Collegedunia Team Content Curator
Content Curator
Chemical Equations are common interpretations used in chemistry. Chemical equations are the interpretation of chemical reactions that encompasses numbers and symbols of various compounds and elements. In a chemical reaction, the reactants are on the left side of the chemical equation while the products or results are on the right side. The arrow in the equation represents the direction of the reaction i.e., the reaction can be unidirectional or bidirectional (or reversible).
| Table of Content |
Also check: Difference Between Electrophile and Nucleophile
What is Chemical Equation?
Chemical Equations are worded or symbolic representations of the reaction that will take place when two or more elements will be combined. Chemical Equations need to be balanced in order to satisfy all the conditions including the law of conservation of matter.
Also read: Precipitation Titration
The number of elements on the left side of an arrow is the reactant elements and elements on the right side are product (or resultants). A balanced equation happens when the number of atoms of an element on the reactant side is equal to the number of atoms of elements on the product side.
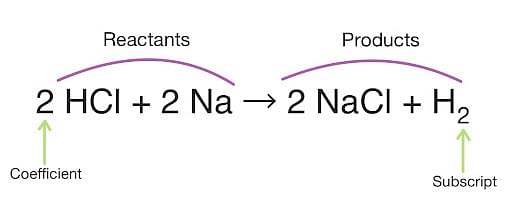
What are Chemical Equations?
An example of a chemical reaction is:
An aqueous solution of sodium chloride (NaCl[aq]) and another aqueous solution of silver nitrate (AgNO3[aq]) are mixed together forming sodium nitrate (NaNO3[aq]) and silver chloride (AgCl[s]).
The symbolic representation will be:
NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
Also check:
Methods of Balancing Chemical Equations
There are two methods of balancing a chemical equation. One is by balancing by inspection and the other is balancing by Numerical method. Balancing by inspection is one of the basic and simple methods to balance the chemical equation. This method is used for simpler problems. In the Balancing by Numerical method a reaction is balanced and the coefficients can be seen. Hence, the Balancing by Numerical method is mostly used as it describes if the equation is balanced or not. Balancing by inspection does not represent if the equation is balanced or not.
Read More:
Direction of Chemical Reaction
Every chemical equation can be unique and can be housed with different elements giving different results. The product obtained can be by different reactions. There are four different representations of chemical equations that are used in chemical equations.
- The symbol ‘→’ represents net forward reaction.
- \(\rightleftharpoons\)’ is used to represent the chemical equilibrium state.
- ‘=’ symbol is used to denote stoichiometric relationships
- ‘⇔’ represents reactions occurring in both forward and backward reactions.
Different entities in a chemical reaction are separated by the ‘+’ symbol and it should be noted that ‘→’ is read as ‘gives rise to’ or ‘yields to’.
Read More:
Representation of Reacting Entities
Physical State Representation of Reacting Entities
All the elements are of different forms of matter i.e., Solid, liquid, and gas. The elements are represented as:
- ‘(s)’ represents Solid State.
- ‘(l)’ represents Liquid State.
- ‘(g)’ represents the Gaseous State.
- ‘(aq)’ represents an Aqueous solution.
- ‘↓’ symbol describes the elements as precipitates.
Read More:
Chemical Representations of Reacting Entities
Some chemical reactions require energy from an external source or yield energy during the reaction. The chemical representation of the energies are as follows:
- ‘Δ’ represents the requirement of heat energy during the chemical reaction.
- ‘Hv’ represents the photon energy (or light energy) to yield a result.
These coefficients are assigned to obey the law of conservation of charge and the law of conservation of mass.
Read More:
Ionic Equation
When electrolytes are written as dissociated ions the equation so formed is called the Ionic equation. Ionic equations are used to represent single and double displacement reactions that occur in an aqueous solution.
Read More:
Enthalpy FormulaExample
The ionic representation of CaCl2 + 2AgNO3 → Ca(NO3)2 + 2AgCl↓ is
Ca2+ + 2Cl– + 2Ag+ + 2NO3– → Ca2+ + 2NO3– + 2AgCl↓.
From the above ionic representation, it can observe that Ca2+ and NO3- are present as separate entities on both sides of the reaction. The elements that are present on both sides of the reaction are the elements that do not take part in the chemical reaction and are called spectator ions. hence the above ionic equation without the spectator ions can be written as ‘2Cl– + 2Ag+ → 2AgCl↓’ or ‘Ag+ + Cl- →AgCl’.
Also Read:
Things to Remember
- Chemical Equations are worded or symbolic representations of the reaction that will take place when two or more elements will be combined.
- There are two methods to balance a chemical equation. These are Balancing by Inspection and Balancing by Numerical method.
- When electrolytes are written as dissociated ions the equation so formed is called the Ionic equation.
Read More:
Sample Questions
Ques. What is a Chemical Equation? (1 Mark)
Ans. Chemical Equation is a symbolic representation of the reaction that will occur when two or more than two elements will be combined. Chemical Equations need to be balanced in order to satisfy all the conditions including the law of conservation of matter.
Ques. What is the difference between a chemical reaction and a chemical equation? (1 Mark)
Ans. Chemical reactions are the experimentation reactions that reactants will become after the reaction. Chemical Equations are the symbolic representation of the chemical reaction.
Read More:
Ques. What is Ionic Equation? (1 Mark)
Ans. When electrolytes are written as dissociated ions the equation so formed is called the Ionic equation.
Ques. What is the chemical equation for photosynthesis? (1 Mark)
Ans. The chemical equation of Photosynthesis is:
6CO2 + 6H2O → C6H12O6 + 6O2.
Read More:
Ques. What is the position of reactants and products? (1 Mark)
Ans. The reactants are on the left side of the chemical equation while the product is on the right side of the chemical equation.
Ques. What are the chemical representations used while writing a chemical equation? (2 Marks)
Ans. The chemical representation that can be used during the chemical equation is:
- ‘Δ’ represents the requirement of heat energy during the chemical reaction.
- ‘Hv’ represents the photon energy (or light energy) to yield a result.
Read More:
Ques. What is the physical representation used during the chemical equation? (2 Marks)
Ans. Physical Representations used during the chemical reactions are:
- ‘(s)’ represents Solid State.
- ‘(l)’ represents Liquid State.
- ‘(g)’ represents Gaseous State.
- ‘(aq)’ represents an Aqueous solution.
- ‘↓’ symbol describes the elements as precipitates.
Ques. What are various symbols used while writing a chemical equation? (2 Marks)
Ans. Various symbols used during the chemical equation writing are:
- the symbol ‘→’ represents net forward reaction.
- \(\rightleftharpoons\)’ is used to represent the chemical equilibrium state.
- ‘=’ symbol is used to denote stoichiometric relationships
- ‘⇔’ represents reactions occurring in both forward and backward reactions.
Read More: Collision Theory
Ques. What are various methods of balancing a chemical equation? (3 Marks)
Ans. There are two methods of balancing a chemical equation. One is by balancing by inspection and the other is balancing by Numerical method. Balancing by inspection is one of the basic and simple methods to balance the chemical equation.
This method is used for simpler problems. In the Balancing by Numerical method a reaction is balanced and the coefficients can be seen. Hence, the Balancing by Numerical method is mostly used as it describes if the equation is balanced or not. Balancing by inspection does not represent if the equation is balanced or not.
Ques. Give some examples of balanced chemical equations. (3 Marks)
Ans. Following are some of the examples of a chemical equation:
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
- CaO(s) + H2O (l) → Ca(OH)2(s)
- CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O (l)
- CaCO3(s) → CaO(s) + CO2(g)
- Fe2+(aq) + 2 OH−(aq) → Fe(OH)2(s)
- Al3+(aq) + PO43−(aq) → AlPO4(s)
Also Read:



Comments