Collegedunia Team Content Curator
Content Curator
Sodium metabisulfite is also called Sodium pyrosulfite. It is an inorganic compound having the chemical formula Na2S205. It is an antioxidant and a pharmaceutical chemical. The substance is also referred to as disodium. It is used as a disinfectant and a preservative agent. Another synonym for it is sodium disulfite, and it is an inorganic salt composed of sodium and disulfite ions in a 2:1 ratio.
| Table of Content |
Preparation of Sodium Metabisulfite
Sodium bisulfite is prepared by treating sodium hydroxide with sulfur dioxide. When mixed with warm water, Na2SO3 precipitates and turns yellow solid. This solid then dissolves to give disulfite, which then crystallizes on cooling.
SO2 + 2NaOH → Na2SO3 + H2O
SO2 + Na2SO3 → Na2S2O5
Hence yield the colorless solid residue Na2S2O5.
| Also, read: | |
|---|---|
Structure of Sodium Metabisulfite
Sodium metabisulfite is made up of two sodium ions and one metabisulfite ion. Na+ helps to balance the charge in the structure. The metabisulfite ion is directly connected to the sulfur atoms. The first sulfur atom is bonded to three oxygen atoms and has an oxidation state of +5. The second sulfur atom is bonded to two oxygen atoms and has an oxidation state of +3.
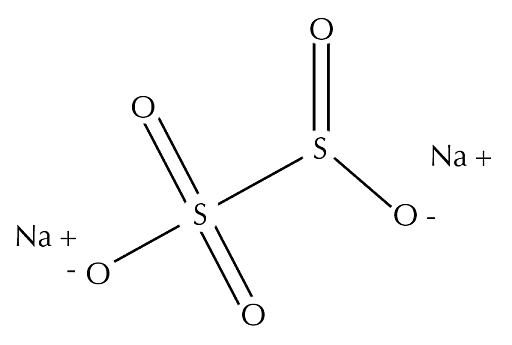
Structure of Sodium Metabisulfite
| Also, read: | |
|---|---|
Physical Properties of Sodium Metabisulfite
- Sodium metabisulfite is white in color and is in powdered form.
- It has a faintly pungent odor.
- It can also cause allergic reactions to humans who are hypersensitive to sulfites.
- It solubilizes easily in water and glycerol, having a solubility of 65.3g/100ml at 20° temperature.
- It does not solubilize easily with ethanol.

Appearance of Sodium Metabisulfite
| Also, read: | |
|---|---|
Chemical Properties of Sodium Metabisulfite
- The anion is a hybrid of dithionite and dithionate.
- The anion consists of a SO2 linked to a SO3 group, having a negative charge which is more localized on the SO3.
- The S-S bond length is 2.22 Å.
- For thionate and dithionite, the S-O distance is 1.46 and 1.50 Å respectively.
- When sodium metabisulfite is introduced to water, it liberates sulfur dioxide gas, which has an unpleasant, pungent smell.
- When heat is introduced to sodium metabisulfite, sodium sulfite and sulfur dioxide are formed by decomposition.
- When strong acids are exposed to sodium metabisulfite, it produces SO2 gas.
| Also, read: | |
|---|---|
Important Facts
- Sodium metabisulfite has a molecular mass of 190.107g/mol.
- It has a melting point of 180°C.
- It decomposes at 150°C.
- Sodium contains a positive charge and metabisulfite has a negative charge.
- Due to the presence of S atoms, it is called Metabisulfite. The direct bond between two S atoms refers to meta.
- Naturally, it is non-combustible.
- Because sodium metabisulfite is an antioxidant, it is widely used in the production of makeup and skincare products.
- Too much exposure to this compound in any form can cause irritation to the eyes, skin, throat, and mucous membrane.
- The acceptable daily intake of sodium metabisulfite is up to 0.7g per kg of body weight.
| Also, read: | |
|---|---|
Uses of Sodium Metabisulfite
- Most commonly sodium metabisulfite is used as a preservative for baking. It has reducing agent properties which help the dough in baking get soft.
- Sodium metabisulfite and potassium metabisulfite are the primary ingredients in Campden tablets used for wine and beer production.
- It is used in medical tests for sickle cell anemia.
- Wastewater treatment projects use it for its reducing agent properties.
- It is used for corrosion inhibition in industries.
- And nowadays, due to pandemics and the large production of sanitizers, sodium metabisulfite is the disinfectant consumed in pharmaceuticals.
| Also, read: | |
|---|---|
Things to Remember
- It solubilizes easily in water and glycerol, having a solubility of 65.3g/100ml at 20° temperature.
- For thionate and dithionite, the S-O distance is 1.46 and 1.50 Å respectively.
- The S-S bond length is 2.22 Å.
- Sodium metabisulfite has a molecular mass of 190.107g/mol.
- It has a melting point of 180°C, and It decomposes at 150°C.
- Because sodium metabisulfite is an antioxidant, it is widely used in the production of makeup and skincare products.
| Also, read: | |
|---|---|
Sample Questions
Ques: Is sodium metabisulfite hazardous for humans?
Ans: Yes, it is hazardous for humans. Ingestion of pure sodium metabisulfite can lead to gastrointestinal damage by releasing sulfurous acid. Inhalation of the substance causes shortness of breath and coughing. It is also a skin and eye irritant. Decomposition of sodium metabisulfite releases toxic gas which causes major medical issues like asthma.
Ques: Is sodium metabisulfite soluble in water? And what does this sodium metabisulfite react with? (2 Marks)
Ans: Sodium metabisulfite solubilizes easily in water and glycerol, having a solubility of 65.3g/100ml at 20° temperature. It is well known that this substance reacts with acids and water releasing toxic sulfur dioxide gas. The SO2 gas reacts with respiratory tissues forming sulfurous acid which causes hypoxemia.
Ques: Explain the structure of sodium metabisulfite. (2 Marks)
Ans: Sodium metabisulfite is an inorganic compound having the chemical formula Na2S205. It is made up of two sodium ions and one metabisulfite ion. Na+ helps to balance the charge in the structure. The metabisulfite ion is directly connected to the sulfur atoms. The first sulfur atom is bonded to three oxygen atoms and has an oxidative state of +5. The second sulfur atom is bonded to two oxygen atoms and has an oxidation state of +3.

Ques: How is sodium metabisulfite prepared? (2 Marks)
Ans: Sodium bisulfite is prepared by treating sodium hydroxide with sulfur dioxide. When mixed with warm water, Na2SO3 precipitates and turns yellow solid. This solid then dissolves to give disulfite, which then crystallizes on cooling.
SO2 + 2NaOH → Na2SO3 + H2O
SO2 + Na2SO3 → Na2S2O5
Hence yield the colorless solid residue Na2S2O5.
Ques: Give the uses of the compound Sodium metabisulfite. (3 Marks)
Ans: Sodium metabisulfite is most commonly used as a preservative for baking. It has reducing agent properties which help the dough in baking get soft. Sodium metabisulfite along with potassium metabisulfite are the primary ingredients in Campden tablets used for wine and beer production.
It is also used in medical tests for sickle cell anemia. Wastewater treatment projects use it for its reducing agent properties. It is used for corrosion inhibition in industries. And nowadays, due to pandemics and the large production of sanitizers, sodium metabisulfite is the disinfectant consumed in pharmaceuticals.
Ques: Write a note on sodium metabisulfite. (4 Marks)
Ans: Sodium metabisulfite is also called Sodium pyrosulfite. It is an inorganic compound having the chemical formula Na2S205. It is an antioxidant and a pharmaceutical chemical. The substance is also referred to as disodium. It is used as a disinfectant and a preservative agent. Another synonym for it is sodium disulfite, and it is an inorganic salt composed of sodium and disulfite ions in a 2:1 ratio.
The substance is noncombustible but can emit toxic oxide fumes of sodium and sulfur. It is a white crystalline or powdered solid, with a slightly pungent odor. It can also cause allergic reactions to humans who are hypersensitive to sulfites. It solubilizes easily in water and glycerol, having a solubility of 65.3g/100ml at 20° temperature.
It does not solubilize easily with ethanol. Most commonly sodium metabisulfite is used as a preservative for baking. It has reducing agent properties which help the dough in baking get soft. Sodium metabisulfite and potassium metabisulfite are the primary ingredients in Campden tablets used for wine and beer production.
Ques: Discuss the chemical properties of sodium metabisulfite. (4 Marks)
Ans: The anion is a hybrid of dithionite and dithionate. It consists of a SO2 linked to a SO2 group, having a negative charge which is more localized on the SO2. The S-S bond length is 2.22 Å. For thionate and dithionite, the S-O distance is 1.46 and 1.50 Å respectively.
When sodium metabisulfite is introduced to water, it liberates sulfur dioxide gas, which has an unpleasant, pungent smell. When heat is introduced to sodium metabisulfite, sodium sulfite and sulfur dioxide are formed by decomposition. When strong acids are exposed to sodium metabisulfite, it produces SO2 gas. It is inorganic in nature and toxic if inhaled.
The anion is a hybrid of dithionite and dithionate. The anion consists of a SO2 linked to a SO2 group, having a negative charge which is more localized on the SO2. The S-S bond length is 2.22 Å. For thionate and dithionite, the S-O distance is 1.46 and 1.50 Å respectively.



Comments