Jasmine Grover Study Abroad Expert
Study Abroad Expert
Micelles are aggregates that are formed in an aqueous solution by molecules containing both polar and nonpolar regions. A micelle has two ends and is almost spherical in shape. Ellipsoid, cylinder or bilayer are some other phases of a micelle.
| Table of Content |
Keyterms: Micelles, Aqueous solution, Molecules, Ellipsoid, Cylinder, Bilayer, Hydrophilic End, Hydrophobic End, Amphiphilic
Read more: Tranquilizers
Micelle Structure & Formation
[Click Here for Sample Questions]
The structure of a micelle has two parts: Hydrophilic End & Hydrophobic End. In simple terms, it can be said a head and a tail. The molecule or ion that has this type of structure is called amphiphilic.
Micelles are formed in water at a particular temperature known as Kraft temperature (Tk) and above critical micelle concentration (CMC).
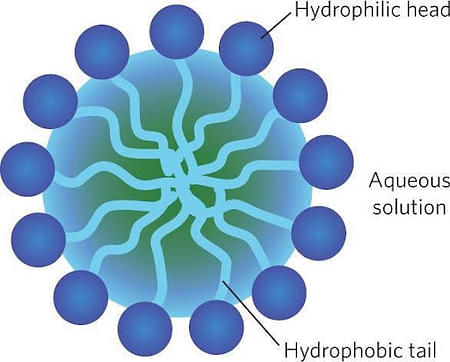
Micelle Structure & Formation
Let’s understand few terms:
- Hydrophilic end: ‘Hydro’ denotes water and ‘philic’ denotes fondness. The head, which is the polar or ionic part of the micelle, is affined to water. This forms the outer structure of the micelle.
- Hydrophobic end: ‘Hydro’ denotes water and ‘phobic’ denotes fear. The tail, which is the nonpolar end of the micelle, is water repulsive. It is not soluble in water and is attracted to dirt.
When an amphiphilic molecule arranges itself in an aqueous solution, in a form that hydrophilic part remains outside and hydrophobic part forms the core, then a micelle is formed.
- Surfactant: This comes from two words- surface & active agent. It has the characteristic to decrease the surface tension between two liquids, gas & liquid or liquid & solid. Any detergent or foaming agent can be an example of surfactants.
- Critical Micelle Concentration: The concentration above which the surfactant forms micelles is known as critical micelle concentration. It is important because the surface tension changes dynamically below CMC.
Read More: Chemical Bonding
Micelle Cleansing action of Soaps & Detergents
[Click Here for Sample Questions]
To understand the cleansing action, let us say that oil and water are mixed in a container and allowed to settle. Oil being a less dense substance will float over water. If you shake them vigorously there will be a temporary layer of emulsion. Now if we add soap or detergent, which acts as a surfactant, it makes the emulsion stable. This process is known as emulsification and is used to remove dirt.
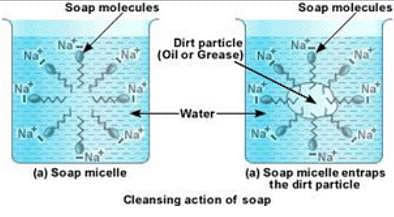
We know that we cannot clean a dirty cloth only by using water. That is because water cannot dissolve oil or dirt. Hence we require a surfactant, like soap or detergent. The hydrophilic head of soap is attracted by water and the hydrophobic end is attracted to non-polar particles like dirt. This forms a micelle.
The outer surface of the micelle is charged. All the micelles formed in the solution have similar charges and they repel each other and are suspended in the water. When agitated, they remain suspended and you get squeaky clean clothes.
Also Read:
| Related Articles | ||
|---|---|---|
| Decomposition Reaction | Classification | Optical Isomerism |
Things to Remember
- Micelles are structures formed in an aqueous solution due to polar and nonpolar regions of the molecules present in the solution.
- Micelles have hydrophobic tails and hydrophilic heads.
- Formation of micelle due to a surfactant is the reason how soap cleansing works.
Important Notes On Redox Titration
Sample Questions
Ques 1. What are micelles? (2 marks)
Ans. Micelles are associated colloids or aggregated colloids which behave as electrolytes at lower concentrations and form colloids at higher concentrations. It is formed at a particular temperature known as Kraft Temperature and above a concentration which is known as critical micelle concentration (CMC). Micelle has hydrophobic and hydrophilic ends as shown in the picture.

Micelle Structure & Formation
Ques 2. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents like ethanol also? (3 marks)
Ans. When soap is added to water, a micelle is formed because of the hydrophobic tails and hydrophilic heads and hydrophobic tails. Tails are sequestered on the surface of dirt particles while heads form the outer surface.
No. Micelle can never be formed in organic solvents like ethanol because soap has a hydrocarbon chain that dissolves in ethanol. The hydrophilic head of soap is attracted by water and the hydrophobic end is attracted to non-polar particles like dirt.
The outer surface of the micelle is charged. All the micelles formed in the solution have similar charges and they repel each other and are suspended in the water. When agitated, they remain suspended and you get squeaky clean clothes.
Ques 3. Comment on the statement that “colloid is not a substance but a state of substance”.
OR Explain the sentence: The same substance can act both as colloids & crystalloids. (1 mark)
Ans. A substance can act as a colloidal substance under certain circumstances. For instance, sodium chloride is a true solution in water but is a colloid in benzene. Similarly, soap is a colloid in an aqueous solution but a crystalloid in alcohol. This clearly indicates that colloidal is a state and does not define a class of substance.
Ques 4. Describe (i) Oil dispersed in water (O/W type) (ii) Water dispersed in oil (W/O type) (1 mark)
Ans.
- Oil dispersed in water (O/W): The dispersion medium is water, and the dispersed phase is oil in this case. Milk and disappearing cream, for example. The liquid fat is distributed in water in milk.
- Water dispersed in oil (W/O): The dispersion medium is oil, and the dispersed phase is water in this case. Butter and cream are two examples.
Ques 5. Define Kraft temperature (1 mark)
Ans. Micelles form only at a certain temperature, known as the Kraft temperature (Tk), and at a certain concentration, known as the crucial micelle concentration (CMC).
Ques 6. Explain the mechanism of cleansing action of soaps. (2 marks)
Ans. Soaps are surfactants, basically, sodium salts, which are used to remove dirt and oil. The structure has a polar hydrophilic head and a nonpolar hydrophobic tail. If we have to clean a dirty cloth then putting it in just water doesn’t help because dirt is insoluble in water. Hence, we need soap.
The hydrophobic end of the soap attaches itself to the dirt and the hydrophilic end forms the outer surface. All the micelles present in the solution repel each other. When the whole solution is agitated you get the cleaned cloth free from dirt. This happens because dirt is trapped in the micelle and a colloidal solution is formed.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:



Comments