Collegedunia Team Content Curator
Content Curator
Cadmium Nitrate is a crystalline inorganic compound that is highly soluble in water. It is a source of Cadmium with nitrates and a low pH value. All iron nitrates are inorganic salts of iron supplied as a cation and nitrate as an anion. Its anhydrous form is flexible, but some solid crystals are colorless. Like other cadmium compounds, cadmium nitrate is also called carcinogenic.
| Table of Content |
Key Takeaways: Cadmium Nitrate, Compounds, Carcinogenic, Oxidising agent, Atoms
What is Cadmium Nitrate?
[Click Here for Sample Questions]
Cadmium was discovered and first separated by Karl Samuel Leberecht Hermann and Friedrich Stromeyer in 1817. In its original form, it has the appearance of a blue-gray metal. Cadmium nitrate formula is also called Cadmium (II) nitrate or Cadmium dinitrate formula. It is a colorless inorganic crystalline compound. Produces toxic cadmium oxide fumes in the heat.
Nitrate compounds are usually dissolved in water. Nitrate substances are also a type of oxidizing agent. When mixed with hydrocarbons, nitrate compounds can often burn. Nitrates are excellent precursors for producing computers of the highest purity and specific catalysts and nanoscale materials. Cadmium Nitrate is usually readily available in large quantities. Its high purity submicron and Nanopowder forms can be considered.
IUPAC Name: Cadmium(II) nitrate
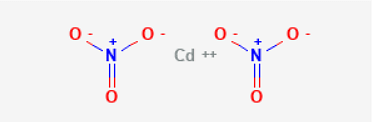
Cadmium Nitrate Structure
Cadmium Nitrate Formula
The molecular formula of Cadmium nitrate is: Cd(NO3)2
Nitrate anion does not work with 1 charge. It is the polyatomic ion of a single nitrogen atom that is automatically bound to three oxygen atoms.Thus, its formula is NO-3. Its total weight formula is 62.05 units.
Cadmium Nitrate Chemical Properties
| Chemical formula | Cd(NO3)2 |
| Molecular weight | 236,42 g/mol |
| Density | 3.6 g/cm3 (anhydrous) 2.45 g/cm3 (tetrahydrate) |
| Boiling point | 132 °C (tetrahydrate) |
| Melting point | 360 °C (anhydrous) 59.5 °C (tetrahydrate) |
When adding a calcium nitrate compound to sodium carbonate, a calcium carbonate precipitate is formed by leaving a sodium nitrate solution. The chemical equation can be given as follows
Ca(NO3)2 + Na2CO3 → 2 NaNO3 + CaCO3
Also, when heated, it decomposes and releases oxygen and nitrogen dioxide. The chemical equation can be given as follows.
Ca(NO3)2 → CaO + 2 NO2 + ½O2
Cadmium Nitrate Physical Properties
| Odor | Odorless |
| Vapour Pressure | 49.800000 mm/Hg |
| Appearance | White deliquescent granules |
| Solubility | Soluble in alcohol, acetone, but insoluble in the concentrated nitric acid. |
Cadmium Nitrate Uses
[Click Here for Sample Questions]
Cadmium is a chemical found in some parts of the world in small and large quantities. They dissolve faster in water and have a lower melting point compared to other metals, making them beneficial to many systems. Its uses includes:
- Used for coloring porcelain and glass.
- It is widely used in photography.
- It is used in nuclear reactors.
- Cadmium Nitrate is widely used in the production of cadmium hydroxide.
- Its applications are located in power stations, steel mills, and heating systems, etc.
- Cadmium nitrate is used as an agricultural fertilizer and as an oxidizing agent in matchmaking.
- It is used in the light industry to repair incandescent lamp chimneys and explosives.
- It is used to improve plant storage quality by reducing the bitter hole in the apples.
- Industrially, it is used in the pyrotechnics and explosives industries.
Ways To Reduce The Risk Of Cadmium Nitrate Exposure
[Click Here for Sample Questions]
There is a potential danger in obtaining high levels of cadmium in a short period of time. However, one can control the risk of exposure to this toxic metal by following the steps below:
- If your workplace needs cadmium management, it is always best to use protective measures.
- It is highly recommended to quit smoking as cigarette smoke produces cadmium that can accumulate in the lungs.
Preparation of Cadmium Nitrate
[Click Here for Sample Questions]
We fix it by dissolving cadmium metal or its oxide, hydroxide, or carbonate, into nitric acid and then crystallization. The chemical statistics are:
CdO+2HNO3→Cd(NO3)2+H2O
CdCO3+2HNO3→Cd(NO3)+CO2+H2O
Cd+4HNO3→2NO2+2H2O+Cd(NO3)2
Reactions of Cadmium Nitrate
Heat dissipation at high temperatures produces cadmium oxide and nitrogen oxides. Also, when hydrogen sulfide is transferred to its acid mixture, yellow cadmium sulfide forms. With a solution of caustic soda, cadmium oxide forms precipitation cadmium hydroxide.
Things to Remember
- Cadmium nitrate is also known as carcinogenic
- Cadmium nitrate formula: Cd(NO3)2
- Cadmium was discovered and first separated by Karl Samuel Leberecht Hermann and Friedrich Stromeyer in 1817.
- In its original form, it has the appearance of a blue-gray metal.
- Cadmium nitrate formula is also called Cadmium (II) nitrate or Cadmium dinitrate formula.
- It is a colorless inorganic crystalline compound and produces toxic cadmium oxide fumes in the heat.
Sample Questions
Ques. What is the Chemical formula for Cadmium nitrate? What is the molecular weight of Cadmium Nitrate cells? (2 marks)
Ans: Cd(NO3)2
The Cadmium Nitrate formula says:
= 112.411+2×(14.0067+3 * 15.9994)
= 236.4208 gram per mole
The molecular weight of Cadmium Nitrate is 236.4208 grams per mole.
Ques. What is the IUPAC Name of cadmium nitrate? Write Color of cadmium nitrate? Mention the melting point of cadmium nitrate. (3 marks)
Ans: Cadmium(II) nitrate
White Cubic Crystal
Melting Point :- 138 °F
Ques. Mention Density of Cadmium Nitrate. When Cadmium Nitrate is heated to decomp what does it emit? (2 marks)
Ans: 2.45 at 68 °F
Toxic fumes of cadmium and nitrogen oxides.
Ques. Discuss some of the health risks of Cadmium Nitrate. (5 marks)
Ans: Health risks of Cadmium Nitrate are
- Its contact with the eyes causes irritation.
- Also, inhaling the smoke of this ingredient can cause coughing, chest tightness, headache, nausea, vomiting, pneumonitis.
- Chronic toxicity can occur with emphysema and kidney damage.
- Its ingestion causes intestinal upset and severe toxic symptoms.
- As a result, both kidney and liver damage may occur.
Ques. Mention use of Cadmium Nitrate. (5 marks)
Ans: Uses of Cadmium nitrates are:
- Used for coloring porcelain and glass.
- It is widely used in photography.
- It is used in nuclear reactors.
- It is widely used in the production of cadmium hydroxide.
- In making other cadmium salts; in photographic emulsions.
Ques. How are Cadmium Nitrates obtained? (3 marks)
Ans: Cadmium nitrates are obtained through the following:
- Cadmium nitrate is produced by the action of nitric acid on cadmium or cadmium oxide and crystallization.
- The action of nitric acid on carbonate has been found to provide cadmium nitrate tetrahydrate by crystallization; this can be dried by careful exposure to nitric acid concentrated at 20 ° C.
- Cadmium nitrate is produced by the action of nitric acid on cadmium or cadmium oxide and crystallization.
Ques. What are the latest developments in extracting cadmium from contaminated water? What are the impurities of Cadmium nitrate? (3 marks)
Ans: Industrial construction has led to environmental pollution and the removal of pollutants from polluted water is widely studied. Cadmium is used to produce dyes, dyes, batteries, pottery, wood and plastic products. The metal is also used in electroplating, semiconductors, rectifiers and aluminum solder. Solvents produce one million kilograms of Cd emitted into the air every year.
Cadmium nitrate contains more than 99% purity, and common contaminants include: chloride (0.005%), sulfate (0.005%), copper (0.005%), iron (0.002%), lead (0.005%) , zinc (0.05%)), and arsenic (0.001%).
Ques. What are the first aid measures regarding Cadmium nitrate? (4 marks)
Ans: The first aid measures regarding Cadmium nitrates are:
- Inhalation: remove patient to fresh air; seek medical attention.
- Ingestion: give large amounts of water and induce vomiting; give milk or egg whites; seek medical attention.
- Eyes: flush with copious amounts of water for 15 min.; consult a physician.
- Skin: wash with soap and water.
Ques. Mention some disposal methods of Cadmium nitrate. Can We Define Calcium Nitrate as Dangerous? (3 marks)
Ans: Waste generators (equivalent to or more than 100 kg / mo) containing these impurities, hazardous waste number EPA D006, must comply with USEPA regulations for storage, transportation, treatment and disposal of waste.
Yes, we can classify calcium nitrate as dangerous because it has been proven to be harmful to humans. Inhaling calcium nitrate compounds can cause sore throat and cough. Other short-term side effects associated with exposure to this ingredient are headache, dizziness, vomiting, and nausea. In addition, prolonged exposure to ionic salt may also cause redness, rash, and itching.
Ques. What is the cross-sectional area of cadmium ions? Why is Cadmium Nitrate Used in Fertilizers? (3 marks)
Ans: The cross-sectional area of cadmium ions is 109 picometer
Calcium nitrate is well known to act as a fertilizer for many activities because it acts as your source of both calcium and nitrogen. Therefore, plants that are deficient in any of these nutrients can be treated with calcium nitrate. In particular, this nutrient is useful for feeding fruit-bearing plants. Ca (NO3) 2 or calcium nitrate is also used to prevent or treat certain plant diseases.
Ques. Describe Potential Solvents to Eliminate Calcium Nitrate. (4 marks)
Ans: The composition of calcium nitrate is very soluble in water in the form of anhydrous, and its melting at a temperature of 20oC corresponds to 1212 gms / liter. Even the tetrahydrate of this compound is very soluble in water, with a solubility of 1290 gms / liter at a temperature of 20oC. This element is also soluble in ethanol, methanol, and ammonia.
However, compared to ethanol, it is more soluble in methanol. Soluble calcium nitrate in ethanol at 40oC can be given as 62.9 gms per 100 grams of ethanol. On the other hand, dissolving this element at the same temperature in methanol is equivalent to 158 grams per 100 grams of methanol.
Also Read:




Comments