Collegedunia Team Content Curator
Content Curator
MCQs on pH cover questions related to pH scale, pH indicators, and the importance of pH in everyday life. The pH value of a solution represents its acidic and basic levels. Solutions are generally acidic, neutral, or basic according to their pH value. The pH value of a solution is numerically equal to the logarithm of the inverse of the hydrogen ion (H+) concentration.
Some of the key concepts covered in these MCQs are:
- pH Value
- Importance of pH in Everyday Life
- pH Value in our Surroundings
Learn About: pH Of Acids and Bases
Ques 1. Which of the following statement is correct regarding the pH Scale?
- It is the negative logarithm of the H+ ion concentration of a given solution.
- It is the positive logarithm of the H+ ion concentration of a given solution.
- It is a 14-point scale.
- pH is an example of an extrinsic property.
Correct options can be:
- 1 and 3
- 2 and 3
- 1, 3 and 4
- Only 2
Click here for the answer
Ans. Option A. is the correct answer.
Explanation: pH scale is the negative logarithm of the H+ ion concentration of a given solution. It is a 14-point scale.
Ques 2. What is the neutral value of the pH scale?
- Less than 5
- Equal to 7
- Less than 8
- Less than 10
Click here for the answer
Ans. Option B. is the correct answer
Explanation: pH ranges from 0 to 14, where 7 is Neutral. pH value less than 7 is acidic and pHs greater than 7 are alkaline (or basic in nature).
- At pH 7, concentrations of H+ and OH- are equal. Thus, pH 7 is considered the neutral pH.
- Below pH 7, concentration of H+ is greater than the concentration of OH-, making the water acidic.
- Above pH 7, OH- concentration is greater than the concentration of H+, making water basic.
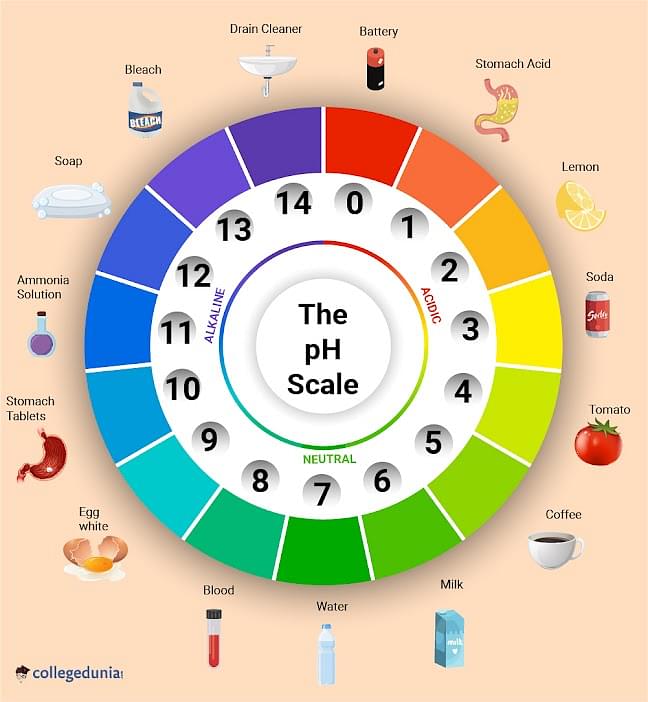
Here’s a diagram to help you understand the pH scale.
Ques 3. Who invented the pH Scale?
- S.P.L Sorenson
- Benjamin Franklin
- Henry Moseley
- Wilhelm Rontgen
Click here for the answer
Ans. Option A. is the correct answer.
Explanation: In 1909 S.P.L. Sorensen, a Danish chemist, introduced the concept of pH as a convenient way of expressing acidity. pH scale tests the acidity or alkalinity of a substance or solution.
Ques 4. In which of the following field pH scales is important for measurements?
- Medicine
- Forestry
- Food Science
- All of the above
Click here for the answer
Ans. Option D. is the correct answer.
Explanation: pH Scales are important for various applications including Medicine, Forestry, and Food Science. pH is an important quantity that reflects the chemical conditions of a solution. The pH can control the availability of nutrients, microbial activity, biological functions, and the behavior of chemicals.
Ques 5. What is the pH value of very strong acid solution?
- Less than 7
- Less than 5
- Less than 2
- Less than zero
Click here for the answer
Ans. Option D. is the correct answer.
Explanation: Solutions with a pH value equal to 0 are known to be strongly acidic solutions. The acidity decreases as the value of pH increases from 0 to 7. Solutions with a pH value equal to 14 are strongly basic solutions.
Ques 6. Why do we measure the pH of seawater?
- It helps in corrosion research.
- It helps in agricultural activity.
- It helps in fermentation.
- It helps in sterilization.
Click here for the answer
Ans. Option A. is the correct answer.
Explanation: Seawater pH measurement helps in understanding that changes in pH can affect organisms living in the sea, and, as a result, humans can also get affected.
Check out:
Ques 7. Which statement is correct regarding Buffer Solution?
- It is a solution whose pH changes when a small amount of an acid or base is added to it.
- It is a solution whose pH does not change when a small amount of an acid or base is added to it.
- It does not use pH value as a constant in a wide variety of chemical applications.
- The solution of methanoic acid is an example of an effective buffer solution.
Click here for the answer
Ans. Option B is the correct answer.
Explanation: pH Buffer solutions help in calibrating a pH controller with a pH sensor (probe). The most common pH buffer solutions are pH4, pH7, and pH10. These buffer solutions have different colors which make them easy to distinguish. pH 4 is often red, pH7 green, and pH10 blue.
Ques 8. What is the pH value of saliva after meal?
- 4.8
- 5.8
- 6.8
- Less than 4
Click here for the answer
Ans. Option B. is the correct answer.
Explanation: After every meal, sugar in food items breaks down to corresponding acids by the bacteria’s actions. When the acidity increases, the pH decreases.
Ques 9. What is the pH value of pure water?
- Less than 7
- Greater than 7
- Equal to 7
- Greater than 14
Click here for the answer
Ans. Option C. is the correct answer.
Explanation: Pure water has a pH value of 7. It is considered “neutral” because it has neither acidic nor basic qualities.
Ques 10. How to identify if a solution is acidic?
- If its pH value is less than 7
- If its pH value is greater than 7
- If its pH value is less than 5
- If its pH value is 5
Click here for the answer
Ans. Option A. is the correct answer.
Explanation: pH value that falls below 7, indicates acidity. A pH level higher than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic.
Also check:




Comments