Muskan Shafi Education Content Expert
Education Content Expert
Tyndall Effect is a phenomenon that involves scattering of a light beam by fine suspended particles, like water drops, dust particles, and molecules of air. Tyndall effect is named after John Tyndall, the Irish physicist who first discovered it. The phenomenon occurs when a beam of light passes through colloidal/fine particles, scattering the light and making the particles as well as the path of the light beam visible. For instance, dust in a room makes the light beam entering a window visible.
Read More: The Human Eye and The Colourful World Important Questions
Key Terms: Tyndall Effect, Light, Colloidal Solution, Scattering of Light, Wavelength, Spectrum of Light, Particles, Blue Light
What is Tyndall Effect?
[Click Here for Sample Questions]
Tyndall effect is a phenomenon in which a light beam is scattered in such a manner that the path or trajectory of the light beam can be observed. Tyndall effect is exhibited by all colloidal solutions as well as some very fine suspensions. Tyndall effect can thus be used to verify whether a given solution is a colloid.
- The colour and density of scattered light depend on the size and weight of the scattering particles and the frequency of the beam of light.
- If the particles are very fine then the light scattering through them will appear mainly as blue light.
- Another reason for blue light's appearance is that when compared with red light, blue light can be scattered to a greater extent and its wavelength is smaller.
- If the particles are of a larger size then the scattered light can appear as white light.
For the Tyndall effect to occur, the particles’ diameter should range from 40 to 900 nanometers (1 nanometer = 10-9 meters) and the wavelength of the light spectrum should range between 400 to 750 nanometers.
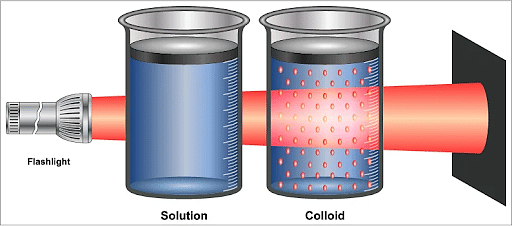
Tyndall Effect
The video below explains this:
Human Eye One Shot Detailed Video Explanation:
Cause of Tyndall Effect
[Click Here for Previous Years Questions]
Tyndall Effect is an outcome of the interaction between the visible spectrum of light and the constituent particles of a colloidal solution.
- The greater the interaction between the particles and the light beam would be, the greater the amount of light will scatter and we will be able to see the Tyndall effect clearly.
- Tyndall effect is not observed in a true solution as the size of its constituent particles is smaller than 1 nm, which is the wavelength of the visible spectrum, and thus, the solution does not exhibit the Tyndall effect.
Read More: Colour of the Sun at Sunrise and Sunset
Examples of Tyndall Effect
[Click Here for Sample Questions]
Here are a few examples of the Tyndall Effect in our day-to-day lives:
- Tyndall effect is the reason behind the blue colour of the sky.
- When a beam of light passes through a glass of milk, light is scattered through milk because milk is a colloidal substance.
- In a foggy environment, when the light hits the molecules of air, it collides with the particles, causing the light to scatter and diffuse in multiple directions because fog is a colloidal substance.
- Similarly, when the sunlight passes through multiple layers of the cloud, the light scatters and diffuses on the ground in multiple directions.
- The colour of the iris of an eye is blue or green or hazel. There are no blue, green, or hazel pigments present in the iris, then how? It is because of the Tyndall effect, light scattering in the stroma and reflecting the light in blue, green, or hazel colour.
- Another example is when the sun sets and the sky colour changes, it also complies with the Tyndall effect, which depends on the sun’s position and the atmosphere when the light is passing through the sunset.

Things to Remember
- Tyndall Effect is the scattering of a light beam by small suspended particles in such a way that its path becomes visible.
- The phenomenon was named after John Tyndall, an Irish physicist.
- Tyndall effect is observed when the visible spectrum of light and the constituent particles of a colloidal solution interact with each other.
- Tyndall effect can be used to identify a colloidal solution as it is a characteristic feature of them.
- Particles with diameters from 40 to 900 nanometers can cause the Tyndall effect.
Read More: NCERT Solutions for Class 10 Science
Previous Years’ Questions (PYQs)
- The Tyndall effect, associated with colloidal particle is due to…
- Tyndall effect is shown by…
- The right option for the statement ''Tyndall effect is exhibited by'', is…
- The phenomenon observed when a beam of light is passed through a…
- Tyndall effect would be observed in a…
- Near and far points of human eye are…
- Scattering of light takes place in…
- The innermost layer of the human eye… (KEAM)
- Random motion of colloid particle is known as…
- Milk is an example of…
Sample Questions
Ques. Illustrate some examples of the Tyndall effect. (3 Marks)
Ans. Here are some examples of the Tyndall effect:
- When the sunlight passes through a large number of dust particles in a room or a canopy of forests, the path of the sunlight becomes visible.
- The scattering of light by water droplets in the air is also an example of the Tyndall effect.
- On switching on a torch in a foggy environment, the path of the light becomes visible. It happens because the water droplets in the fog scatter the light.
Ques. What is Tyndall Effect? (2 Marks)
Ans. Tyndall effect is the scattering of a beam of light through fine suspended particles, such as dust or smoke in a room causing the beam of light entering the room to become visible.
Ques. State the reason why Tyndall Effect is responsible for blue eye colour. (3 Marks)
Ans. The blue colour of the iris is the most exciting example of the Tyndall effect. The translucent layer present over the iris causes the scattering of blue light, leading to making the appearance of blue eyes due to the scattering of blue light. There is a high concentration of melanin in this layer, which is generally opaque. But in the case of blue eyes, this layer is translucent, which contributes to the blue colour of the eye.
Ques. Why is the blue colour scattered the most in the Tyndall effect? (2 Marks)
Ans. Blue light is scattered to a larger extent when compared to red light. It is because the wavelength of blue light is much smaller than that of red light.
Ques. Explain Tyndall Effect in Solutions, Colloids and Suspension. (3 Marks)
Ans. Tyndall Effect in Solutions, Colloids and Suspension will be as follows:
- Solution: Do not scatter light.
- Colloids: Scatter light.
- Suspension: Either scatter light or be opaque.
Ques. Why do true solutions not display the Tyndall effect? (1 Mark)
Ans. True solution doesn’t show the Tyndall effect because the diameter of the particles is smaller than the wavelength of the light, which results in no scattering of light.
Ques. Describe the change observed when the light passes through NaCl (Sodium Chloride) solution followed by a sol. (1 Mark)
Ans. When the beam of light passes through NaCl (Sodium Chloride) solution followed by a sol, the Tyndall effect is observed in the sol.
Question: Which among the following does not show the Tyndall effect?
(A) Emulsion
(B) Blood
(C) Milk
(D) Sugar Solution (1 Mark)
Ans. (D) Sugar Solution. The sugar solution is not a colloidal solution but a true solution. The Tyndall effect can only be shown when the solution is colloidal and the particles in it can scatter the light passing through it, hence proving the Tyndall effect can’t be shown by the sugar solution.
Ques. Pyrometer works on the basis of:
(A) Laser technology
(B) Photo-conduction
(C) Thermal emission
(D) Tyndall effect (1 Mark)
Ans. (D) Tyndall effect.
Ques. Optical fibre operates on the basis of which of the following principle:
(A) Total internal reflectance
(B) Tyndall effect
(C) Photo-electric effect
(D) Laser technology (1 Mark)
Ans. (B) Tyndall effect.
Ques. In the Tyndall phenomenon can we see light only at a right angle? (1 Mark)
Ans. In the Tyndall phenomenon, we see light not only at the right angle but also in different directions. When the light hits the colloidal solutions and gets scattered by the colloidal particles, it doesn’t just depend on the solutions but also on the wavelengths and the light frequency.
Check-Out:




Comments