Jasmine Grover Study Abroad Expert
Study Abroad Expert
The valency of Carbon is four and hence one atom of Carbon can form 4 covalent bonds. Valency can be understood as the measure of the combining power of the element when it forms other chemical molecules or compounds. Thus, tetravalency of Carbon refers to its ability to bond with four other atoms of Carbon of other elements. Carbon is versatile in nature and is capable of forming covalent bonds with itself and other elements such as hydrogen, sulphur, oxygen, chlorine, and nitrogen. Carbon also forms compounds containing double and triple bonds between many carbon atoms. These chains of carbon might be straight chains, branched chains, or in the form of rings.
| Table of Content |
Key Terms: Valency of Carbon, atoms, catenation, compounds, valency, covalent bonds, electronic configuration, tetravalency of carbon
What is the Valency of Carbon?
[Click Here for Sample Questions]
The valency of carbon is 4. The ability of carbon to bond with the other four atoms of carbon or atoms of other monovalent elements is known as the Tetravalency of Carbon. The word “Tetra” means “Four” and the word “Valency” means “Combining capacity”. When carbon is said to be Tetravalent, it means that the valency of carbon is 4 and it can form 4 covalent bonds with other atoms. The ability of carbon to form bonds of covalent nature with other carbon atoms is known as catenation. With Carbon’s Tetravalency and Catenation property, it is able to form a large number of compounds. The formations of covalent bonds by Carbon are explained in terms of its electronic configuration and hybridization. Carbon is an important element due to its following properties:
- Catenation
- Tetravalency
- Carbon atom size

Why the valency of carbon is 4?
[Click Here for Sample Questions]
The valency of carbon is 4 as it has 4 electrons in its outermost shell and therefore needs 4 more electrons to complete its octet configuration.
The atomic number of Carbon = 6
Electronic configuration of Carbon = 2, 4 = 1s2 2s2 2p2 = [He] 2s2 2p2
The outermost cells have 4 electrons which means that the carbon atom can neither lose 4 electrons nor it can gain 4 electrons because it requires a large amount of energy. So, the atoms of carbon share their 4 electrons with other atoms. Since the electron is being shared and the number of electrons that is being shared is 4. Hence, the valency of the Carbon is = 4, therefore “Carbon is Tetravalent”.
Carbon can neither give nor gain any electrons, it can only share them. The tetravalent nature (4 valency of carbon) affects many compounds it forms.

Electronic Configuration and Valency of Carbon
[Click Here for Sample Questions]
The valency of carbon can be explained by understanding the Electronic Configuration of Carbon. The electronic configurations of carbon are of two types:
- Carbon at ground state: A ground-state atom is an atom in which the total energy of electrons cannot be lowered by transferring one or more electrons to different orbitals. All electrons are in the lowest possible energy levels in the ground state carbon. Although carbon has 4 electrons, it can make only 2 bonds since it has only 2 unpaired electrons in ground state carbon.
- Carbon at excited state: A carbon is said to be in an excited state when the total energy of electrons can be lowered by transferring an electron from the 2P orbital to the 2S orbital. In the excited state of carbon, there are 4 unpaired electrons so it can make bonds with 4 other atoms.

Let us take the example of methane (CH4) to understand this:
- We know that the Ground-state electronic configuration of carbon is 1s2, 2s2, 2p2 and it has 4 valence electrons (the valency of carbon is four), so it can form 4 bonds at maximum.
- But in the ground state, the carbon has only 2 unpaired electrons which means that it can only form 2 bonds.
- Whereas in the excited state the carbon has 4 unpaired electrons which means that it can form 4 bonds.
- However, if the carbon uses these 2s and 2p orbitals to form a formation, the shape will not be tetrahedral because the bond formed will be shorter since the 2s orbital will be more stable and closer but 2p will be longer as it will be less stable and further away from the nucleus.
So, this means that there will be 1 shorter and 3 longer bonds in Methane.
Hybridization refers to combining atomic orbitals in order to form new hybrid orbitals that are appropriate to represent their properties of bonding. And since the carbon in methane (CH4) has 4 sigma bonds so it will mix 4 of its valence orbitals to form 4 identical orbitals. When carbon is bonded to four other atoms the hybridization is sp3 and the arrangement is tetrahedral. This hybridized orbital will repel each other equally and give sp3 hybridized carbon in methane (CH4) its linear shape.

Hybridization of Carbon
[Click Here for Sample Questions]
Hybridization refers to the concept of combining atomic orbitals in order to form new hybrid orbitals that are appropriate to represent their bonding properties. Hybridization influences the bond length and bond strength in organic compounds.
- When carbon, due to its tetravalency, is bonded to four other atoms, it has sp3 hybridization and tetrahedral arrangement
- When the carbon atom is bound to three other atoms, the hybridization is sp2.
- When the carbon atom is bound to two other atoms the hybridization is known to be sp.
The shape of molecules like methane (CH4), ethane (C2H4), ethyne (C2H2) is explained in terms of the use of sp3, sp2, and sp hybrid orbitals by carbon atoms.
Let us understand it through an example of Ethyne (C2H2):
- Carbon in ethyne (C2H2) forms 2 sigma bonds and 2 pi bonds.
- Since carbon forms 2 sigma bonds it will mix 2 of its valence orbitals to form 2 identical orbitals.
- When the carbon atom is bound to two other atoms with the same shape and energy the hybridization is known to be sp hybridized orbitals.
- This hybridized orbital will repel each other equally and give sp hybridized carbon in ethyne (C2H2) its linear shape.

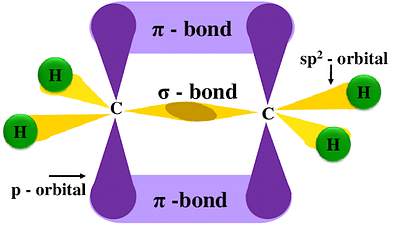
Sp2 hybridization
[Click Here for Sample Questions]
When three equivalent orbitals are formed by mixing one s and two p orbitals of an atom’s same shell, it is known to be sp2 hybridized. In this, the sp2 hybridized orbitals form a flat triangular arrangement with a 120° angle between bonds.
An example of sp2 hybridization is Ethene (C2H4).
- A single carbon atom in Ethene (C2H4) forms 3 sigma bonds and 1 pi bond.
- Since the carbon has 3 sigma bonds, so it will mix 2 of its valence orbitals to form 3 identical orbitals.
- When the carbon atom is bound to three atoms the hybridization is known to be sp2 hybridized orbitals.
- This hybridized orbital will repel each other equally and give sp hybridized carbon in ethene (C2H4) its linear shape and will form a flat triangular arrangement with a 120° angle between bonds.
Things to Remember
[Click Here for Sample Questions]
- The ability of carbon to bond with the other four atoms of carbon or atoms of other monovalent elements is known as the Tetravalency of Carbon.
- The valency of carbon is 4.
- Carbon’s ability to form bonds of covalent nature with other carbon atoms is known as catenation.
- The outermost cells have 4 electrons which means that they can neither lose 4 nor gain 4 electrons because it requires a large amount of energy. Hence, they form compounds by sharing the electrons.
- Hybridization refers to the concept of combining atomic orbitals in order to form new hybrid orbitals that are appropriate to represent their bonding properties.
Sample Questions
Ques. What is the valency of Carbon? (1 mark)
a) One
b) Two
c) Three
d) Four
Answer: (d) Four
Explanation: Carbon has a valency of 4 and is known as tetravalent. This is because it has 4 electrons in its outermost shell and needs 4 more electrons to attain a noble gas configuration. Hence, the valency of carbon is 4.
Ques. What is the hybridization of CH3CH2CH2CN? (1 mark)
a) sp, sp3
b) sp2, sp3
c) sp
d) sp, sp2
Ques. How does tetravalency help carbon? (2 marks)
Ans. Carbon’s tetravalency permits it to generate valuable and useful compounds like diamond and graphite. It may make bonds with four additional atoms because of its valency of four. Carbon may create compounds with oxygen, chlorine, hydrogen, sulphur, and various other elements due to this property.
Ques. Explain the Tetravalency of carbon? (2 marks)
Ans. The word “Tetra” means “Four” and the word “Valency” means “Combining capacity”. When carbon is said to be Tetravalent it means that the valency of carbon is 4 and it can form 4 covalent bonds with other atoms.
Ques. What do you mean by the versatile nature of carbon? (2 marks)
Ans. Carbon is Versatile in Nature. It forms bonds of covalent nature with itself and other elements such as hydrogen, chlorine, oxygen, sulphur, and nitrogen. Carbon also forms compounds containing double and triple bonds between many carbon atoms.
Ques. Does Carbon lose 4 electrons to form compounds? (2 marks)
Ans. No, Carbon can neither lose 4 nor gain 4 because it requires a large amount of energy to do so. Hence, the atoms of carbon can share their 4 electrons with each other.
Ques. How is tetravalency of carbon responsible for its versatile nature? (2 marks)
Ans. Carbon has an atomic number of 6. Two electrons are present in the completely filled inner orbital, while four electrons are in the outermost shell. Therefore, carbon has a valency of four. Consequently, it is capable of bonding four other atoms of carbon or atoms of some other mono-valent elements.
Ques. Write the state of hybridization of carbon in the following compounds and shapes of each of the molecules. (3 marks)
(a) H2C=O,
(b) CH3F,
(c) HC≡N.
Ans. (a) sp2 hybridized carbon, trigonal planar;
(b) sp3 hybridized carbon, tetrahedral;
(c) sp hybridized carbon, linear.
Ques. What is the type of hybridization of each carbon in the following compounds? (5 marks)
(a) CH3Cl,
(b) (CH3) 2CO,
(c) CH3CN,
(d) HCONH2,
(e) CH3CH=CHCN
Ans. (a) sp3,
(b) sp3, sp2,
(c) sp3, sp,
(d) sp2
(e) sp3, sp2, sp2, sp
Read More:
Chemistry Study Guides:



Comments