Jasmine Grover Study Abroad Expert
Study Abroad Expert
Copper is material used by us on a daily basis whether directly in the form of wiring and motors, or indirectly in alloys. There are so many things around us that are made of copper and copper based alloys. Copper is all around us, whether you turn on a tap or use any appliances in your home, it’s copper which helps in delivering water and electricity to us. As it is a good conductor of electricity, it is mainly used in generators and motors for the purpose of wiring and also are used in the electric goods. Copper is also used in water pipes as it does not corrode easily and also because of its high malleability, the copper pipes can be bent and given shape easily, without breaking. The element copper, denoted by Cu, is an important metal in our life. In this article, we will look at the chemical formula of copper, its properties and oxidation state.
| Table of Content |
What is copper?
Copper is a chemical element, which is denoted by symbol Cu (derived from Latin word cuprum) and has an atomic number 29. It is present in the Group 11) of the periodic table. Copper is a very good conductor of electricity as well as heat and is soft, malleable and ductile in nature. Copper is reddish-gold in color but freshly exposed, pure copper is pinkish-orange. If we talk about its usability, copper is one of the few metals which is directly usable in natural metallic form.

Copper
- It’s the first metal that was smelted in 5000 BC from the ores of sulfide.
- Copper is the first metal which was put in a shape with the help of a mold in 4000 BC.
- It is also the very first metal which was alloyed with another metal, tin, to produce bronze (3500 BC).
Read More: Thermal Conductivity of Copper
Oxidation state of Copper
Oxidation state refers to the number allotted to an element in a compound representing the number of electrons gained or lost by an atom.
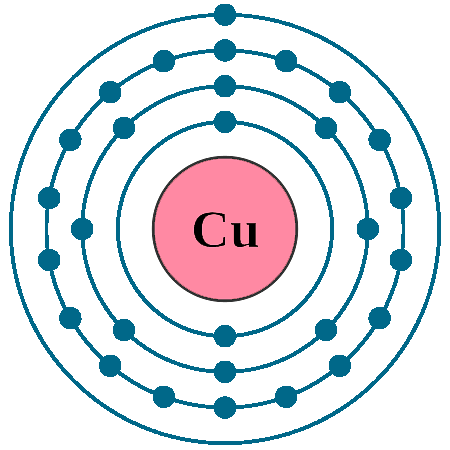
Atomic Structure of Copper
The electron configuration of copper is [Ar] 3d10 4s1. In order to attain noble gas configuration, it takes copper to lose one electron. The energy required to lose one more electron is very less, so copper loses 2 electrons and forms Cu2+ ion, hence copper displays +1 and +2 oxidation states. Oxidation state +1 and +2 are often called cuprous and cupric, respectively.
Copper also forms coordination complexes with ligands. The simplest compounds of copper are binary compounds which are those that only contain two elements. Examples are oxides, sulfides and halides.
Also Read: Electrophilic Aromatic Substitution
Chemical formula of copper
- Copper has high thermal and electrical conductivity and has malleable and ductile properties.
- As copper is an element it doesn't have any chemical formula, though it is represented by the symbol Cu.
- When it combines with other elements or radicals, then it could be represented by formulas like CuSO4.
Check out:
Production and Extraction
Copper is extracted from copper sulfides that contain only 0.5 - 1.0% of copper, from the large pits of the porphyry copper reserves sites. Most of these sites and mines are situated in Chili, Utah in the United States and Mexico.
In order to extract copper from the nodules, methods like sulfuric leaching, smelting are used which is then followed by the cuprion process. In order to remove much of the iron in the ore flash, melting of the material with silica is done to remove iron as slag. The iron sulfides are now turned into oxides that react with silica to form silicate slag that floats up to the surface of the heated material.

Extraction of Copper from copper pyrites
What is left is copper matte in the form of copper sulfide. The compound name is Cu2S. This compound is then roasted to convert the sulfides into copper (I) oxide which is another copper oxide formula with +1 oxidation stage that is unstable in nature and readily decomposes on heating to pure copper.
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu2O → 4Cu + O2
There is another process which requires reduction of CuO, in the laboratory. This process is also called hydrolysis of CuO as, in this reaction, copper oxide gets reduced in presence of hydrogen where it loses its oxygen that gets attached to hydrogen to form water.
CuO + H2 → Cu + H2O
In another process, copper sulfate is dropped in a container which is known as autoclave and then it is pressurized with the hydrogen gas at 25 bar. Then the copper sulfate solution (CuSO4 compound) is heated at around 150 degree Celsius for an hour that results in the formation of copper powder.
CuSO4 (aq) + H2 (g) → Cu(s) + H2SO4 (aq)
Read More:
Characteristics of copper
The physical and chemical properties of copper are as given below:
Physical Properties
- Gold, copper, and silver all fall in group 11 of the periodic table. These metals also have one s-orbital electron on top of a d-electron shell that is filled. All these three metals have high ductile characteristics. Also there is a little contribution of the filled d-shells to the interatomic interactions.
- Copper is a soft metal, thus it has high thermal and electrical conductivity. Copper has 2nd highest electrical and thermal conductivity at room temperature among pure metals.
- Pure copper naturally has an orange-red color. But after exposure to air, it acquires reddish tarnish. The reason behind its color is the electronic transition of the filled 3d atomic shell and half empty 4s atomic shell.
Chemical Properties
- Copper forms a brown-black copper oxide layer when it reacts with atmospheric oxygen. This brown-black oxide layer protects the underlying metal from further corrosion and is not at all the rust which forms on iron in moist air.
- Further, copper does not react with water.
- Copper tarnishes when exposed to some sulphur compounds. It forms various copper sulfides consequently when it reacts with these sulphur compounds.
Read More:
Density of copper
Whenever we say that copper is a heavier metal than aluminum, we are talking about their densities. Density refers to the mass of the substance per its unit volume.
Unless specified usually at room temperature the density of a pure solid chemical element is always numerated as the density of the equilibrium crystalline state of that element.
Density of copper is 8.96 g/cm3.
The density of copper in the unstructured solid state at a given room temperature can be predicted by the ratio of packing fraction.
Read More:
Properties of Copper
The various properties of copper are as tabulated below:
| Properties | Values |
|---|---|
| Atomic weight | 63.546 g/mol |
| Atomic number | 29 |
| Phase | Solid |
| Melting point | 1084.62oC or 1357.77 K |
| Boiling point | 2562oC or 2835 K |
| Density | 8.96 g/cm3 8.02 g/cm3 when in liquid |
| Heat of fusion | 13.26 KJ/mol |
| Heat of vaporization | 300.4 KJ/mol |
| Molar heat capacity | 24.440 J (mol.K) |
| Electronegativity | 1.90 at Pauling scale |
| Ionization energy | 1st: 745.5 KJ/mol 2nd: 1957.9 KJ/mol 3rd: 3555 KJ/mol |
| Atomic radius | 128 pm |
| Thermal expansion | 16.5 μm / (m.K) at 25oC |
| Thermal conductivity | 401 W/(m.K) |
| Hardness | 3.0 Mohs scale |
| Molar magnetic susceptibility | -5.46 x 10-6 cm3/mol |
| Electrical resistivity | 16.78 nΩ•m at 20oC |
| Young’s modulus | 110-128 GPa |
Read More: Covalent bonds
Things to Remember
- Copper is a chemical element, which is denoted by symbol Cu (derived from Latin word cuprum) and has an atomic number 29.
- Copper is material used by us on a daily basis whether directly in the form of wiring and motors, or indirectly in alloys.
- Oxidation states +1 and +2 of copper are often called cuprous and cupric, respectively.
- Copper has malleable and ductile properties.
- Copper has 2nd highest electrical and thermal conductivity at room temperature among pure metals.
- The density of copper is 8.96 g/cm3.
Sample Questions
Ques. Why is it ideal to use copper in making wires? (5 marks)
Ans. Properties of copper that make it ideal for home wiring are its:
- High electrical conductivity
- High tensile strength
- Highly ductile nature
- Excellent resistance to deformation and creep
- Do not get corroded easily
- High thermal conductivity
- Highly malleable, that's why it is used in wiring
Ques. What is the electronic configuration of Cu? (2 marks)
Ans. The atomic no. of Cu is 29. The electronic configuration of Copper is written as [Ar]3d¹º 4s¹. It does not follow the Aufbau principle. It is explained by the relatively low energy gap between 3d and 4s orbital and the stability added by the completely filled d orbital.
Ques. What are the effects of Cu on humans? (2 marks)
Ans. Being in contact with Cu for a long time can cause burning in the nose, throat, causes headaches, feels dizzy, diarrhea. Intensively high copper absorption can damage liver and kidney leading to death. Copper is carcinogenic or not is yet to be determined.
Ques. What are the names of copper's ore? (2 marks)
Ans. It's rare to find copper in its natural state. There are various processes required to extract copper from its ore. It has several ores in the world. Cuprite, bornite, chalcopyrite are some examples of copper ores.
Ques.Name the sulfide ore of copper. Write the technique needed to purify copper from its ores? (1 mark)
Ans. Chalcocite is the sulfide ore of copper. Several processes are needed to extract copper from its ores that include roasting (Heating in the presence of Air), refining, smelting, crushing etc.
Ques. What are the factors which make copper choose between either of the oxidation state +1 or +2? (3 marks)
Ans. "For more stability copper chooses to always be in +1 state" this statement is not entirely correct. In copper chemistry the stability of the oxidation states +1 and +2 entirely depends on whether we have a solution or we have a solid-state material.
Copper (I) is stable only in the solid state (e.g. Cu2O, CuI, CuCl), whereas copper(II) is more stable in solution or when it is in water e.g. in hydrated copper(II) salts. Higher oxidation states (+3 and +4) can be stabilized in combination with O2 or F.
Ques. Explain in brief Metallurgy of copper. (5 marks)
Ans. Extraction of copper
Copper is mainly extracted from pyrites(CuFeS2). Sulphide ore is very low grade and contains many impurities in it. Various steps involved in extraction of copper are:
- Crushing and concentration
In this process ores are crushed in a jaw crusher and then powdered and concentrated through froth floatation. The earthy and siliceous impurities are removed and they settle at the bottom of the tank.
- Roasting
In this process the concentrated ore is then roasted, i.e. heated strongly, in the presence of air. During roasting
- Moisture is then removed and the ore becomes dry
- As metal impurities are volatile oxides, they are removed.
- Copper pyrite is converted into ferrous sulfide(FeS) and Cuprous Sulfide (Cu2S) which are partially oxidized.
2CuFeS2 + O2 → Cu2S+ FeS + SO2
Copper pyrite
2FeS + 3O2 → 2FeO + 2SO2
2CuS + 3O2 → 2Cu2O + 2SO2
- Smelting
In this process the roasted ore is then mixed with coke and silica SiO2 and is introduced into a blast furnace. The hot air is then blasted and FeO is converted into ferrous silicate (FeSiO3).
FeO + SiO2 → FeSiO3
Cu2O + FeS → Cu2S + FeO
The slag i.e. FeSiO3 floats on the copper’s molten matte.
- Bessemerization
Bessemerization is the process through which copper metal is extracted from molten matte. The matte is put into a Bessemer converter which is upheld by tuyers. The air is blown through the molten matte. Blasts of air convert Cu2S partly into Cu2O which then reacts with remaining Cu2S to give out molten copper.
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu2O + Cu2S → 6Cu + SO2
The copper now obtained is called "Blister copper".
- Refining of Cu
Blister copper is refined by electrolysis. Thin sheets of pure copper act as cathodes and blocks of blister copper are used as anodes. In order to remove depositing copper, the cathode plates are coated with graphite. The electrolyte is copper sulphate (CuSO4) mixed with a little amount of H2SO4. This is done to increase the electrical conductivity. For this electrolytic process the optimum potential difference is 1.3 volt. During the process of electrolysis, pure copper is deposited on the cathode plates and impurities, which are soluble, fall to the bottom of the cell as anode mud or sludge.
ELECTROCHEMICAL CHANGES DURING ELECTROLYSIS
Cu --> Cu+2 + 2e- (at the anode)
Cu+2 + 2e- → Cu (at the cathode)
Ques. Explain electroplating of zinc on copper. (5 marks)
Ans. During electroplating, Electrodes of copper and zinc are placed in the beaker containing zinc sulfate, ammonium citrate, and ammonium chloride as electrolyte. Electrodes are connected to DC power supply and a voltage is applied, the positive moves on the zinc electrode and the negative moves on the copper electrode. A white coating of zinc starts covering on the copper electrode, and after some time, the copper electrode has a fine zinc plating on it. Faraday's experiment is used to demonstrate it.
Reduction Half-reaction
Zn2+ + 2e- → Zn
Zn2+ + 2e- → Zn
Ques. Explain in brief about the change of copper surface over time. (3 marks)
Ans. The more rough the metal surface; the greater the contact surface of corrosive media. The surface is then dynamically and more rapidly corroded. This is a general rule for most cases. In addition, corrosion of rough surfaces is increased due to long exposure of metal with retention of water in depressions. In these cases this may lead to pitting corrosion. Another characteristic of the corrosion of rough surfaces is the state of corrosion products. Rough surface is directly proportional to the layer of corrosion products and the better this layer adheres to the metal.
Read More:



Comments