahedral geometry.
- It exists as a three-dimensional network with strong carbon-carbon covalent bonds.
- In a diamond, each carbon atom is connected to four neighbouring carbon atoms through a single covalent bond.
- The density of diamond is 5.5 g/cc
- Diamond has a very high refractive index of 2.5.
- It is a poor conductor of electricity and a good conductor of heat.
- In the presence of light, the diamond shines. It is mainly used in jewellery making.
- The nature of diamond is hard and it has a high melting point.
- Diamond is also used in cutting and drilling tools.
Graphite
- Graphite is formed from weak van der waal forces.
- Each carbon atom is bonded covalently to three other carbon atoms and hexagonal rings are formed in graphite.
- The density of graphite is 2.25 g/cc
- Graphite is a good conductor of both electricity and heat
- It is used as a dry lubricant for machine parts and in lead pencils.
Fullerene
- Fullerene is also known as C60 and it is very stable
- Along with hexagonal rings, it sometimes has pentagonal or heptagonal rings.
Properties of Carbon
[Click Here for Sample Questions]
There are two main properties of carbons namely Catenation and Tetravalent nature.
Catenation:
- Catenation is the self linking property of carbon via covalent bonds in order to form straight-chain, branched-chain and closed rings of different sizes.
- This property is due to the small size of the carbon atom.
Straight Chain: Propane

Branched Chain: 2-Methylpropane

Rings: Cyclohexane

Tetravalent Nature
- This property is because carbon has a valency of 4.
- Carbon has the ability to bond with four other atoms of carbon or some other heteroatoms with a covalent bond.
Example: Chloroethane

Compounds
A combination of two or more elements forms a Compound. Organic Compounds and Inorganic Compounds are the two types of compounds. Carbon and Hydrogen combine together to form the organic compound.
Hydrocarbons
Hydrocarbons are compounds that are formed by the combination of carbon and hydrogen. The two types of hydrocarbons are Saturated Hydrocarbon and Unsaturated Hydrocarbons. Carbon atoms that have a single bond between them are called saturated hydrocarbons. Alkanes are examples of saturated hydrocarbons which are represented by CnH2n+2. The carbon atoms with double or triple bonds between them are called Unsaturated Hydrocarbons. Alkenes are examples of unsaturated hydrocarbons.
Types of Carbon Compounds
[Click Here for Sample Questions]
Based on the structure carbon compounds are of three types namely Straight Chain Compounds, Branched Chain Compounds and Cyclic Compounds.
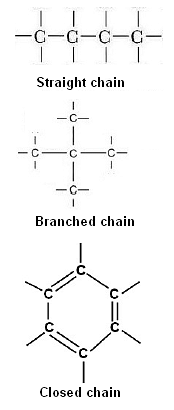
Types of Carbon Compounds
Functional Groups and Nomenclature
[Click Here for Sample Questions]
Functional Groups
An atom or a group of atoms that are present in a compound that determines its physical and chemical properties are called Functional Groups.
The following tabulation gives the functional groups and their formulas.
| Functional Group | Formula of Functional Group |
|---|---|
Halogen Group
| -Cl -Br |
Hydroxyl Group
| -OH |
| Aldehyde Group | -CHO |
| Ketone Group | -C=O |
| Carboxyl Group | -COOH |
Nomenclature
[Click Here for Sample Questions]
There are millions of compounds and it is difficult to remember them by their individual common name. Hence the International Union of Pure and Applied Chemistry (IUPAC) has given certain rules to systemize the nomenclature of organic compounds. The following section gives the rules assigned by IUPAC.
- Identify the Number of Carbon Atoms in a Compound
| S.No | Number of Carbon Atoms | (Suffix) | Single Bond |
|---|---|---|---|
| 1 | One Carbon atom (1-C) | Meth | +ane |
| 2 | Two Carbon Atoms (2-C) | Eth | +ane |
| 3 | Three Carbon Atoms (3-C) | Prop | +ane |
| 4 | Four Carbon Atoms (4-C) | But | +ane |
| 5 | Five Carbon Atoms (5-C) | Pent | +ane |
| 6 | Six Carbon Atoms (6-C) | Hex | +ane |
- Identify the Functional Group
| S.No | Functional Group | Prefix | Suffix |
|---|---|---|---|
| 1 | Double Bond (=) | - | ene |
| 2 | Triple Bond | - | yne |
| 3 | Chlorine (-Cl) | Chloro | - |
| 4 | Bromine (-Br) | Bromo | - |
| 5 | Alcohol (-OH) | - | ol |
| 6 | Aldehyde (-CHO) | - | al |
| 7 | Ketone (-CO-) | - | one |
| 8 | Carboxylic Acid (-COOH) | - | oic acid |
Chemical Properties of Carbon Compounds
[Click Here for Sample Questions]
There are four important properties of carbon compounds namely Combustion, Oxidation, Addition Reaction and Substitution Reaction.
Combustion
Combustion is the scientific word for burning. The complete combustion of carbon compounds in the air produces carbon dioxide water, heat and light.
C(s) + O2(g) → CO2(g) + Heat and light
In the presence of sufficient air or oxygen, saturated hydrocarbons burn with a blue flame.
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) + Heat and light
Oxidation
In the presence of the oxidizing agent, the oxidation of ethanol gives ethanoic acid. An oxidizing agent is a substance that is capable of adding oxygen to others. For example, Alkaline KMnO4
Addition Reaction
In the presence of catalysts like nickel or platinum or palladium, the addition of hydrogen with unsaturated hydrocarbons causes an addition reaction. Catalysts are the substance that causes a reaction to occur or proceed at a faster rate.
CH2 (Ethene) = CH2 + H2 →(Ni catalyst) CH3 – CH3 (Ethane)
Substitution Reaction
Substitution Reaction is the replacement of one or more hydrogen atoms of an organic compound by another atom or group of the atom. Hydrogen atoms are replaced by other elements in Alkanes.
CH4 (g) (Methane) + Cl2 (g) →(sunlight) CH3Cl (Chloromethane) + HCl (g)
Important Carbon Compounds: Ethanol and Ethanoic Acid
[Click Here for Sample Questions]
Ethanol
- Ethanol is an unstable liquid with a low melting point.
- In order to form sodium ethoxide, ethanol reacts with sodium.
- The presence of ethanol can be tested by the evolution of hydrogen gas by using the following reaction.
2Na + CH3CH2OH → 2CH3CH2\(\bar{O}\)Na + (Sodium ethoxide) + H2
- In the presence of hot sulphuric acid, dehydration of ethanol forms alkene.
CH3CH2OH →(Hot conc.)(H2SO4) CH2 = CH2 + H2O
Ethanoic Acid
- Ethanoic Acid is a colourless liquid that is formed at a temperature of about 16.6- degree centigrade.
- If the pure Ethanoic acid is frozen like ice, it is known as Glacial Acetic Acid.
- While reacting with ethanol, ethanoic acid/acetic acid forms ester. It can be found by its sweet smell.
- Soap is formed with the reaction of an ester with a strong base and the process is known as Saponification.
FAQs
Ques: Are Covalent bonds good conductors of electricity? (2 marks)
Ans: No, Covalent Compounds form covalent bonds. Since all the electrons are used to create the covalent bond, they do not give rise to free electrons. So covalent compounds are poor conductors of electricity because there are no ions to move the electrical charge.
Ques: What are the properties of covalent compounds? (2 marks)
Ans: The properties of covalent compounds include low melting and boiling points, low enthalpies of fusion and vaporization and poor electrical and thermal conductivity.
Ques: What is saturated hydrocarbon? (2 marks)
Ans: Saturated hydrocarbons are the simplest class of hydrocarbons. They are hydrocarbons that only contain single bonds between carbon atoms. In saturated hydrocarbons, each carbon atom is bonded to as many hydrogen atoms as possible.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Also Check:




Comments