Gaurav Goplani Content Writer
Content Writer
Versatile nature of carbon can be understood by the fact that it can form single, double, and triple bonds. When connected to other carbon atoms, it can also form chains, branched chains, and rings. The word Carbon is derived from the Latin word “carbo” which means charcoal and is found in various chemical compounds, including those found in space. In this article, we will explore the versatile nature of carbon.
| Table of Content |
Key takeaways: Versatile nature of carbon, tetravalency, catenation, isomerism, carbon atoms, covalent bond.
NCERT Solutions of: Class 10 Science Chapter 4 Carbon and its Compounds
What is the Versatile Nature of Carbon?
[Click Here for Sample Questions]
The existence of an infinite number of carbon compounds in nature is due to the unique nature of carbon atoms. As it possesses a tetravalent as well as catenation property, it can form so many compounds.

Versatile Nature of Carbon
Organic compounds are basically formed by carbon, oxygen, hydrogen, and some other elements. However, due to the existing nature of carbon, it gains the ability to form a huge number of compounds than any other element. The chapter Carbon and its Compound, mentions a discrete topic on the Versatile Nature Of Carbon. Today, we are going to discuss the same topic and see the salient characteristics of the element carbon and why it is so versatile.
Read also: Isomerism in Coordinating Compounds
Explanation: Versatile Nature of Carbon
[Click Here for Sample Questions]
Carbon exists in nature in two different states: free state and compound state. In the free state, it occurs in the form of Diamond, Graphite, and BuckminsterFullerene. These compounds are carbon-only compounds which is why they show similar chemical properties. However, they differ in their physical appearances due to the distinct formation of carbon atoms. They are also known as the allotropes of carbon.
On the other hand, in the compound state, carbon forms bonds with other elements to form new compounds. They may exist in a solid, liquid, or even in a gaseous state as well. This ability of carbon to readily form bonds with other atoms gives rise to the formation of a huge number of organic compounds.
According to a recent estimation, the total number of chemical formulas of carbon known till today is expected to be about 3 million. This is far more than the number of compounds formed by all other elements together.
Read More: Combustion Reaction
Reasons for the Versatile Nature of Carbon
The versatile nature of carbon can be better explained with the salient features of this element. The specific features of a carbon atom are given below:
- Catenation: Carbon atoms show this property according to which they can form bonds by self-linking to other carbon atoms.

Catenation
(i) Compounds formed by the catenation property in carbons are much more stable than others. This leads to the formation of long chains, branched chains, and rings giving rise to a large number of carbon compounds.
(ii) Carbon atoms may form single, double, or triple bonds through self-linking. The compounds formed by single bonds are called saturated compounds; for example methane(CH4). Carbon compounds having double or triple bonds are called unsaturated compounds; for example, alkene (C2H4) has double bonding and ethyl (C2H2) has triple bonds between the carbon atoms
- Tetravalency: Carbon atoms possess a valency of 4. As it cannot lose or gain 4 electrons to fulfill its octet, it shares its valence shell electrons to form covalent bonds with other elements. Carbon forms covalent bonds with hydrogen, oxygen, chlorine, nitrogen, etc forming various compounds.
Tetravalency
- Isomerism: Carbon compounds show isomerism. Isomers are compounds having the same or identical chemical formula but different chemical structures. This effect is mainly seen in the carbon compounds because of the varied arrangements in the carbon atoms. The three isomers of pentane (C5H12) are an example of structural isomerism in carbon compounds.
Conclusion
[Click Here for Sample Questions]
A carbon atom possesses the following properties which make it the most versatile element in the periodic table - Catenation, Tetravalency, and Isomerism. Each of these properties is responsible for why carbon atoms can form such a huge amount of compounds. Compared to the organic compounds, the number of inorganic compounds is much lesser, as they do not form any bonds.
Read More: Tetravalency of Carbon
Things to Remember
- The versatile Nature of Carbon is the topic that is covered in the fourth chapter i.e, Carbon and Its Compounds of CBSE Class 10 Science.
- The entire unit 1 i.e., Chemical Substances- Nature and Behaviour will carry 26 marks in CBSE Class 10 Board Examination.
- Carbon is referred to as a chemical element with the symbol C and atomic number 6, which is a versatile element and is found in many different chemical compounds.
- The versatile nature of carbon can be understood better with its features such as tetravalency and catenation.
- Tetravalency is when Carbon has a valency of four so it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element.
- Catenation can be defined as the property of a carbon element due to which its atom can join one another to form long carbon chains.
Sample Questions
Ques: List and explain in brief the major properties of carbon that are responsible for the formation of a large number of compounds.(2 marks)
Ans: The major properties of a carbon atom that enables it to form numerous compounds are:
Catenation: The property to self-link with other carbon atoms forming long chains.
Tetravalency: Carbon atoms are tetravalent which is why they form bonds with other elements like hydrogen, oxygen, nitrogen, etc to complete its octet.
Ques: Why are carbon compounds so stable?(2 marks)
Ans: One of the major reasons behind the stability of the carbon compounds is the size of the element. Carbon atoms are smaller in size, which is why the nucleus of the atom can hold the shared pair of electrons more firmly resulting in the formation of much stronger bonds between the elements.
Ques: Give an example each for the single, double, and triple bonds in carbon compounds.(4 marks)
Ans: Methane is an example of a single bond carbon compound. The chemical formula of methane is CH4.
Structure of Methane:

Ethane is an example of a double bond carbon compound. The chemical formula of methane is C2H4.
Structure of Ethane:

Propyne is an example of a single bond carbon compound. The chemical formula of propyne is C2H2.
Structure of propyne:

Ques: Mention the structural formula of the three isomers of pentane(3 marks)
Ans: The structural formula of the three isomers of pentane are:

Solved Examples of NCERT
Ques: Write the two properties of carbon that lead to the huge number of carbon compounds we see around us?(2 marks)
Ans: The two properties of carbon due to which we see huge number of carbon compounds are:
(i) tetravalency
(ii) catenation
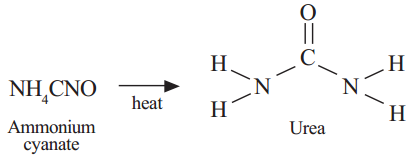
Ques: Which among the following is not a straight chain hydrocarbon?(2 marks)

Ans:
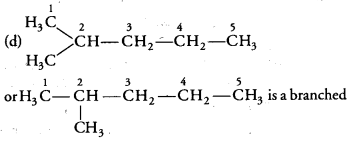
This is not a straight chain hydrocrbon. The rest three are straight chain hydrocarbons.
Ques: Which among the following are unsaturated hydrocarbons:
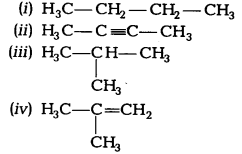
(a) (i) and (iii)
(b) (ii) and (iii)
(c)(ii) and (iv)
(d) (iii) and (iv) (2 marks)
Ans: Unsaturated hydrocarbons possess double or triple bond in the structure. Both (ii) and (iv) structures have triple and double carbon- carbon bonds respectively.
Ques: Draw the structures of the following compounds:
(i) Ehanoic acid
(ii) Bromopentane
(iii) Butanone
(iv) Hexanal(3 marks)
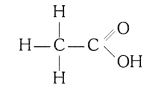
Ans: (i) Ethanoic acid (CH3COOH)
(ii) Bromopentane
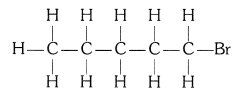
(iii) Butanone
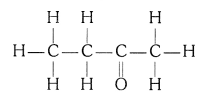
(iv) Hexanal
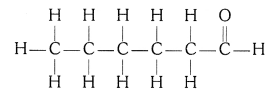
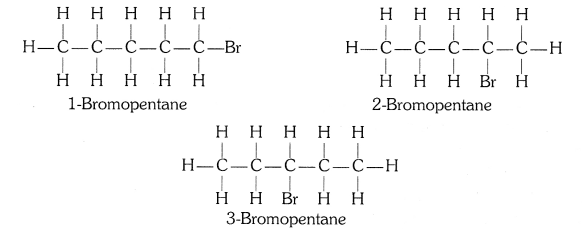
Structural isomers for bromopentane: There are three structural isomers for bromopentane based on the position of Br at carbon, 1 , 2, 3.
Ques: Explain the nature of the covalent bond by using the bond formation in CH3Cl. (2 marks)
Ans: Covalent bond is formed by sharing of electrons in a way that the combining atoms complete their outermost shell.
In CH3Cl, C = 6, H =1 and Cl = 17 and their electronic configuration is C – 2,4, H – 1, and Cl – 2, 8, 7.
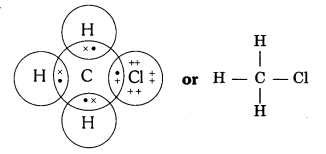
Three hydrogen atoms complete their shells by sharing three electrons of carbon atoms. Chlorine completes its outer shell by sharing its one out of seven electrons with one electron of carbon atom. Therefore, carbon atom shares all its four electrons with three hydrogen atoms and one chlorine atom and completes its outermost shell and single covalent bonds are formed in CH3Cl.
Ques: Draw the electron dot structure of the following:?
(i) ethanoic acid
(ii) propanone
(iii) H2S
(iv) F2 (2 marks)
Ans:
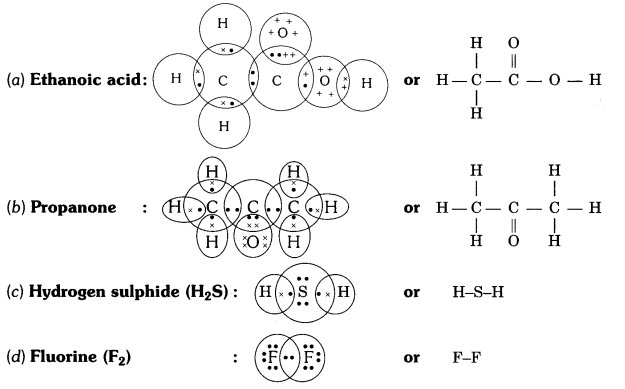










Comments