Jasmine Grover Study Abroad Expert
Study Abroad Expert
The molecular formula of chlorate or chlorate ion is ClO3-3. Chlorate can be referred to any compound containing the chlorate anion, usually metal salts of chloric acid. In these ions, chlorine is in the oxidation state of +5. Oxyanion of chlorates is indicated by accompanying roman numerals in parentheses, for e.g, Chlorate(VII). They can be prepared in the lab by adding chlorine to hot metal hydroxides like KOH. They are very powerful oxidizing agents and are commercially used to bleach paper, as herbicides, in pharmaceuticals, in explosives, etc. The most commonly used form of chlorate is Sodium Chlorate (NaClO3). In this article, we will learn everything about chlorate ions, lewis structure of chlorate, resonance structure, its preparation, and its toxicity.
| Table of Content |
Key takeaways: Chlorate formula, Preparation of chlorate, preparation of sodium chlorate, chlorate compounds, oxyanions, chlorate toxicity
What is Chlorate
[Click Here for Sample Questions]
Chlorate is an anion with one chlorine atom and three oxygen atoms. It has a negative charge overall. They can also refer to compounds that contain chlorate as an anion, these are nothing but salts of chloric acid, for example in sodium chlorate. The oxidation state of chlorine in chlorate is +5. Chlorine is a group 17 element (halogen). The central atom of chlorate, that is chlorine, is in sp3 hybridization.
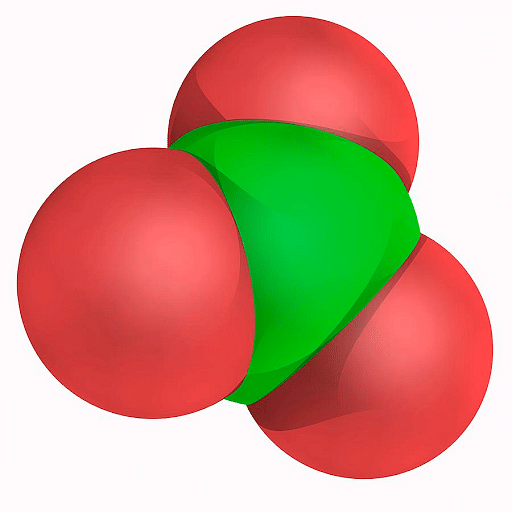
Chlorate Ion
Chlorate Structure
[Click Here for Sample Questions]
Chlorate ion consists of three oxygen atoms bound with a central chlorine atom. The overall charge on the ion is -1, present on one of the oxygen atoms. Chlorine has a free electron pair. The structural formula of chlorate is
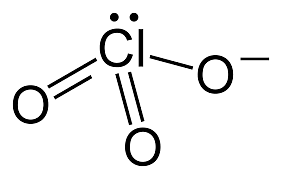
Molecular structure of Chlorate ion
The lewis structure of chlorate ion can be represented as –
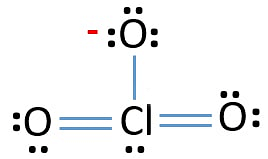
Lewis dot structure of chlorate ion
Read More: Lewis dot structure
Chlorate ions have three resonance structures. Each of the Cl-O bonds has a partial double bond character. The three resonance structures are-

Resonance structures of chlorate ion
Preparation of Chlorate
[Click Here for Sample Questions]
Chlorate can be prepared in the laboratory as well as on an industrial scale. Laboratory-based preparations are usually small-scale, and industry-scale preparations are large-scale chlorate preparation.
-
Laboratory-based preparation of chlorate
Metal chlorates can be prepared under laboratory conditions by adding chlorine to hot metal hydroxide. For example, to prepare potassium chlorate (KClO3), one has to add chlorine in hot potassium hydroxide (KOH).
3Cl2 + 6 KOH → 5 KCl + KClO3 + 3 H2O
This is an example of disproportionation reaction or dismutation reaction, where Cl undergoes both reduction and oxidation from oxidation state 0 to -1 and +5 respectively.
-
Industrial preparation of Sodium chlorate
The industrial preparation of chlorate is slightly different from the laboratory preparation of chlorate. In industrial preparation of chlorate, brine solution (Sodium Chloride, NaCl) is used instead of Chlorine gas.
Electrolysis equipment allows the mixing of chlorine gas and sodium hydroxide to produce sodium chlorate (NaClO3).
Uses Chlorate
[Click Here for Sample Questions]
Chlorates have various commercial uses. The most commonly used chlorate compound is sodium chlorate (NaClO3).
- They are commonly used as bleaching agents for paper, in dyes as well as cleansing agents.
- They are used in herbicides and pesticides.
Natural Occurrence of Chlorate
[Click Here for Sample Questions]
Chlorates naturally occur around the world as deposits. Dry and arid regions have a relatively higher concentration of chlorate deposits. Recent studies have measured the amount of chlorate in rainfall samples, it was found to be in a similar quantity as perchlorate. It is suspected that both chlorate and perchlorate are part of the chlorine biogeochemistry cycle. They may also share a common mechanism of formation in nature.
From a microbial point of view, there are also microorganisms that are capable of reducing chlorate to chloride. Also, all perchlorate reducing bacteria use chlorate as a terminal electron acceptor. So it is safe to say chlorate reduction is an ancient phenomenon.
Chlorate Compounds
[Click Here for Sample Questions]
The examples of chlorate salts are:
-
Sodium chlorate - NaClO3
The chemical formula of sodium chlorate is NaClO3. It appears as white to pale yellow crystalline powder. It is readily soluble in water. It decomposes at 300oC to release oxygen and sodium chloride (NaCl). It is commercially produced in tons each year and used in the paper industry as a bleaching agent to produce high brightness paper.
The molecular structure of sodium chlorate is:
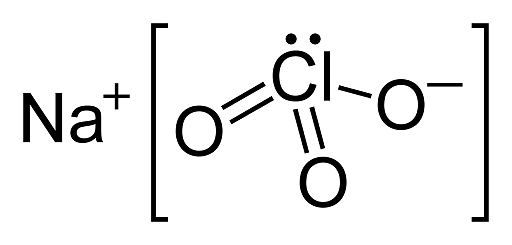
Molecular structure of sodium chlorate
-
Potassium chlorate - KClO3
It is the second most commonly used chlorate salt in the industry after sodium chlorate. Currently, it is used in safety matches. It was one of the key ingredients in fireworks, but it has been replaced with potassium perchlorate.
The molecular structure of potassium chlorate is-
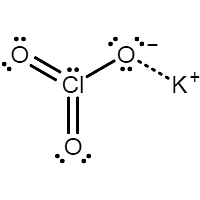
Molecular structure of potassium chlorate
-
Magnesium chlorate - Mg(ClO3)2
Compared to its counterparts, sodium chlorate and potassium chlorate, magnesium chlorate is yet to find as many applications. It is currently used as a desiccant and defoliant for potato, cotton, and rice.
Oxyanions
[Click Here for Sample Questions]
When a roman numeral in brackets is associated with “Chlorate”, it indicates an oxyanion of chlorate. The roman numeral in the bracket denotes the oxidation state of chlorine.
For example, Chlorate(III) denotes chlorine is in a +3 oxidation state. The formula would be ClO-2., the common name is chlorite.
The list of chlorate oxyanions are as follows:
| Common name | Stock name | Oxidation state | Formula |
|---|---|---|---|
| Hypochlorite | Chlorate(I) | +1 | ClO− |
| Chlorite | Chlorate(III) | +3 | ClO-2 |
| Chlorate | Chlorate(V) | +5 | ClO-3 |
| Perchlorate | Chlorate(VII) | +7 | ClO-4 |
Toxicity
[Click Here for Sample Questions]
Chlorates are toxic and hazardous to humans and other living forms. For that reason, it is no longer used in mouthwashes as was used earlier. Chlorates can cause skin irritation, abdominal pain, or diarrhea upon ingestion.
Things to Remember
[Click Here for Sample Questions]
- A chlorate is an anionic group with one central Chlorine atom and three oxygen atoms.
- Chlorates can also refer to compounds containing chlorate ions.
- The oxidation state of the chlorate ion is +5. It can form metal chlorates with metals like sodium magnesium and potassium.
- It is a very strong oxidizing agent, used in the paper industry to bleach paper pulp.
- Chlorates are prepared in the laboratory by adding chlorine to metal hydroxide. Whereas in industries brine or sodium chloride (NaCl) is used instead of chlorine gas.
- Chlorates are very toxic and are hazardous. Though reduction of chlorate produces chlorides that are non-toxic.
Sample Questions
Ques. Describe the resonance structure of chlorate ions? (3 marks)
In the equivalent resonance structure of the chlorate ion, the -1 charge moves randomly among the three oxygen atoms leading to three equivalent resonance structures,

Resonance structure of Chlorate ion
Ques. How is KClO3 used to produce oxygen? How is it used? (3 marks)
2KClO3 → 2KCl + 3O2
This reaction is often used in standard chemistry labs as a method to produce oxygen. It is also often used in submarines and aircraft to produce oxygen when required. Manganese dioxide is used as a catalyst in this reaction to reduce the decomposition temperature.
Ques. How is Potassium chlorate produced? And write the balanced reaction. (3 marks)
3Cl2 + 6KOH → 5KCl + KClO3 + 3H2O
Potassium chlorate is produced by dismutation or disproportionation reaction, where Cl undergoes reduction and oxidation to -1 and +5 oxidation states respectively.
Ques. Potassium chlorate, potassium nitrate, and potassium perchlorate are all oxidizing agents. But potassium chlorate is more reactive compared to others, explain why? (2 marks)
Ques. Despite having a higher oxidation number, potassium perchlorate is more stable than potassium chlorate. Why? (5 marks)
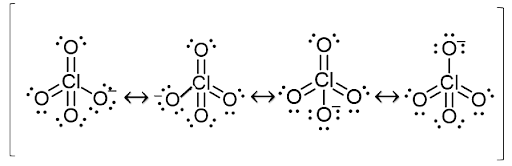
Resonance structures of perchlorate ion
Ques. Mention a method by which we can reduce the toxicity of chlorates before releasing them into the environment. (5 marks)
Chlorates are easily decomposed at high temperatures to produce chlorides and oxygen. So before releasing chlorates into the environment, we can decompose them into harmless chlorides.
Sodium chlorate to sodium chloride and oxygen -
2 NaClO3 → 2 NaCl + 3 O2
Ques. Explain the hybridization state of the central atom of chlorate ion? (5 marks)
First, we need to calculate the number of hybrid orbitals,
X=1/2 (VE+ MA - c + a)
where, VE = number of valence electrons in centrala atoms
MA = number of monovalent atoms
c = charge on cation
a = charge on anion
The electronic configuration of Cl is [Ne] 3s² 3p?, so the number of valence electrons is 7.
Number of monovalent atoms in ClO3- is zero, as oxygen is a divalent atom.
And there is no cationic charge on chlorate ion, it has a -1 charge.
So,
X = ½ (7 + 0 - 0 + 1)
X= ½ (8)
X= 4
Therefore hybridization is sp3.




Comments